Abstract
OBJECTIVE
To examine the impact of hormones used for controlled ovarian hyperstimulation (COH) on normal and malignant breast cell growth and proliferation.
DESIGN
In vitro study of cultured normal and malignant breast cell lines.
SETTING
Academic medical center
INTERVENTIONS
Normal and malignant breast cell lines were cultured in two- (2D) and three-dimensional (3D) systems and treated with follicle-stimulating hormone (FSH), luteinizing hormone (LH), or FSH with LH or human chorionic gonadotropin (hCG).
MAIN OUTCOME MEASURES
Effects of treatment on cell proliferation in 2D culture using the MTS assay and on colony growth in 3D culture.
RESULTS
Compared with untreated cells, normal MCF-10A cells showed a decrease in proliferation and colony size when exposed to a combination of FSH and hCG. HCC 1937 cells treated with FSH and LH also showed a decrease in colony growth but no change in proliferation. None of the treatments had an effect on the proliferation or colony size of the MCF-7 cells.
CONCLUSION
FSH, LH, and hCG do not appear to cause an increase in cell proliferation or colony growth in either normal or malignant mammary epithelial cell lines. The potential risk for mammary cell transformation associated with these agents may be related to indirect endocrine effects on breast cell physiology.
Keywords: FSH, LH, infertility, breast cancer, fertility treatment, fertility preservation
Introduction
Reflecting their evolving roles in modern society, more women are delaying childbearing. As a woman ages her fertility rate declines. In the United States, 20% of women will have their first child after age 35, with one third of these women having issues with infertility (1,2). Simultaneously, the risk for developing breast cancer increases with age. There are approximately 200,000 new cases of breast cancer diagnosed in the U.S. annually, and over 18,000 women are diagnosed under the age of 45 (3). Currently, infertility treatment implementing in vitro fertilization (IVF) involves controlled ovarian hyperstimulation (COH) in which combinations of gonadotropin hormones, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and human chorionic gonadotropin (hCG), are used to stimulate follicle growth, steroid hormone production, oocyte maturation, and ovulation. When results from the Women’s Health Initiative (WHI) study suggested that hormone replacement therapy may increase the risk for breast cancer in 2002, concern was raised for both patients and physicians regarding the use of hormone therapies, not only during menopause, but also for oral contraception and infertility treatment (4,5). The impact of infertility regimens on breast cancer risk is largely unknown, with some studies suggesting a possible correlation while others have found no definitive association (6–10). In particular, there have been reports of a trend toward increased breast cancer risk for patients with a family history of the disease undergoing IVF treatment (11,12).
From a mechanistic standpoint, more is known about the indirect effects of COH, mediated through estrogen, on mammary epithelium, than the direct effects of FSH, LH, and hCG. Estradiol (E2), a known cell cycle mitogen and promoter of breast cell proliferation (13–15), is elevated to supraphysiologic levels during COH for IVF. Previous work assessing the effects of E2 in estrogen receptor-positive (ER+) breast cancer cells in vitro showed a proliferative effect at treatment levels similar to those encountered during IVF cycles (16). Furthermore, escalating doses of E2 correlated with increased ER+ breast cancer cell proliferation. These data point toward a possible estrogen-driven association between COH, high-risk ER+ breast lesions, and breast cancer risk in women undergoing treatment for infertility. Regarding the direct effects of COH, there is data suggesting that β-hCG/LH receptors are upregulated in invasive ductal tumor cells compared with non-invasive components (8). Conversely, other data show that the expression of LH receptors is undetectable or very low in most breast cell lines, suggesting a limited role for LH signaling in breast tissue (17).
At this point, the independent proliferative effects of FSH and LH on breast epithelial cells have not been thoroughly examined. Thus, the aim of this study was to examine the impact of widely used hormones for COH—FSH, LH, and hCG—on breast cell growth and proliferation in vitro, independent of the E2 induction present in vivo. The cells utilized for these studies included normal immortalized MCF-10A breast cells, MCF-7 ER+ cancer cells, and HCC 1937 breast cancer cells, which are characterized as ER− with a BRCA1 mutation, consistent with a hereditary breast cancer subtype.
