Abstract
Background
Strain-specific effects of probiotics in pro- or anti-inflammatory immune responses have been well recognized. Several proinflammatory Lactobacillus strains have been shown to act as adjuvants to enhance the immunogenicity of vaccines. However, dose effects of probiotics in modulating immune responses are not clearly understood. This study examined the dose effects of Lactobacillus acidophilus (LA) NCFM strain on T cell immune responses to rotavirus vaccination in a gnotobiotic (Gn) pig model.
Methods
Frequencies of IFN-γ producing CD4+ and CD8+ T cell and IL-10 and TGF-β producing CD4+CD25+ and CD4+CD25- regulatory T (Treg) cell responses were determined in the intestinal and systemic lymphoid tissues of Gn pigs vaccinated with an oral human rotavirus vaccine in conjunction with low dose (5 feedings; up to 106 colony forming units [CFU]/dose) or high dose (14 feedings; up to 109 CFU/dose) or without LA feeding.
Results
Low dose LA significantly promoted IFN-γ producing T cell responses and down-regulated Treg cell responses and their TGF-β and IL-10 productions in all the tissues compared to the high dose LA and control groups. To the contrary, high dose LA increased the frequencies of Treg cells in most of the tissues compared to the control groups. The dose effects of LA on IFN-γ producing T cell and CD4+CD25- Treg cell immune responses were similar in the intestinal and systemic lymphoid tissues and were independent from the vaccination.
Conclusion
Thus the same probiotic strain in different doses can either promote or suppress IFN-γ producing T cell or Treg cell immune responses. These findings have significant implications in the use of probiotic lactobacilli as immunostimulatory versus immunoregulatory agents. Probiotics can be ineffective or even detrimental if not used at the optimal dosage for the appropriate purposes.
Keywords: Probiotics, Lactobacilli, Treg cells, Rotavirus vaccine, Gnotobiotic pigs
1. Introduction
Intestinal commensal bacteria and probiotics, including lactobacilli, help maintain gut homeostasis by balancing proinflammatory and antiinflammatory mucosal responses. Many Lactobacillus strains are known to have immune regulatory functions and have been tested clinically or experimentally for therapeutic effects on controlling inflammatory diseases, autoimmune diseases and allergies, such as using L. gasseri, L. fermentum and L. salivarius for inflammatory bowel disease [1, 2], L. casei for rheumatoid arthritis [3, 4], L. rhamnosus GG for eczema [5], and L. fermentum for atopic dermatitis [6]. However, same Lactobacillus strains have sometimes been shown to have opposite immune modulatory functions by different studies. L. casei was reported by one study to be a pure Th1 inducer [7] while it was reported by another study to promote regulatory T (Treg) cell response and suppress Th 1 type responses [3]. L. acidophilus (LA), L. gasseri, L. fermentum, L. casei, L. plantarum, L. johnsonii, and L. rhamnosus were all reported to stimulate human or murine dendritic cells (DCs) to produce increased levels of proinflammatory cytokines (IL-2, IL-12, TNF-α) that favored Th1 and cytotoxic T cell polarization, and decreased levels of the regulatory cytokine TGF-β [7-13]. Such immunostimulatory effects are common characteristics to vaccine adjuvants. Adjuvanticity of various Lactobacillus strains in enhancing cellular and/or humoral immune responses has been reported in studies of influenza, polio, rotavirus and cholera vaccines and rotavirus and Salmonella typhi Ty21a infections [14-21].
Although the strain-specific effects of probiotics in up- or down-regulating proinflammatory immune responses [22] or in inducing Treg cell responses [23] have been well recognized, the dose effects of probiotics on such immune responses are not clearly understood. A recent study on the dose-dependent immunomodulation of human DCs by the probiotic L. rhamnosus Lcr35 showed that very different profiles of gene expression were induced with different doses [24]. The authors suggested that depending on the doses ingested and their frequency, the effects of probiotics could be very different and suitable for the treatment of different diseases via pro- or anti-inflammatory responses [24].
The objective of the present study is to evaluate the dose effects of immunomodulation of probiotic LA on T cell responses and protection induced by an oral rotavirus vaccine. Our overall goal is to identify the LA dose that is most effective in potentiating the vaccine-induced protective immunity. Gnotobiotic pig monoassociated with LA provides an ideal animal model for the study. The distinctive advantages of using the Gn pig model to study the immunomodulating mechanism of different doses of lactobacilli include: (1) the highly recognized similarities between human and porcine intestinal physiology and mucosal immune system; (2) the gnotobiotic status prevents confounding factors from commensal microflora that are present in conventionally reared animals or in humans; and, (3) unlike Gn mice, Gn pigs are devoid of maternal antibodies thus providing an immunologically naïve background that allows clear identification of the immune responses to a single vaccine in hosts colonized with a qualitatively and quantitatively defined probiotic bacterial strain. The Gn pig model of human rotavirus (HRV) infection and probiotic lactobacilli colonization has been well established in our previous studies [17, 25, 26].
The prominent cell types involved in transducing immunomodulating signals from probiotics to protective effector/memory T and B cell immune responses include epithelial cells, macrophages, DCs and Treg cells. Natural Treg cells (nTreg cells) develop in the thymus and enter the periphery sites as CD4+CD25+ T cells [27]. The transcription factor FoxP3 is used to phenotypically define the suppressor population of CD4+CD25+ cells [28], therefore nTreg cells in our study were defined as CD4+CD25+FoxP3+ cells. Inducible Treg cells (iTreg cells) are developed from naive CD4+CD25- T cells under specific antigen exposure and/or cytokine stimulation in the periphery sites, and express FoxP3 and CTLA-4 and secrete IL-10 and TGF-β [28]. A major subset of iTreg cells are phenotypically defined as CD4+CD25-FoxP3+ cells (with the stimulation of highly antigenic epitopes [29]). However, another subset of iTreg cells are defined as CD4+CD25+FoxP3+ cells (with the stimulation of weak antigenic epitopes [30]). Therefore, CD4+CD25+ Treg cells may include both nTreg and iTreg cells. In this study, we investigated the effects of high dose and low dose LA feeding on virus-specific IFN-γ producing CD4+ and CD8+ T cell responses, and IL-10 or TGF-β producing CD4+CD25+ Treg and CD4+CD25- Treg cell responses in Gn pigs vaccinated with an oral attenuated HRV (AttHRV) vaccine and challenged with the virulent HRV (VirHRV).
There is accumulating evidence that probiotics’ immunomodulating effects are not limited to the gut. Oral administration of L. fermentum CECT5716 potentiated the Th1 type response in blood and virus-neutralizing antibody levels in serum of adults vaccinated parentally with an influenza vaccine [15]. LA feeding significantly increased IFN-γ producing CD8+ T cell responses to AttHRV vaccine not only in the intestine but also in spleen and blood in Gn pigs [17]. An important question we also aim to address is whether varying oral doses of probiotics differentially modulate the intestinal versus systemic immune responses. We measured the T cell responses in the intestinal (ileum and intraepithelial lymphocytes [IEL]) and systemic (spleen) lymphoid tissues and blood of Gn pigs and associated the enhanced intestinal IFN-γ producing T cell with increased protection in the low dose LA group. We demonstrated that high dose and low dose LA can exert qualitatively different immune modulating effects on IFN-γ producing T cell and Treg cell responses with and without rotavirus vaccines and the effects were similar at the intestinal and systemic sites.
2. Materials and methods
2.1. Virus
The cell culture-adapted Wa strain (G1P1A[8]) HRV, derived from the 34th passage in African green monkey kidney cells (MA104), was used as the AttHRV vaccine to inoculate Gn pigs at a dose of 5 × 107 fluorescent focus-forming units (FFU) [31]. The AttHRV was also used as detector antigens in the enzyme-linked immunosorbent assay (ELISA) and as stimulating antigens in the intracellular IFN-γ staining assay as described previously [32].
