Abstract
The concept of the “mnemonic scotoma,” a spatially circumscribed region of working memory impairment produced by unilateral lesions of the prefrontal cortex (PFC), is central to the view that the PFC is critical for the short-term retention of information. Presented here, however, are previously unpublished data that offer an alternative, nonmnemonic interpretation of this pattern of deficit. In their study, K. Wajima and T. Sawaguchi applied the GABAA antagonist bicuculline methiodide unilaterally to the PFC of two monkeys while they performed an oculomotor delayed-response task. Consistent with previous studies, errors for the initial memory-guided saccade were markedly higher when the cued location fell into the region of the visual field affected by the infusion. These erroneous saccades tended to select an alternative target location (out of a possible 16) that had not been cued on that trial. By extending the analysis window, however, it was observed that the second, “corrective” saccade often acquired the location that had been cued on that trial. Further analysis of the erroneous initial saccades indicated that they tended to be directed to a location that had been relevant on the previous trial. Thus, the deficit was not one of “forgetting” the cued location. Rather, it was one of selecting between currently and previously relevant locations. These findings suggest a need for a reconsideration of the concept of the mnemonic scotoma, which in turn invites a reconsideration of functional interpretations of sustained neuronal activity in the PFC.
Introduction
The prefrontal cortex (PFC) comprises an interconnected set of areas in the anterior part of the frontal lobes, lying in front of the motor and premotor areas (Fuster, 2008; Petrides & Pandya, 2002). It sends and receives projections from most, if not all, cortical sensory and motor systems, as well as a wide range of subcortical structures (Fuster, 2008; Goldman-Rakic, 1987; Miller & Cohen, 2001). For the past 75 years, the functions of the PFC in many species have been studied with delay tasks (e.g., delayed response, delayed recognition, delayed alternation) that require the subject to guide behavior with trial-specific information that is no longer accessible to the senses (Fig. 1A). Because these tasks require short-term (or working) memory (for the cued information and/or the impending response, for the sample, or for the most recent response and/or reward), the idea of an explicitly mnemonic role for the PFC has been a potent one. It has also, however, been controversial, and the history of the study of PFC function can be portrayed in terms of periods of relative waxing vs. waning of the mnemonic hypothesis1.
Figure 1.
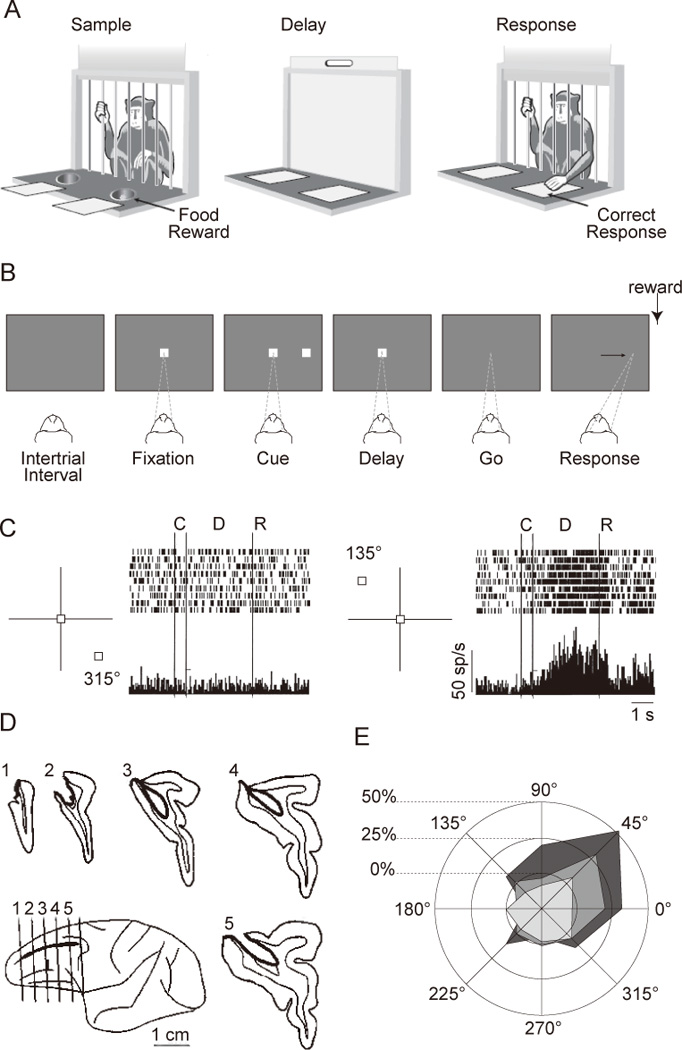
Delayed-response tasks, and neuropsychology and neurophysiology using such tasks. A. Schematic drawing of a classic delayed-response task administered with the Wisconsin General Test Apparatus. In the Sample phase, the monkey observes while one of the food wells is baited with a food reward. During the Delay phase, an opaque screen is lowered, and both food wells are covered by identical objects. The Response phase is initiated by the lifting of the screen, upon which the monkey selects one of the food wells by displacing the cover in order to retrieve the reward. Illustration adapted from Curtis and D’Esposito (2004). B. Sequence of a common version of the oculomotor delayed-response (ODR) task. Each rectangle shows the screen at a time during the trial. Dashed lines and arrow show the monkey’s point of fixation. C. Activity from a DLPFC neuron located in the right hemisphere. Only trials with upper left targets (135°) and those with the opposite direction (315°) are shown. From Funahashi et al. (1989) D. Reconstruction of the lesion that gives rise to the behavioral deficit shown in panel E. From Funahashi et al. (1993). E. Changes in performance on the ODR task after unilateral lesion to the DLPFC. From Funahashi et al. (1993).
In this commentary, we will briefly review some of the important landmarks in the history of PFC research in nonhuman primates, with an emphasis on how, with neuropsychological, neurophysiological, and neuropharmacological methods, subtle differences in experimental procedures can lead to radically different conclusions. Within this framework we will introduce previously unpublished findings from a neuropharmacological study that, like many studies before it, may necessitate a reinterpretation of the implications of a longstanding, highly influential finding. Finally, we will propose an interpretation that can bridge the gap between neurophysiology and results from disruption studies (such as lesion and neuropharmacological interventions).
Brief history of the PFC and delay tasks
Jacobsen was the first to systematically examine PFC function in the monkey using a delay task (Jacobsen, 1936). His experiments established two important findings. First, animals with large bilateral PFC lesions had little difficulty retrieving food from one of two covered wells when they could do so immediately after a well was baited. This meant that the lesions affected neither vision, nor motor control, nor motivation. Strikingly, however, performance dropped to chance levels if an opaque screen was lowered to prevent a response for even a few seconds. Jacobsen interpreted the results (with laudable caution) as evidence for a deficit in “immediate memory” (p. 11). Subsequent work by Pribram et al. (1952) established that lesions of area 46 of the dorsolateral PFC (DLPFC) were sufficient to produce this delayed-response deficit. These and related findings (e.g. Bauer & Fuster, 1976; Butters & Pandya, 1969; Goldman & Rosvold, 1970; Stamm, 1969) provided a strong foundation for the mnemonic hypothesis of DLPFC function. However, although they were unequivocal demonstrations of deficits on short-term memory tasks, it took less than 10 years after Jacobsen’s initial study for a follow-up to call into question whether the impairment that they demonstrated was one of memory per se.
In his 1942 study, Malmo closely followed the procedure of Jacobsen (1936), but with the additional manipulation of whether or not the testing room was illuminated during the delay period. The finding, that the performance of PFC-lesioned monkeys was at chance with the lights on (a replication of Jacobsen, 1936), but was rescued to near pre-operative levels with the lights off during the delay period, led Malmo (1942) to conclude that the delayed-response deficit was one of susceptibility to interference, rather than of short-term memory. For the next few decades, neuropsychological investigations in several species explored the role of PFC in the control of behavior, as assessed, for example, with tests of delayed alternation in nonhuman animals and the Wisconsin Card Sorting Task in humans (e.g. Warren & Akert, 1964).