Materials and Methods
Cell lines, culture media, and hormones
MCF-10A, MCF-7, and HCC 1937 cell lines were obtained from American Type Cell Culture (Manassas, VA). MCF-10A cells were maintained in DMEM-F12 containing 10% horse serum, MCF-7 cells in DMEM-F12 with 2.5% FBS, and HCC 1937 cells in RPMI containing 10% FBS. The media for all cell lines was phenol red-free and supplemented with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA); serum used in culture media for the MCF-10A and HCC 1937 cells was charcoal stripped to remove estrogenic compounds, whereas reduced regular serum (2.5%) was used in culture media for the MCF-7 cell line. The cells were maintained at 37°C in a humidified incubator containing 5% CO2. Pituitary-derived FSH, LH, and purified hCG were obtained from Sigma (St. Louis, MO).
2D cell culture and MTS assay
Cells were seeded in 96-well plates at 5×103 cells/well for MCF-10A cells and at 3×103/well for MCF-7 and HCC1937 cells, which grow and reach confluency more rapidly than MCF-10A cells. Five wells were seeded for each treatment, for each time point, and the cells were allowed to attach overnight. Cell proliferation was measured the following day (day 0) with a MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI), which was then repeated on days 2, 4, 6, and 10 of culture. For each reading, 20 μl of MTS solution was added, plates were incubated for 3 hours, and absorbance read on a Bio Rad Microplate Reader Model 680 (Hercules, CA) at 490 nm. Treatment regimens included the following: no treatment, 40 mIU/ml FSH, 40 mIU/ml LH, FSH combined with LH, or FSH combined with 0.5 IU/ml hCG. Cells treated with FSH and either LH or hCG were primed with FSH on days 1, 3, and 5, treated with the combined hormones on day 7, then treated with media on day 9. All experiments (5 wells per treatment per time point) were repeated in triplicate.
3D Matrigel cell culture
In 96-well plates, 30 μl of growth factor-reduced phenol red-free Matrigel (BD Biosciences, San Jose, CA) was spread evenly along the bottom of each well. Plates were then incubated at 37° C for 30 minutes to allo w the Matrigel to solidify. Cells from each line were trypsinized, spun down, and resuspended in media containing 2% v/v Matrigel, and then plated at a density of 1.25×103 cells/well. This was considered day 0. Culture media was freshly prepared and replaced every other day beginning on day 1. On days 4 and 10, an image of each well was taken at 10x magnification using an Axiovert 200 inverted microscope (Zeiss, Thornwood, NY). The same treatment regimens were used as described above for the cell proliferation studies and all experiments were performed in triplicate.
Statistical analysis
For the MTS assays, proliferation was measured as absorbance at 490 nm and reported as raw values with or without treatment at each time point. To assess 3D Matrigel colony growth, the number of colonies was recorded and the size of each colony measured for each well, allowing us to determine the average colony size. These values were reported as a ratio of treated to untreated cells at each time point. The average absorbance (proliferation in 2D culture), average ratios of colony size (growth in 3D culture) and standard errors of the mean (SEM) were calculated using GraphPad Prism 4 software (La Jolla, CA). Comparisons were made between treated and untreated cells at each time point using a two-sided Student’s t-test. P-values of <0.05 were considered statistically significant.