The VirHRV Wa strain was passaged through Gn pigs and the pooled intestinal contents from the 23th passage were used to challenge Gn pigs at a dose of 1 × 105 FFU. The median infectious dose (ID50) and median diarrhea dose (DD50) of the VirHRV in Gn pigs were determined as approximately 1 FFU [33].
2.2. Bacteria
The Lactobacillus acidophilus NCFM (NCK56) strain (LA) was used in this study and was propagated in lactobacilli MRS broth (Weber, Hamilton, NJ, USA). LA inoculums were prepared and titrated as we previously described [26]. The enumeration of LA in Gn pig fecal samples was performed throughout the experiment to confirm colonization as previously described [26].
2.3. Treatment groups and inoculation of Gn pigs
Near-term pigs were derived by hysterectomy and maintained in germ-free isolator units as described [34]. All pigs were confirmed seronegative for rotavirus antibodies and germ-free prior to LA and AttHRV exposure. Gn pigs (both males and females) were randomly assigned to six treatment groups: (1) high dose LA plus AttHRV (HiLA+AttHRV+), (2) low dose LA plus AttHRV (LoLA+AttHRV+), (3) AttHRV only (LA-AttHRV+), (4) high dose LA only (HiLA+), (5) low dose LA only (LoLA+), and (6) control (LA-AttHRV-). Pigs in HiLA+ groups were orally dosed daily with 103 to 109 CFU/dose of LA in 3 ml of 0.1 % peptone water (BD Biosciences, Sparks, MD) for 14 days with 10-fold incremental dose increase every other day from 3-16 days of age (Table 1). Pigs in LoLA+ groups were orally dosed with 103, 104,105,106, and 106 CFU/dose of LA on 3, 5, 7, 9 and 11 days of age, respectively. Pigs in non-LA fed groups (LA-AttHRV+ and LA-AttHRV-) were given an equal volume of 0.1 % peptone water. Pigs in AttHRV+ groups were orally inoculated with two doses of 5 × 107 FFU AttHRV at 5 and 15 days of age [post-inoculation day (PID) 0 and 10, respectively]. At PID 28, subsets of pigs from all treatment groups were orally challenged with 105 FFU VirHRV [33]. Pigs were given 5 ml of 100 mM sodium bicarbonate to reduce gastric acidity 20 min before HRV inoculation. Pigs were examined daily postchallenge for clinical signs, including prevalence, duration and severity of diarrhea using fecal consistence scores as we previously described [31]. Pigs were euthanized on PID 28 (postchallenge day [PCD] 0) or PID 35 (PCD 7) to isolate mononuclear cells (MNCs) from ileum, IEL, spleen and peripheral blood as we previous described [31]. All animal experimental procedures were conducted in accordance with protocols approved by Institutional Animal Care and Use Committees of Virginia Polytechnic Institute and State University.
Table 1.
Probiotic LA high dose and low dose feeding regimens and fecal LA shedding
| Age (PPD)a | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 26 | 33 | Accumulative |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIDb | -5 | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 21 | 28 | total dose |
| HiLA+ dose (CFU) | 103 | 103 | 104 | 104 | 105 | 105 | 106 | 106 | 107 | 107 | 108 | 108 | 109 | 109 | 2.22 × 109 | |||||
| LoLA+ dose (CFU) | 103 | 104 | 105 | 106 | 106 | 2.11 × 106 | ||||||||||||||
| HiLA+AttHRV+c | 3.2 × 107* | 6.8 × 106 | 1.9 × 106 | 4.7 × 105* | ||||||||||||||||
| LoLA+AttHRV+c | 7.9 × 105 | 6.0 × 106 | 5.4 × 106 | 8.8 × 106 | ||||||||||||||||
PPD, post-partum day.
PID, post primary AttHRV inoculation day.
Mean fecal LA count (CFU/ml)
Indicates significant difference compared to the low dose LA group (Kruskal-Wallis test on log transformed data, p<0.05).
2.4. Detection of rotavirus and LA shedding and assessment of rotavirus diarrhea
Rectal swabs were collected on PID 5, 10, 21, and 28 for enumeration of LA shedding and collected weekly for sterility test as described previously [25]. Rectal swabs were also collected for 7 days after VirHRV challenge to assess rotavirus shedding and diarrhea. Virus shedding was detected by ELISA and cell culture immunofluorescence (CCIF) assay in processed rectal swab fluids as described previously [35, 36].
2.5. Intracellular cytokine staining and flow cytometry analysis of IFN-γ producing CD4+ and CD8+ T cells
Flow cytometry was used to determine frequencies of HRV-specific and non-specific IFN-γ producing CD4+ and CD8+ T cells in ileum, IEL, spleen and blood of Gn pigs as we previously described [32]. At least 100,000 cells were acquired on a FACSAria flow cytometer (BD Biosciences). Data were analyzed using FlowJo 7.2.2 software (Tree Star, Ashland, Oregon). The frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ T cells were expressed as percentages among total CD3+ T cells. All mean frequencies are reported after subtraction of the background frequencies.
2.6. Intracellular cytokine staining and flow cytometry analysis of TGF-β and IL-10 producing CD4+CD25+ and CD4+CD25- Treg cells
The MNCs were stained freshly on the same day of MNC isolation. The MNCs (2 × 106 cells/tube) were first stained at 4°C for 15 min with fluorescein isothiocyanate (FITC) conjugated mouse anti-porcine CD4α (IgG2b, BD Pharmingen), SpectralRed™ (SPRD) conjugated mouse anti-porcine CD8α (IgG2a, BD Pharmingen), and mouse anti-porcine CD25 (IgG1, AbDSerotec), followed by allophycocyanin (APC) conjugated rat anti-mouse IgG1 (IgG1, BD pharmingen). After staining of cell surface markers, the MNCs were permeabilized with FoxP3 Staining Buffer Set (eBiosciences) at 4°C for 30 min. Then the cells were washed with FoxP3 Staining Buffer Set and stained with the phycoerythrin-cyanine tandem fluorochrome (PE-Cy7) conjugated rat anti-mouse/rat FoxP3 (IgG2a, eBioscience), biotin conjugated mouse anti-porcine IL-10 (IgG1, Cell Sciences), and PE conjugated mouse anti-human TGF-β1 (IgG1, R&D Systems), followed by staining with streptavidin conjugated pacific blue (Invitrogen) at 4°C for 30 min. The rat anti-mouse/rat-FoxP3 antibody cross-reacts with porcine FoxP3, allowing the identification of porcine Treg cells [37]. The frequencies of CD4+CD25+FoxP3+ and CD4+CD25-FoxP3+ Treg cells were expressed as percentages among total MNCs. The frequencies of IL-10+ and TGF-β+ cells were expressed as percentages among CD4+CD25+FoxP3+ and CD4+CD25-FoxP3+ Treg cells, respectively. All mean frequencies are reported after subtraction of the background frequencies.
2.7. Statistical analysis
Non-parametric Kruskal-Wallis rank sum test was performed to compare frequencies of T cell subsets in different tissues among treatment groups at each time point. When differences among the groups were detected, the same test was used in a pairwise fashion to identify the nature of the differences. One-way analysis of variance (ANOVA-general linear model [GLM]), followed by Duncan's multiple range test, were used to compare mean duration of virus shedding and diarrhea, and mean cumulative fecal consistence scores. All statistical significance was assessed at p < 0.05.
3. Results
3.1. Intestinal bacterial load reached an equilibrium at PID 10 in both low and high dose LA fed Gn pigs but increased in low dose LA group and decreased in high dose LA group at PID 28
LA colonization in all LA-fed Gn pigs were confirmed by LA enumeration in rectal swab samples collected on PID 5, 10, 21, and 28. The HiLA+AttHRV+ pigs shed significantly higher titers of LA than the LoLA+AttHRV+ pigs at PID 5 (Table 1). At PID 10 and PID 21, the mean LA shedding titers in both HiLA+AttHRV+ and LoLA+AttHRV+ pigs were maintained between 1.9 × 106 and 6.8 × 106 CFU/ml. There was no significant difference in LA shedding titers between the HiLA+AttHRV+ and LoLA+AttHRV+ pigs at these time points. Therefore, after colonization, the intestinal bacterial load reached equilibrium quickly. At PID28, however, LA shedding titers in the HiLA+AttHRV+ pigs were significantly lower than that of the LoLA+AttHRV+ pigs.
3.2. Representative responses in four treatment groups detected by flow cytometry analyses
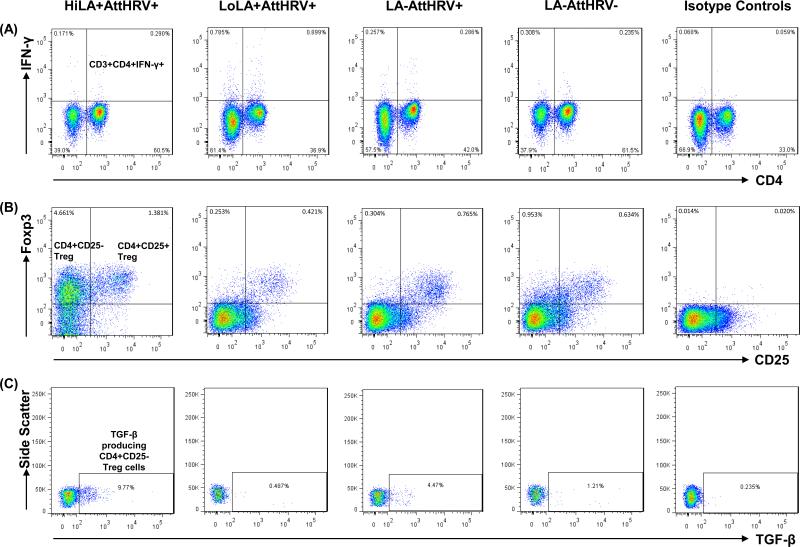
Flow cytometry analyses of HRV-specific IFN-γ+CD4+ T cells, CD4+CD25+ Treg cells (CD4+CD25+FoxP3+), CD4+CD25- Treg cells (CD4+CD25-FoxP3+), and TGF-β producing CD4+CD25- Treg cells in spleen of Gn pigs from four treatment groups are illustrated in Fig. 1. Analyses of TGF-β producing CD4+CD25+ Treg and IL-10 producing CD4+CD25+ and CD4+CD25- Treg cells followed the same gating strategy (not shown). Fig. 1A shows representative dot plots of the HRV-specific IFN-γ+CD4+ T cells in the four treatment groups and the isotype control. LoLA+AttHRV+ pigs had the highest frequencies of IFN-γ+CD4+ T cells in spleen at PID35 (PCD7) compared to other treatment groups. Detailed comparison of IFN-γ producing CD4+ and CD8+ T cell responses in the intestinal and systemic lymphoid tissues and blood among the treatment groups are presented in Fig. 2 and supplemental Fig. 1S. Fig. 1B shows representative dot plots of CD4+CD25+and CD4+CD25- Treg cells among MNCs in the four treatment groups and the isotype control. Fig. 1C shows the frequencies of TGF-β producing cells among CD4+CD25- Treg cells. The Gn pigs in the HiLA+AttHRV+ group had the highest frequencies of CD4+CD25+ and CD4+CD25- Treg cells and TGF-β producing CD4+CD25- Treg cells in spleen compared to Gn pigs in other treatment groups. Detailed comparison of Treg cell responses in the intestinal and systemic lymphoid tissues and blood among the treatment groups are illustrated in Figs. 3-5 and 2S-4S.
Fig. 1. Representatives of IFN-γ producing CD3+CD4+ T cells, CD4+CD25+ and CD4+CD25- Treg cells and TGF-β producing CD4+CD25- Treg cells in spleen of Gn pigs fed high or low dose LA, vaccinated with AttHRV and challenged with VirHRV at PID35/PCD7.
MNCs were isolated from pigs infected with high dose LA plus AttHRV (HiLA+AttHRV+), low dose LA plus AttHRV (LoLA+AttHRV+), AttHRV only (LA-AttHRV+), or mock-inoculated controls (LA-AttHRV-). A representative dot plot was shown for each inoculation group and isotype control staining. The numbers at the upper right corners of dot plots of panel (A) are the frequencies of HRV-specific IFN-γ+CD4+ T cells among CD3+ T cells. The numbers at the upper left and right corners of dot plots of panel (B) are the frequencies of CD4+CD25-FoxP3+ Treg cells and CD4+CD25+FoxP3+ Treg cells among MNCs, respectively. The numbers in the rectangles in dot plots of panel (C) are the frequencies of TGF-β+ cells among CD4+CD25-FoxP3+ Treg cells. The data were summarized by subtracting the frequencies of non-specific cross-reaction shown in the isotype control staining from the frequencies of samples.
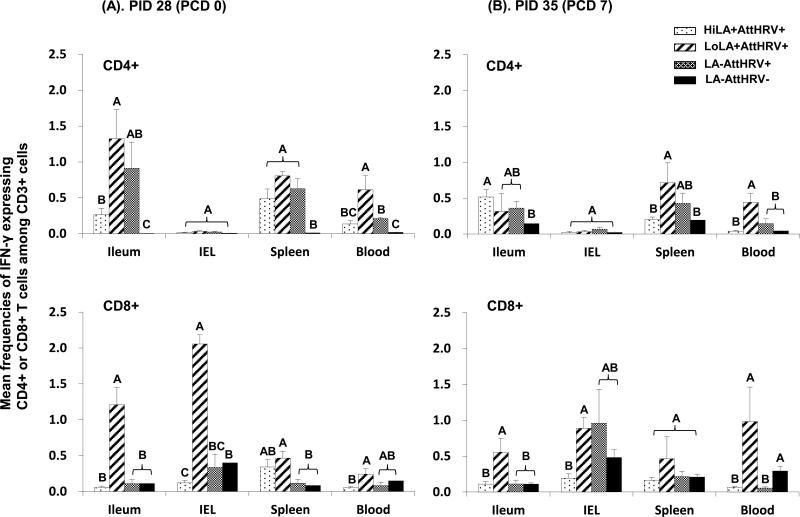
Fig. 2. Virus-specific IFN-γ producing T cell responses in Gn pigs vaccinated with AttHRV with or without high or low dose LA and the control.
MNCs were stimulated with semi-purified AttHRV antigen in vitro for 17 hrs. Brefeldin A was added for the last 5 hrs to block secretion of cytokines produced by the T cells. IFN-γ production was detected by intracellular staining and flow cytometry. Data are presented as mean frequency ± standard error of the mean (n = 3-13). The top two figures show the frequencies of IFN-γ+CD3+CD4+ T cells and the bottom two show those of IFN-γ+CD3+CD8+ T cells. Two figures in panel (A) show the prechallenge data and those in panel (B) show the postchallenge data. Different letters on top of bars indicate significant differences in frequencies among groups for the same cell type and tissue (Kruskal–Wallis test, p < 0.05), while shared letters indicate no significant difference.