Results
Effects of infertility agents on breast cell proliferation
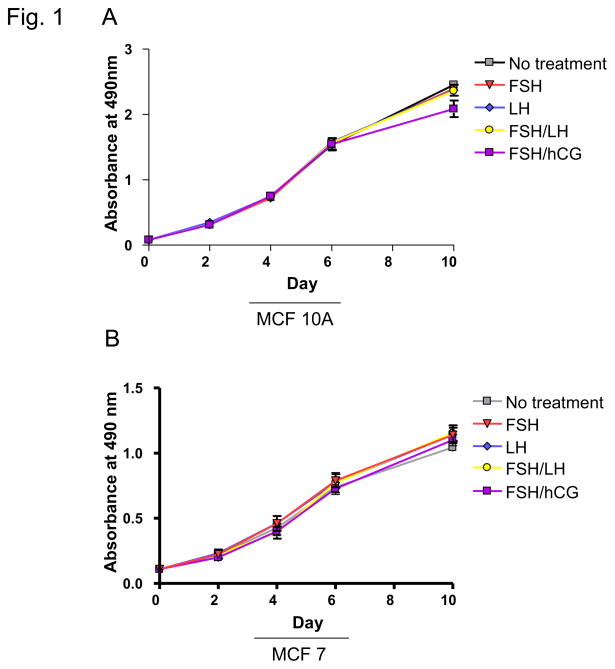
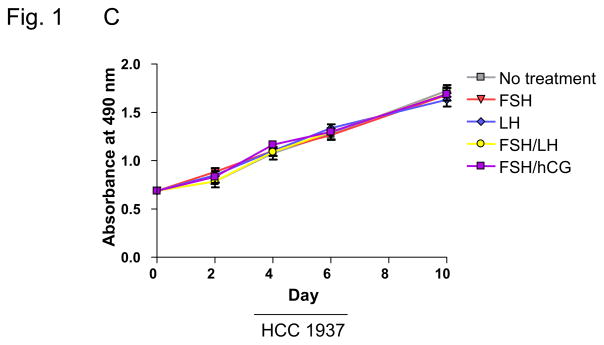
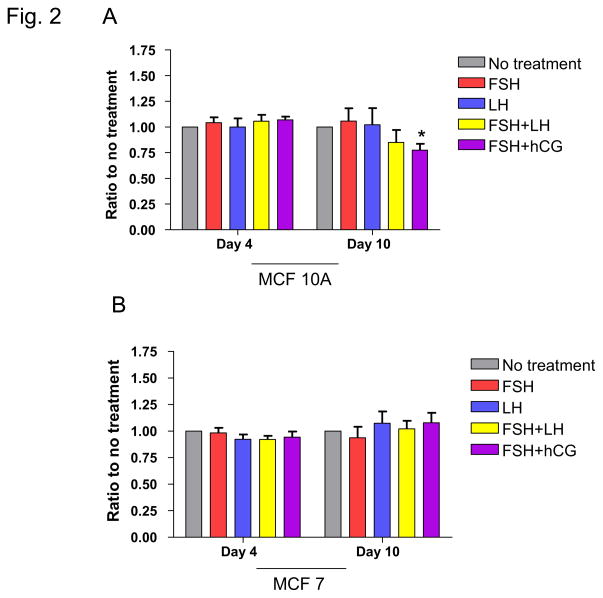
MTS assays were performed to determine the effect of treatment with hormones used for COH on breast cell proliferation. MCF-10A cells, which approximate normal immortalized mammary epithelium, were studied along with MCF-7 and HCC 1937 breast cancer cell lines. MCF-7 cells are well-differentiated and ER+, whereas HCC 1937 cells are ER− and harbor a BRCA1 gene mutation. For MCF-10A cells, the only observed treatment effect was for cells treated with a combination of FSH and hCG, which resulted in a decrease in proliferation (p=0.04; Figure 1A; Table 1). Compared with untreated cells, there was no effect found for FSH or LH alone or FSH in combination with LH or hCG on proliferation of MCF-7 or HCC 1937 cells (Figure 1B, C; Table 1).
Fig. 1. Effects of infertility agents on cell proliferation in 2D culture.
(A–C) MTS assay in MCF-10A, MCF-7, and HCC 1937 cell lines; absorbance indicates number of viable cells at each time point. Asterisks indicate a statistically significant difference (p<0.05) between treated and untreated cells at each time point. All values are given as means ± SEM for n=3 experiments.
Table 1.
Proliferation in 2D cultures of MCF-10A, MCF-7, and HCC 1937 cells treated with various infertility agents.
| MCF-10A | MCF-7 | HCC 1937 | ||||
|---|---|---|---|---|---|---|
| Abs490 | p-value | Abs490 | p-value | Abs490 | p-value | |
| No Treatment | 2.34 | n/a | 0.97 | n/a | 1.76 | n/a |
| 40 mlU/ml FSH | 2.39 | 0.09 | 1.14 | 0.14 | 1.68 | 0.35 |
| 40 mIU/ml LH | 2.37 | 0.09 | 1.14 | 0.16 | 1.60 | 0.08 |
| FSH/LH | 2.36 | 0.78 | 1.15 | 0.13 | 1.69 | 0.49 |
| FSH/0.5 IU/ml hCG | 2.09 | 0.04* | 1.10 | 0.23 | 1.69 | 0.49 |
Abs490, absorbance at 490 nm.
p<0.05, treated vs. untreated on culture day 10 (Student’s two-tailed t-test).