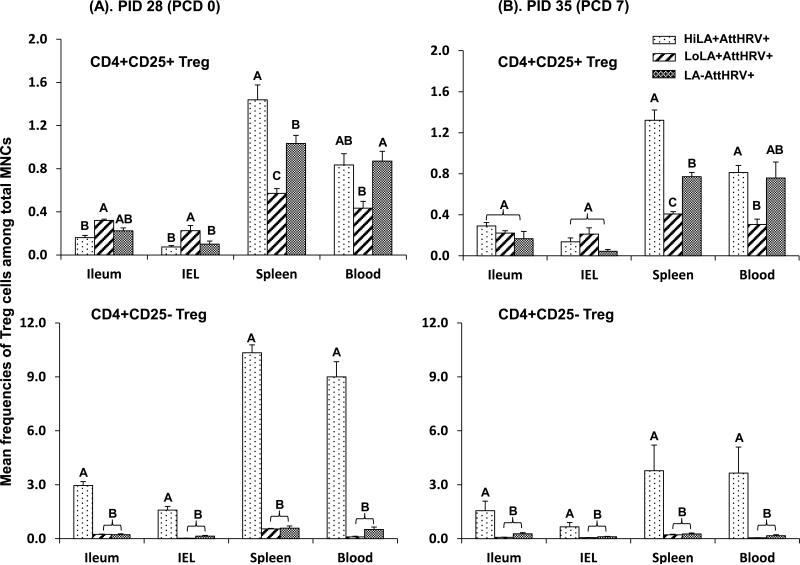
Fig. 3. Frequencies of CD4+CD25+FoxP3+ Treg and CD4+CD25-FoxP3+ Treg cells among total MNCs from Gn pigs vaccinated with AttHRV with high or low dose LA or without LA.
MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-9). The top two figures show the frequencies of CD4+CD25+ Treg cells and the bottom two show those of CD4+CD25- Treg cells. See Fig. 2 legend for panel description and statistical analysis.
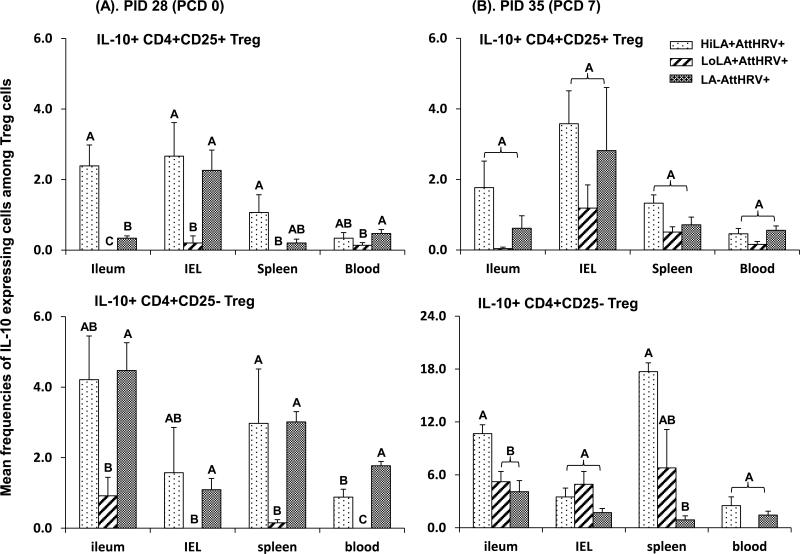
Fig. 5. IL-10 expressing CD4+CD25+ and CD4+CD25- Treg cell responses in Gn pigs vaccinated with AttHRV with high or low dose LA or without LA.
MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-9). The top two figures show the frequencies of IL-10+ cells among CD4+CD25+ Treg cells and the bottom two show the frequencies of IL-10+ cells among CD4+CD25- Treg cells. See Fig. 2 legend for panel description and statistical analysis.
3.3. Low dose LA enhanced HRV-specific and non-specific IFN-γ producing T cell responses
The magnitude of HRV-specific IFN-γ producing T cell responses in Gn pigs was differentially modulated by low versus high dose LA at both prechallenge (PID 28 [PCD 0]) and postchallenge (PID 35 [PCD 7]). As shown in Fig. 2, high dose LA did not enhance the HRV-specific IFN-γ producing CD4+ and CD8+ T cell responses in the HiLA+HRV+ pigs compared to AttHRV only pigs. In contrast, LoLA+AttHRV+ pigs had significantly higher frequencies of HRV-specific IFN-γ+CD8+ T cells in ileum (11- and 5-fold higher pre- and post-challenge, respectively), IEL (6-fold higher prechallenge), spleen (4-fold higher prechallenge) and blood (20-fold higher postchallenge) compared to the AttHRV only pigs. The LoLA+AttHRV+ pigs also had significantly higher frequencies of HRV-specific IFN-γ+CD4+ T cells in blood (3-fold higher for both pre- and postchallenge) compared to the AttHRV only pigs. Consequently, the AttHRV vaccine with high dose LA induced overall lower HRV-specific IFN-γ producing CD4+ and CD8+ T cell responses in all tissues of Gn pigs than the AttHRV vaccine with low dose LA, pre- and postchallenge. The frequencies of IFN-γ+CD8+ T cells in ileum, IEL, and blood pre- and postchallenge and IFN-γ+CD4+ T cells in ileum and blood prechallenge and in spleen and blood postchallenge of the LoLA+AttHRV+ pigs were significantly higher (ranging from 4- to 24-fold) than the HiLA+AttHRV+ pigs.
In the low dose and high dose LA only groups (Fig. 1S), the non-specific IFN-γ producing CD4+ and CD8+ T cell responses (detected from mock-stimulated MNCs) also differed significantly, with low dose LA inducing higher or significantly higher frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ T cells in most tissues compared to the high dose LA fed group and the non-LA fed control group pre- and postchallenge. The results indicate that the dose effect of LA on the IFN-γ producing T cell response is independent of AttHRV vaccine or VirHRV challenge. Low dose, but not high dose, LA acted as a general immunostimulator to promote the development of IFN-γ producing T cells in the intestinal and systemic lymphoid tissues and blood.
3.4. High dose LA strongly and significantly increased the frequencies of intestinal and systemic CD4+CD25- Treg cells compared to low dose and without LA
Because the magnitude of virus-specific T cell responses are modulated by both CD4+CD25+ and CD4+CD25- Treg cells, we compared frequencies of the Treg cells in the vaccinated pigs fed with high or low dose LA or without LA (HiLA+AttHRV+, LoLA+AttHRV+ and LA-AttHRV+) pre- and postchallenge. HiLA+AttHRV+ pigs had significantly higher frequencies of CD4+CD25- Treg cells (ranging from 6- to 86-fold higher) in all the tissues compared to LoLA+AttHRV+ and LA-AttHRV+ pigs pre- and postchallenge (Fig. 3 bottom panel). Frequencies of CD4+CD25- Treg cells in LoLA+AttHRV+ and LA-AttHRV+ pigs did not differ significantly in any tissue pre- and postchallenge. The dose effects of LA on CD4+CD25+ Treg cells were similar to CD4+CD25- Treg cells but at a reduced magnitude (note the difference in y axis scales in Fig 3). LoLA+AttHRV+ pigs had lower or significantly lower frequencies of CD4+CD25+ Treg cells (2- to 3-fold lower) in spleen and blood compared to HiLA+AttHRV+ and LA-AttHRV+ pigs pre- and postchallenge. Conversely, low dose LA induced slightly higher CD4+CD25+ Treg cell responses in the intestine (2- to 3-fold higher) compared to HiLA+AttHRV+ and LA-AttHRV+ pigs prechallenge and did not have a significant effect on intestinal CD4+CD25+ Treg cells postchallenge (Fig. 3 top panel).
In the pigs fed high or low dose LA without AttHRV vaccine, LA had similar dose effects on the Treg cell responses compared to the AttHRV vaccinated pigs (Figs 2S and 3). Consistent with the dose effect of LA on the IFN-γ producing T cell response, these results showed that the dose effect of LA on Treg cells is independent of AttHRV vaccine or VirHRV challenge. The dose effect on CD4+CD25- Treg cells was substantially stronger postchallenge than prechallenge in the non-vaccinated pigs (note the difference in y axis scales of the bottom panel in Fig. 2S).
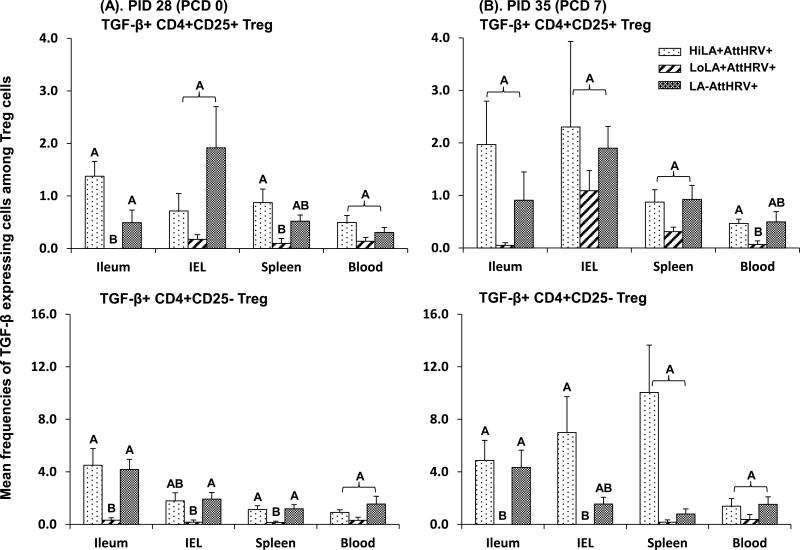
3.5. Low dose LA decreased TGF-β producing Treg cell responses in all tissues
Because Treg cells exert regulatory functions through mechanisms involving TGF-β, we compared frequencies of the Treg cell subsets that produced TGF-β among the low and high dose LA and non-LA groups. LoLA+AttHRV+ pigs had lower or significantly lower frequencies of TGF-β producing CD4+CD25+ and CD4+CD25- Treg cells than HiLA+AttHRV+ and LA-AttHRV+ pigs in all tissues pre- and post-challenge (Fig. 4). The differences were greater among the groups for CD4+CD25- Treg cells than CD4+CD25+ Treg cells. Frequencies of TGF-β producing CD4+CD25+ and CD4+CD25- Treg cells between HiLA+AttHRV+ and LA-AttHRV+ pigs did not differ significantly in any tissue pre- and postchallenge.
Fig. 4. TGF-β expressing CD4+CD25+ and CD4+CD25- Treg cell responses in Gn pigs vaccinated with AttHRV with high or low dose LA or without LA.
MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-9). The top two figures show the frequencies of TGF-β+ cells among CD4+CD25+ Treg cells and the bottom two show the frequencies of TGF-β+ cells among CD4+CD25- Treg cells. See Fig. 2 legend for panel description and statistical analysis.
In the pigs fed high or low dose LA without AttHRV vaccine (Fig 3S), low dose LA fed pigs also had significantly reduced frequencies of TGF-β producing CD4+CD25- Treg cells in ileum, spleen and blood prechallenge and TGF-β producing CD4+CD25- Treg cells in ileum and CD4+CD25+ Treg cells in IEL postchallenge compared to the non-LA control pigs. Taken together, low dose LA, with or without AttHRV vaccine, down-regulated the TGF-β production in CD4+CD25+ or CD4+CD25- Treg cells in the intestinal or systemic lymphoid tissues. With few exceptions, high dose LA did not significantly influence the TGF-β production in Treg cells.
3.6. Low dose LA significantly decreased IL-10 producing Treg cell responses compared to high dose LA and non-LA in vaccinated pigs prechallenge
Because IL-10 is the most important regulatory cytokine in virus infection and immunity; increased IL-10 production has been associated with reduced anti-viral immunity, we compared frequencies of the Treg cell subsets that produced IL-10 among the low dose, high dose LA and non-LA groups. Low dose LA reduced or significantly reduced frequencies of IL-10 producing CD4+CD25+ and CD4+CD25- Treg cells compared to high dose LA and non-LA pre- and postchallenge (except for IL-10+CD4+CD25- Treg cells postchallenge) (Fig. 5). The high dose LA induced significantly higher frequencies of IL-10 producing CD4+CD25+ Treg cells in ileum prechallenge and IL-10 producing CD4+CD25- Treg cells in ileum and spleen postchallenge compared to non-LA (Fig. 5). HiLA+AttHRV+ pigs had slightly, but significantly, lower frequencies of IL-10 producing CD4+CD25- Treg cells in blood compared to LA-AttHRV+ pigs prechallenge (Fig. 5).
In the pigs fed with high or low dose LA without AttHRV vaccine, low dose LA significantly reduced the frequencies of IL-10 producing CD4+CD25+ Treg cells in ileum and CD4+CD25-Treg cells in blood prechallenge and CD4+CD25+ Treg cells in IEL and blood postchallenge compared to the non-LA fed control pigs (Fig. 4S). There was a trend for higher frequencies of IL-10 producing CD4+CD25- Treg in the high dose LA group prechallenge, but lower frequencies of IL-10 producing CD4+CD25+ and CD4+CD25- Treg cells postchallenge compared to the non-LA fed controls. However, the differences were not statistically significant.
3.7. Low dose LA slightly enhanced protection conferred by the AttHRV vaccine against rotavirus diarrhea upon VirHRV challenge
To examine the effects of low and high dose LA on improving the protection conferred by the AttHRV vaccine, subsets of Gn pigs from each treatment group were challenged with VirHRV at PID 28. Clinical signs and virus shedding were monitored for 7 days postchallenge (Table 2). After VirHRV challenge, although the proportion of pigs that developed virus shedding and diarrhea did not differ significantly among the three AttHRV vaccinated groups (31-50%, data not shown), the LoLA+AttHRV+ group had the shortest mean durations of fecal virus shedding and diarrhea and the lowest mean cumulative fecal consistency scores among all the treatment groups. The durations of diarrhea in the LoLA+AttHRV+ pigs were significantly shorter compared to the LA-AttHRV+ and the mock-vaccinated control pigs. The durations of virus shedding in the LoLA+AttHRV+ pigs were significantly shorter compared to the HiLA+AttHRV+ and the mock control pigs. The mean cumulative fecal consistency scores in all the pigs in the LoLA+AttHRV+ and LA-AttHRV+ groups (8.4 and 9.0, respectively) were significantly lower than the control group, indicating significant protection against the severity of diarrhea. Although the fecal scores in the HiLA+AttHRV+ group were lower than the control group (12.5 versus 16.9) they did not differ significantly. Thus, low dose LA slightly but clearly improved the protection conferred by the AttHRV vaccine against rotavirus diarrhea. In contrast, high dose LA reduced the protection conferred by the AttHRV vaccine as indicated by the significantly longer mean duration of virus shedding (3.8 versus 1.3 days) and higher mean cumulative fecal scores compared to the AttHRV only pigs.
Table 2.