Effects of infertility agents on breast cell growth in 3D culture
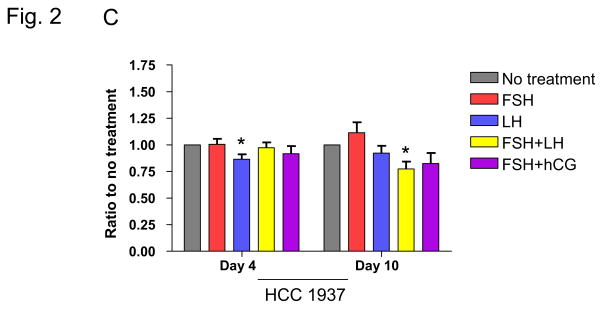
Three-dimensional matrices more closely mimic the in vivo environment, providing a scaffold to study cellular events in the context of more physiologic cell-cell interactions. We thus also studied the impact of infertility regimens on cell proliferation in this 3-dimensional context. Cells were embedded in Matrigel and then treated with FSH or LH alone or FSH in combination with LH or hCG to determine the effect of treatment on breast cell growth. Cells in 3D culture adopt a clustered, rounded morphology, and growth was assessed by measuring both the number and size of these colonies. For the MCF-10A cell line, treatment with a combination of FSH and hCG led to a decrease in colony size at day 10 (p=0.005; Figure 2A; Table 2). There was no observed effect on colony growth with any of the treatments in MCF-7 cells, whereas treatment with combination FSH/LH resulted in a decrease in colony size for the HCC 1937 cells (Figure 2B, 2C; Table 2).
Fig. 2. Effects of infertility agents on cell colony growth in 3D culture.
(A–C) Ratio of total area of colonies in treated vs. untreated control cell cultures for the MCF-10A, MCF-7, and HCC 1937 cell lines. Asterisks indicate a statistically significant difference (p<0.05) between treated and untreated cells at each time point. All values are given as means ± SEM for n=3 experiments.
Table 2.
Colony growth in 3D cultures of MCF-10A, MCF-7, and HCC 1937 cells treated with various infertility agents.
| MCF-10A | MCF-7 | HCC 1937 | ||||
|---|---|---|---|---|---|---|
| Colony growth | p-value | Colony growth | p-value | Colony growth | p-value | |
| No Treatment | 1.00 | n/a | 1.00 | n/a | 1.00 | n/a |
| 40 mlU/ml FSH | 1.14 | 0.97 | 0.94 | 0.56 | 1.12 | 0.27 |
| 40 mIU/ml LH | 0.88 | 0.97 | 1.07 | 0.52 | 0.92 | 0.29 |
| FSH/LH | 1.00 | 0.25 | 1.02 | 0.79 | 0.77 | 0.009* |
| FSH/0.5 IU/ml hCG | 0.77 | 0.005* | 1.08 | 0.42 | 0.83 | 0.11 |
Average ratio of colony size in treated to untreated cells at day 10 of culture.
p<0.05, treated vs. untreated on culture day 10 (Student’s two-tailed t-test).
Discussion
High levels of ovarian steroid hormones resulting from COH with FSH, LH and hCG have been linked to breast cell proliferation and an increased risk of breast cancer development in women undergoing infertility treatment (6,9). Burkman et al. reported that compared with patients who had never used any infertility medication, women using human menopausal gonadotropin (hMG) for at least 6 months or for 6 cycles had a relative risk of breast cancer ranging between 2.7 to 3.8 (12). In a study of more than 3000 women, Pappo et al. showed that age ≥40 and undergoing ≥4 IVF cycles were associated with an increased risk for breast cancer (11). While compelling, these population-based studies did not investigate whether these associations were related, in part, to the direct effects of the gonadotropins on breast epithelium or due to indirect effects of E2 produced in the ovaries.