Protection against rotavirus shedding and diarrhea after VirHRV challenge
| Treatment groups | N | Mean duration of virus shedding (days) | Mean duration of diarrhea (days)a | Mean cumulative fecal scoreb |
|---|---|---|---|---|
| HiLA+AttHRV+ | 13 | 3.8 (0.3c)Ad | 4.3 (0.7)AB | 12.5 (1.4)AB |
| LoLA+AttHRV+ | 8 | 1.0 (0)B | 2.4 (0.7)B | 8.4 (1.3)B |
| LA-AttHRV+ | 12 | 1.3 (0.2)B | 4.6 (0.5)A | 9.0 (1.8)B |
| LA-AttHRV- | 8 | 4.4 (0.7)A | 5.8 (0.3)A | 16.9 (1.3)A |
Pigs with daily fecal scores of ≥2 were considered diarrheic.
Fecal consistency was scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid.
Mean cumulative score calculation included all the pigs for 7 days postchallenge in each group.
Standard error of the mean.
Means in the same column with different superscript letters (A, B) differ significantly (GLM, p≤0.05); shared letters indicate no significant difference.
4. Discussion
In this study, we demonstrated that the low dose LA feeding regimen promoted IFN-γ producing T cell immune responses and reduced the frequencies and functions (regulatory cytokine TGF-β and IL-10 production) of Treg cells whereas the high dose LA feeding regimen promoted the development of Treg cell responses in Gn pigs vaccinated with the AttHRV vaccine. Corresponding to the differentially altered IFN-γ producing T cell and Treg cell responses, low dose LA slightly but clearly improved the protection conferred by the AttHRV vaccine against rotavirus diarrhea whereas high dose LA significantly prolonged the virus shedding (for 2.5 days) compared to the AttHRV vaccine alone upon challenge with the VirHRV. Thus dose effects of LA on rotavirus vaccine-induced protective immunity were highly significant. The same probiotic LA strain at low or high dose exerted qualitatively different modulating effects on the T cell immune responses induced by the oral AttHRV vaccine.
Our previous studies have demonstrated that the low dose LA was sufficient to colonize the intestine of germ-free pigs [17, 25]. However, it was unexpected in this study that the extra amounts of LA ingested by the HiLA+ pigs did not result in higher numbers of LA fecal shedding beyond PID 5. Innate immune responses induced by the LA (i.e., α-defensins secreted by Paneth cells [38], neutrophil and γδ T cell responses) and LA-specific secretory IgA antibodies may play important roles in controlling the loads of colonizing bacteria in the gut. Studies of Gn mice inoculated with L. johnsonii or L. paracasei at the same dose showed that the two Lactobacillus strains shed in very different titers in feces, suggesting that different Lactobacillus strains colonize the gut at different levels [39]. The study also showed that bacterial counts from fecal samples were similar to the counts from the luminal contents of the colon, indicating that fecal bacterial counts reflect the relative bacterial loads in the gut [39]. In our study, although the additional high dose LA the HiLA+ pigs received from PID 6-11 did not increase the intestinal bacterial load (to the contrary, the HiLA+AttHRV+ pigs had significantly lower bacterial load at PID 28 compared to the LoLA+AttHRV+ pigs), the effects between the high and low dose LA on the IFN-γ producing T cell and Treg cell, especially CD4+CD25- Treg cell, immune responses measured 3-4 weeks after PID 5 (PID 28 and PCD 7) were strikingly different. Therefore the initially different LA doses that the intestinal innate immune cells (epithelial cells, macrophages, DCs, nTreg cells, etc.) interacted with before PID 10 had determined the profile of the IFN-γ producing T cell and CD4+CD25- Treg cell responses developed later on.
It has been suggested that low and high dose microbe-associated molecular patterns (MAMP), i.e. bacterial lipopolysaccharide (LPS) from E. coli and peptidoglycan from LA, engages different receptor conformations, and/or differentially distributes to sub-cellular locations and subsequently, activates different downstream pathways [40]. The effect of low dose LPS was strikingly different as compared to that of high dose LPS on macrophage cell functions: low dose LPS induced a strong inflammatory response in macrophages. It is plausible that similar interaction occurs between the MAMP from LA and innate immune cells in the gut. Future studies are needed to identify the molecular mechanisms of the dose responses of different MAMP.
To our knowledge, this study is the first in vivo study to investigate the dose effects of probiotics on immune responses and the protective efficacy of an oral viral vaccine. Other studies have shown similar qualitative, but not quantitative, differences in the immunological effects between two dosages of probiotics without vaccination. In a double-blind, placebo-controlled, randomized clinical trial, two different doses of L. plantarum CECT7315/7316 were given to elderly subjects for 12 weeks (low dose = 5 × 108 CFU/day and high dose = 5 × 109 CFU/day) [41]. After treatment, high probiotic dose resulted in significant increases in the frequencies of CD8+CD25+ T cells and NK cells, while low probiotic dose increased CD4+CD25+ T cells, B cells, and antigen presenting cells in peripheral blood. Although the study did not examine the function of the different T cell populations, the authors suggested that low dose probiotics might be useful as vaccine adjuvant while higher dose might be useful to prevent infections [41]. Another recent study showed that low concentrations (<1 × 106 CFU/ml) of Lactobacillus and Bifidobacterium mixtures enhanced IFN-γ production and inhibited IL-4 production in mitogen-activated murine and human splenic T cells, whereas high concentrations (≥1 × 106 CFU/ml) inhibited mitogen-induced T cell proliferation [42].
Low dose LA promoted IFN-γ producing T cell and down-regulated Treg cell responses whereas high dose LA promoted Treg cell responses in Gn pigs (with or without AttHRV vaccine). These findings may explain some of the controversies that the same probiotic strains used by different research groups in animal studies showed opposite immunomodulatory functions. For example, administration of L. casei suppressed pro-inflammatory cytokine expression by CD4+ T cells and up-regulated IL-10 and TGF-β levels in rats [3, 4]. To the contrary, Van Overtvelt et al. found that L. casei was a pure Th1 inducer in mice In addition to the difference in animal species, the L. casei doses used by the different studies differed significantly, with much higher doses used in So et al.'s studies [3, 4]. In So et al.'s studies the amount of L. casei was 5 × 109 or 2 ×1010 CFU/dose per rat, three times per week for 11-12 weeks. In Van Overtvelt et al.'s study, the amount of L. casei was 2 × 108 CFU/dose per mouse, twice per week for 8 weeks [7]. Additional caution concerning the dosages is needed in comparing and interpreting results from different probiotic studies.
The dose effect of LA on immune responses to the AttHRV vaccine in Gn pigs may also partly explain why the efficacies of oral rotavirus vaccines are significantly lower in developing countries compared to developed countries. The two licensed rotavirus vaccines, RotaTeq and Rotarix have a protective efficacy of >85% against moderate to severe rotavirus gastroenteritis in middle and high-income countries [43]. However, the protective efficacy of RotaTeq vaccine is only 39.3% against severe rotavirus gastroenteritis in developing countries in sub-Saharan Africa [44] and 48.3% in developing countries in Asia [45]. Rotarix vaccine showed a similar disparity in efficacy in developing countries in Africa [46]. In addition to other factors that contribute to the reduction in rotavirus vaccine efficacy (e.g., higher titers of maternal antibodies, malnutrition), during the initial colonization of human infants, exposure to high dose commensal bacteria (common in countries with lower hygiene standards) would have a suppressive effect on IFN-γ producing T cell responses and promote Treg cell responses, thus leading to the lowered protective immunity after rotavirus vaccination.
We reported previously that protection rates against rotavirus infection and diarrhea are correlated with virus-specific intestinal IFN-γ producing T cell and IgA antibody-secreting cell responses at PID 28 in Gn pigs [31, 32]. A balanced Th1 and Th2 type response is needed for the optimal protective immunity against rotavirus. Although low dose LA further reduced the duration of diarrhea in the AttHRV-vaccinated pigs postchallenge, neither low nor high dose LA significantly altered protection rate against rotavirus challenge (proportions of pigs that were infected and developed diarrhea after challenge). The differences in protection conferred by the HiLA+AttHRV+ versus LoLA+AttHRV+ vaccine against rotavirus shedding and diarrhea were not as substantial as the differences in the magnitudes of the intestinal IFN-γ producing CD8+ T cell responses between the two treatment groups. Because virus-specific intestinal IgA antibody-secreting cell responses probably play a more important role in rotavirus protective immunity than the IFN-γ producing CD8+ T cell responses [31, 32], the dose effect of LA on virus-specific antibody-secreting cell responses also need to be taken into consideration in understanding the differences in the protection conferred by the AttHRV vaccine with high or low dose LA. To improve the AttHRV vaccine efficacy, an intermediate dose of LA may be optimal to promote a balanced Th1 and Th2 response and without increased Treg cell responses.
In vaccine development, co-administration of adjuvants (e.g., bacterial toxins, aluminum salts, monophosphoryl lipid A, and CpG) is the traditional approach to enhance the immunogenicity of vaccine antigens. An increasing number of studies, including ours have shown that certain strains of lactobacilli administered orally around the time of vaccination have similar effects in enhancing humoral and/or cellular immune responses to mucosal as well as parenteral vaccines as conventional adjuvants [15, 17, 20, 21, 25, 47]. Yet, probiotics are safe, even for newborn infants and the elderly [21, 48]; therefore their use will not raise concerns for side effects as for any newly introduced conventional adjuvants. The use of probiotics as adjuvants (with the appropriate strain and optimal dosage) is a promising practical novel approach to enhance vaccine immunogenicity and protective efficacy.
An unaddressed question by this study is that whether the dose effect of probiotics observed in Gn pigs is the same in humans with a normal gut microflora. In future experiments, we will conventionalize Gn pigs with normal pig feces or colonize Gn pigs with human gut microflora and reevaluate the effect of LA on the AttHRV vaccine in those pigs.
Probiotics are increasingly used to improve human health, alleviate disease symptoms and to enhance vaccine efficacies. Our findings suggest that probiotics can be ineffective or even detrimental if not used at the optimal dosage for the appropriate purposes, highlighting the importance of dose selection in probiotic studies. Dose effects of each probiotic product should be fully investigated in clinical trials before use for improving human health or treating diseases. The underlying mechanisms of dose effects of probiotics require further study.
Supplementary Material
Figure legends for supplemental figures
Fig. 1S. Non-specific IFN-γ producing T cell responses in Gn pigs fed with high dose, low dose LA and the control. MNCs were mock stimulated in vitro for 17 hrs. Data are presented as mean frequency ± standard error of the mean (n = 3-8). The top two figures show the frequencies of IFN-γ+CD3+CD4+ T cells and the bottom two show those of IFN-γ+CD3+CD8+ T cells. Two figures in panel (A) show the prechallenge data and those in panel (B) show the postchallenge data. Different letters on top of bars indicate significant differences in frequencies among groups for the same cell type and tissue (Kruskal–Wallis test, p < 0.05), while shared letters indicate no significant difference.
Fig. 2S. Frequencies of CD4+CD25+FoxP3+ Treg and CD4+CD25-FoxP3+ Treg cells among total MNCs from Gn pigs fed with high dose, low dose LA and the control. MNCs were stained freshly without in vitro stimulation. The top two figures show the frequencies of CD4+CD25+ Treg cells and the bottom two show those of CD4+CD25- Treg cells. Data are presented as mean frequency ± standard error of the mean (n = 3-4). See Fig. 1S legend for panel description and statistical analysis.
Fig. 3S. TGF-β expressing CD4+CD25+ and CD4+CD25- Treg cell responses in Gn pigs fed with high dose, low dose LA and the control. MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-4). The top two figures show the frequencies of TGF-β+ cells among CD4+CD25+ Treg cells and the bottom two show the frequencies of TGF-β+ cells among CD4+CD25- Treg cells. See Fig. 1S legend for panel description and statistical analysis.
Fig. 4S. IL-10 expressing CD4+CD25+ and CD4+CD25- Treg cell responses in Gn pigs fed with high dose, low dose LA and the control. MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-4). The top two figures show the frequencies of IL-10+ cells among CD4+CD25+ Treg cells and the bottom two show the frequencies of IL-10+ cells among CD4+CD25- Treg cells. See Fig. 1S legend for panel description and statistical analysis.
Highlights.
>Dose effects of lactobacilli on T cell responses to rotavirus vaccine were studied. >Low dose promoted Th1 and down-regulated Treg cell responses in gnotobiotic pigs. >High dose increased natural and induced Treg cells and their TGF-β/IL-10 production. >Dose effects were similar in the intestinal and systemic lymphoid tissues of the pigs. >The same probiotic strain at different doses exerted qualitatively different effects.
Acknowledgements
We thank Dr. Marlice Vonck, Dr. Kevin Pelzer, Pete Jobst, Andrea Aman and Shannon Viers for animal care. We thank Melissa Makris for assistance in flow cytometry. We acknowledge that the virulent and attenuated Wa human rotavirus strains were provided by Dr. Linda Saif, The Ohio State University. This work was supported by a grant (R01AT004789) from the National Center of Complementary and Alternative Medicine (NCCAM), National Institutes of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geier MS, Butler RN, Giffard PM, Howarth GS. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int J Food Microbiol. 2007 Mar 20;114(3):267–74. doi: 10.1016/j.ijfoodmicro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Carroll IM, Andrus JM, Bruno-Barcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2007 Oct;293(4):G729–38. doi: 10.1152/ajpgi.00132.2007. [DOI] [PubMed] [Google Scholar]
- 3.So JS, Lee CG, Kwon HK, Yi HJ, Chae CS, Park JA, et al. Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol Immunol. 2008 Nov;46(1):172–80. doi: 10.1016/j.molimm.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 4.So JS, Kwon HK, Lee CG, Yi HJ, Park JA, Lim SY, et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol. 2008 May;45(9):2690–9. doi: 10.1016/j.molimm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008 Oct;122(4):788–94. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005 Sep;90(9):892–7. doi: 10.1136/adc.2004.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Overtvelt L, Moussu H, Horiot S, Samson S, Lombardi V, Mascarell L, et al. Lactic acid bacteria as adjuvants for sublingual allergy vaccines. Vaccine. 2010 Apr 9;28(17):2986–92. doi: 10.1016/j.vaccine.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Vitini E, Alvarez S, Medina M, Medici M, de Budeguer MV, Perdigon G. Gut mucosal immunostimulation by lactic acid bacteria. Biocell. 2000 Dec;24(3):223–32. [PubMed] [Google Scholar]