E2 is known to stimulate breast epithelial cell proliferation, and through interaction with other hormones and growth factors, contributes to the activation of proto-oncogenes, including c-myc and cyclins D1 and E (13–15). These proteins mediate the progression from G1 to S phase in the cell cycle. Thus, increased production of estrogens during infertility treatment may promote the malignant potential of key cell cycle regulatory proteins in mammary epithelium (15). Our prior work showed no effect of E2 on ER− MCF-10A and HCC 1937 cells in vitro, with doses of E2 comparable to those observed during COH cycles, whereas an expected increase in proliferation was observed when MCF-7 ER+ cells were treated with escalating does of estrogen (16,17). For breast cancer patients with ER+ lesions, the proliferative effect of E2 on malignant cells is well established. Consequently, breast cancer survivors undergoing treatment for infertility have recently been stimulated with breast cancer therapies including selective estrogen receptor modulators, such as tamoxifen, or aromatase inhibitors, including letrozole or anastrozole, which can have both pro- and anti-estrogenic effects (18). Selected use of letrozole has resulted in lower E2 levels when compared with the conventional fertility therapy, clomiphene, and may reduce the potential risk of fertility treatment-related breast cancer recurrence (19). It has also been shown that stimulation with FSH/letrozole resulted in lower E2 levels when compared to FSH/anastrozole, and therefore the FSH/letrozole strategy may be more favorable for fertility treatment in breast cancer survivors (18). Finally, for a cohort of breast cancer patients undergoing fertility preservation prior to chemotherapy, data showed no increased risk for recurrence when tamoxifen or letrozole was added to an FSH-based COH regimen (18, 20).
For women undergoing treatment for infertility, the direct impact of infertility regimens on breast cells was a concern, and data on the subject has been contradictory. Hudelist et al. has shown that the β-HCG/LH receptor was selectively upregulated in invasive versus noninvasive breast tumor components and hypothesized that β-HCG/LH receptor mediated gonadotropin stimulation could contribute to mammary tumorigenesis (8). Conversely, Kuijper et al. showed that LH receptor expression is very low in most breast tumor cell lines, favoring an indirect ovarian-mediated role for the effect of gonadotropins on tumorigenesis (21). Our current work similarly showed no significant increase in cell proliferation for the 3 breast cell lines tested after exposure to FSH, LH or combinations of FSH with LH or hCG. These preclinical results are reassuring, and represent an initial step in exploring the potential effect of FSH, LH and hCG associated infertility regimens on breast cancer risk, independent of E2.
Taken together, we found that exposure to FSH, LH, and hCG at doses similar to those used for COH in IVF protocols did not result in growth-promoting effects in either normal or malignant breast epithelial cells. The potential risk of breast cancer associated with these agents is thus more likely related to indirect estrogen-associated events. Future work will focus on the effect of elevated E2 levels observed during infertility treatment in high-risk breast cancer models.
Acknowledgments
Grant Support: JSJ is a Lynn Sage Scholar supported by the Lynn Sage and Dixon Foundations and NIH UL1 RRR024926 and K22 CA138776 research grants.
The authors thank Elizabeth Tarasewicz, and Stacey C. Tobin, PhD, for editorial assistance.
Footnotes
The authors have no financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ASRM B. Age and Fertility. 2003. Alabama. [Google Scholar]
- 2.Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–53. doi: 10.1093/humrep/deh304. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Breast Cancer Facts & Figures 2009–2010. American Cancer Society, Inc; Atlanta: [Google Scholar]
- 4.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–8. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 5.Nekhlyudov L, Bush T, Bonomi AE, Ludman EJ, Newton KM. Physicians’ and women’s views on hormone therapy and breast cancer risk after the WHI: a qualitative study. Women Health. 2009;49:280–93. doi: 10.1080/03630240903158446. [DOI] [PubMed] [Google Scholar]
- 6.Zreik TG, Mazloom A, Chen Y, Vannucci M, Pinnix CC, Fulton S, et al. Fertility drugs and the risk of breast cancer: a meta-analysis and review. Breast Cancer Res Treat. 2010;124:13–26. doi: 10.1007/s10549-010-1140-4. [DOI] [PubMed] [Google Scholar]
- 7.Orgeas CC, Sanner K, Hall P, Conner P, Holte J, Nilsson SJ, et al. Breast cancer incidence after hormonal infertility treatment in Sweden: a cohort study. Am J Obstet Gynecol. 2009;200:72 e1–7. doi: 10.1016/j.ajog.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 8.Hudelist G, Wuelfing P, Czerwenka K, Knofler M, Haider S, Fink-Retter A, et al. Beta-hCG/LH receptor (b-HCG/LH-R) expression is increased in invasive versus preinvasive breast cancer: implications for breast carcinogenesis? J Cancer Res Clin Oncol. 2009;135:191–5. doi: 10.1007/s00432-008-0458-3. [DOI] [PubMed] [Google Scholar]
- 9.Chung KH-LL, Hawes D, Taylor D, Paulson R, Pike M. Controlled ovarian hyperstimulation increases cell proliferation in normal breast tissue: implications for the magnitude of possible breast cancer risk from infertility treatment. Fertil Steril. 2009;92:S181. [Google Scholar]
- 10.Gauthier E, Paoletti X, Clavel-Chapelon F. Breast cancer risk associated with being treated for infertility: results from the French E3N cohort study. Hum Reprod. 2004;19:2216–21. doi: 10.1093/humrep/deh422. [DOI] [PubMed] [Google Scholar]
- 11.Pappo I, Lerner-Geva L, Halevy A, Olmer L, Friedler S, Raziel A, et al. The possible association between IVF and breast cancer incidence. Ann Surg Oncol. 2008;15:1048–55. doi: 10.1245/s10434-007-9800-2. [DOI] [PubMed] [Google Scholar]
- 12.Burkman RT, Tang MT, Malone KE, Marchbanks PA, McDonald JA, Folger SG, et al. Infertility drugs and the risk of breast cancer: findings from the National Institute of Child Health and Human Development Women’s Contraceptive and Reproductive Experiences Study. Fertil Steril. 2003;79:844–51. doi: 10.1016/s0015-0282(02)04950-6. [DOI] [PubMed] [Google Scholar]
- 13.Han HJ, Heo JS, Lee YJ. Estradiol-17beta stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol. 2006;290:C1067–75. doi: 10.1152/ajpcell.00222.2005. [DOI] [PubMed] [Google Scholar]
- 14.Mawson A, Lai A, Carroll JS, Sergio CM, Mitchell CJ, Sarcevic B. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Mol Cell Endocrinol. 2005;229:161–73. doi: 10.1016/j.mce.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Butt AJ, Caldon CE, McNeil CM, Swarbrick A, Musgrove EA, Sutherland RL. Cell cycle machinery: links with genesis and treatment of breast cancer. Adv Exp Med Biol. 2008;630:189–205. doi: 10.1007/978-0-387-78818-0_12. [DOI] [PubMed] [Google Scholar]
- 16.Cooley A, Matthews L, Ukeje C, Zelivianski S, Jeruss JS. Effects of Fertility Treatment Regimens and Pregnancy-Related Hormones on Breast Cell Proliferation. Poster session. 5th Annual Oncofertility Consortium Conference; 2009. [Google Scholar]
- 17.Cooley A, Matthews L, Zelivianski S, Hardy A, Jeruss JS. Effect of Infertility treatment and pregnancy-related hormones on breast cell proliferation in vitro. Human Reproduction. doi: 10.1093/humanrep/.2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–53. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Begum MR, Ferdous J, Begum A, Quadir E. Comparison of efficacy of aromatase inhibitor and clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. Fertil Steril. 2009;92:853–7. doi: 10.1016/j.fertnstert.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 21.Kuijper TM, Ruigrok-Ritstier K, Verhoef-Post M, Piersma D, Bruysters MW, Berns EM, et al. LH receptor gene expression is essentially absent in breast tumor tissue: implications for treatment. Mol Cell Endocrinol. 2009;302:58–64. doi: 10.1016/j.mce.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]