- 9.Yazdi MH, Soltan Dallal MM, Hassan ZM, Holakuyee M, Agha Amiri S, Abolhassani M, et al. Oral administration of Lactobacillus acidophilus induces IL-12 production in spleen cell culture of BALB/c mice bearing transplanted breast tumour. Br J Nutr. 2010 Jul;104(2):227–32. doi: 10.1017/S0007114510000516. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G, Rasmussen S, Zeuthen LH, Nielsen BN, Jarmer H, Jespersen L, et al. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology. 2010 Oct;131(2):268–81. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, et al. Well-controlled proinflammatory cytokine responses of Peyer's patch cells to probiotic Lactobacillus casei. Immunology. 2010 Jul;130(3):352–62. doi: 10.1111/j.1365-2567.2009.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002 Jan 1;168(1):171–8. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 13.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):2880–5. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992 Aug;32(2):141–4. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007 Mar;23(3):254–60. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Paineau D, Carcano D, Leyer G, Darquy S, Alyanakian MA, Simoneau G, et al. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol. 2008 Jun;53(1):107–13. doi: 10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008 Jul 4;26(29-30):3655–61. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler P, de Vrese M, Laue C, Schrezenmeir J. Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters. Int J Clin Pharmacol Ther. 2005 Jul;43(7):318–26. doi: 10.5414/cpp43318. [DOI] [PubMed] [Google Scholar]
- 19.Mohamadzadeh M, Duong T, Hoover T, Klaenhammer TR. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev Vaccines. 2008 Mar;7(2):163–74. doi: 10.1586/14760584.7.2.163. [DOI] [PubMed] [Google Scholar]
- 20.Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995 Feb;13(3):310–2. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 21.Boge T, Remigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009 Sep 18;27(41):5677–84. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 22.Wagner RD, Warner T, Roberts L, Farmer J, Dohnalek M, Hilty M, et al. Variable biotherapeutic effects of Lactobacillus acidophilus isolates on orogastric and systemic candidiasis in immunodeficient mice. Rev Iberoam Micol. 1998 Dec;15(4):271–6. [PubMed] [Google Scholar]
- 23.de Roock S, van Elk M, van Dijk ME, Timmerman HM, Rijkers GT, Prakken BJ, et al. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin Exp Allergy. 2010 Jan;40(1):103–10. doi: 10.1111/j.1365-2222.2009.03344.x. [DOI] [PubMed] [Google Scholar]
- 24.Evrard B, Coudeyras S, Dosgilbert A, Charbonnel N, Alame J, Tridon A, et al. Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PLoS One. 2011;6(4):e18735. doi: 10.1371/journal.pone.0018735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, et al. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008 Mar 15;122(1-2):175–81. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008 Feb 15;121(3-4):222–31. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug 1;155(3):1151–64. [PubMed] [Google Scholar]
- 28.Nizar S, Meyer B, Galustian C, Kumar D, Dalgleish A. T regulatory cells, the evolution of targeted immunotherapy. Biochim Biophys Acta. 2010 Aug;1806(1):7–17. doi: 10.1016/j.bbcan.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Geng S, Yu Y, Kang Y, Pavlakis G, Jin H, Li J, et al. Efficient induction of CD25- iTreg by co-immunization requires strongly antigenic epitopes for T cells. BMC Immunol. 2011;12:27. doi: 10.1186/1471-2172-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008 Sep;29(9):429–35. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996 May;70(5):3075–83. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008 Jun 19;26(26):3322–31. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996 Jul;77(Pt 7):1431–41. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 34.Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Seine for Microbiological Investigations. Appl Microbiol. 1964 Jul;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005 May;79(9):5428–36. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, Li G, Wen K, Bui T, Cao D, Zhang Y, et al. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010 Apr;23(2):135–49. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaser T, Gerner W, Hammer SE, Patzl M, Saalmuller A. Detection of Foxp3 protein expression in porcine T lymphocytes. Vet Immunol Immunopathol. 2008 Sep 15;125(1-2):92–101. doi: 10.1016/j.vetimm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000 Aug;1(2):113–8. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 39.Ibnou-Zekri N, Blum S, Schiffrin EJ, von der Weid T. Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect Immun. 2003 Jan;71(1):428–36. doi: 10.1128/IAI.71.1.428-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maitra U, Gan L, Chang S, Li L. Low-dose endotoxin induces inflammation by selectively removing nuclear receptors and activating CCAAT/enhancer-binding protein delta. J Immunol. 2011 Apr 1;186(7):4467–73. doi: 10.4049/jimmunol.1003300. [DOI] [PubMed] [Google Scholar]
- 41.Mane J, Pedrosa E, Loren V, Gassull MA, Espadaler J, Cune J, et al. A mixture of Lactobacillus plantarum CECT 7315 and CECT 7316 enhances systemic immunity in elderly subjects. A dose-response, double-blind, placebo-controlled, randomized pilot trial. Nutr Hosp. 2011 Jan-Feb;26(1):228–35. [PubMed] [Google Scholar]
- 42.Li CY, Lin HC, Lai CH, Lu JJ, Wu SF, Fang SH. Immunomodulatory Effects of Lactobacillus and Bifidobacterium on Both Murine and Human Mitogen-Activated T Cells. Int Arch Allergy Immunol. 2011 May 16;156(2):128–36. doi: 10.1159/000322350. [DOI] [PubMed] [Google Scholar]
- 43.O'Ryan ML, Hermosilla G, Osorio G. Rotavirus vaccines for the developing world. Curr Opin Infect Dis. 2009 Oct;22(5):483–9. doi: 10.1097/QCO.0b013e32833040a9. [DOI] [PubMed] [Google Scholar]
- 44.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Aug 21;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 45.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010 Aug 21;376(9741):615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 46.O'Ryan M, Linhares AC. Update on Rotarix: an oral human rotavirus vaccine. Expert Rev Vaccines. 2009 Dec;8(12):1627–41. doi: 10.1586/erv.09.136. [DOI] [PubMed] [Google Scholar]
- 47.de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005 Oct;44(7):406–13. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 48.Weizman Z, Alsheikh A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: a pilot study. J Am Coll Nutr. 2006 Oct;25(5):415–9. doi: 10.1080/07315724.2006.10719554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure legends for supplemental figures
Fig. 1S. Non-specific IFN-γ producing T cell responses in Gn pigs fed with high dose, low dose LA and the control. MNCs were mock stimulated in vitro for 17 hrs. Data are presented as mean frequency ± standard error of the mean (n = 3-8). The top two figures show the frequencies of IFN-γ+CD3+CD4+ T cells and the bottom two show those of IFN-γ+CD3+CD8+ T cells. Two figures in panel (A) show the prechallenge data and those in panel (B) show the postchallenge data. Different letters on top of bars indicate significant differences in frequencies among groups for the same cell type and tissue (Kruskal–Wallis test, p < 0.05), while shared letters indicate no significant difference.
Fig. 2S. Frequencies of CD4+CD25+FoxP3+ Treg and CD4+CD25-FoxP3+ Treg cells among total MNCs from Gn pigs fed with high dose, low dose LA and the control. MNCs were stained freshly without in vitro stimulation. The top two figures show the frequencies of CD4+CD25+ Treg cells and the bottom two show those of CD4+CD25- Treg cells. Data are presented as mean frequency ± standard error of the mean (n = 3-4). See Fig. 1S legend for panel description and statistical analysis.
Fig. 3S. TGF-β expressing CD4+CD25+ and CD4+CD25- Treg cell responses in Gn pigs fed with high dose, low dose LA and the control. MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-4). The top two figures show the frequencies of TGF-β+ cells among CD4+CD25+ Treg cells and the bottom two show the frequencies of TGF-β+ cells among CD4+CD25- Treg cells. See Fig. 1S legend for panel description and statistical analysis.
Fig. 4S. IL-10 expressing CD4+CD25+ and CD4+CD25- Treg cell responses in Gn pigs fed with high dose, low dose LA and the control. MNCs were stained freshly without in vitro stimulation. Data are presented as mean frequency ± standard error of the mean (n = 3-4). The top two figures show the frequencies of IL-10+ cells among CD4+CD25+ Treg cells and the bottom two show the frequencies of IL-10+ cells among CD4+CD25- Treg cells. See Fig. 1S legend for panel description and statistical analysis.