The pendulum began to swing back in favor of the mnemonic hypothesis of DLPFC function with the advent of electrical recordings from neurons in the DLPFC in the 1970s. The first such studies, using spatial delayed-response with arm movements, discovered sustained, elevated levels of activity that spanned the delay period (Fuster & Alexander, 1971; Kojima & Goldman-rakic, 1982; Kubota & Niki, 1971). Importantly, the magnitude of this sustained activity often differed as a function of the location that was cued on a particular trial, a key characteristic that one would expect to see in a neural correlate of a short-term spatial memory. These were followed by studies using an oculomotor version of the delayed-response task (ODR task; Fig. 1B), with single-neuron recordings revealing directionally tuned delay-period activity (Fig. 1D) (Chafee & Goldman-Rakic, 1998; Constantinidis, Franowicz, & Goldman-Rakic, 2001; Funahashi, Bruce, & Goldman-Rakic, 1989). The remarkable specificity demonstrated in these studies motivated, in turn, a next generation of disruption studies that employed techniques that afforded anatomical and, in some cases, pharmacological precision that had not been previously achievable. These demonstrated that targeted ablation or chemical disruption of the DLPFC produced deficits in memory-guided, but not externally cued, saccades (Funahashi, Bruce, & Goldman-Rakic, 1993; Sawaguchi & Goldman-Rakic, 1991, 1994; Sawaguchi & Iba, 2001). Because the deficits were often restricted to precise target locations (Fig. 1C), they were interpreted as “mnemonic scotomas” -- memory deficits restricted to circumscribed portions of the visual field (Funahashi et al., 1993). Together, these findings were widely accepted as evidence that the DLPFC is a critical substrate for the short-term storage of spatial information (Constantinidis et al., 2001; Funahashi, 2006; Goldman-Rakic, 1995), and the idea of the mnemonic scotoma remains a potent, influential one at the time of this writing.
In recent years, however, continued study of the DLPFC has raised significant problems for this second incarnation of the mnemonic hypothesis. One thread draws on the remarkable plasticity of PFC neurons to suggest that these cells aren’t specialized for the memory of any particular kind of information, but, instead, will modify their response properties to reflect changing environmental exigencies, as can be operationalized by intensive training in the laboratory (e.g. Duncan, 2010; Duncan & Owen, 2000; Fuster, 2002; Rao, Rainer, & Miller, 1997) (but see Meyer, Qi, & Constantinidis, 2007; Meyer, Qi, Stanford, & Constantinidis, 2011; Qi, Meyer, Stanford, & Constantinidis, 2011). Relatedly, it has been shown that location- or object-selective delay-period activity changes its firing rate dynamically according to task requirements, rather than maintaining stable sensory representations (Asaad, Rainer, & Miller, 1998; Genovesio, Tsujimoto, & Wise, 2011; Hussar & Pasternak, 2009; Warden & Miller, 2010). From a different perspective, the conception of the mnemonic specificity of DLPFC neurons has also been eroded by several more recent sets of findings. In one, DLPFC neurons with delay-period activity in the ODR task have been shown to exhibit similar sustained activity during the “delay” period of a visually guided saccade task (Tsujimoto & Sawaguchi, 2004). In a second, these neurons have been shown to dynamically change during a single delay period from retrospectively representing the location of the cue to prospectively representing the target of the impending saccade (Takeda & Funahashi, 2002, 2004, 2007). In a third, which dissociates the focus of spatial attention from the focus of spatial memory, the majority of DLPFC neurons are seen to track the former (Lebedev, Messinger, Kralik, & Wise, 2004).
And so, at the time of this writing, we again find ourselves in an era in which the idea of purely mnemonic functions of the PFC is not endorsed by the majority of researchers in the field. Despite this, however, the mnemonic scotoma findings from the early 1990s (Funahashi et al., 1993; Sawaguchi & Goldman-Rakic, 1991, 1994; Sawaguchi & Iba, 2001) have stood as seemingly incontrovertible evidence for a necessary role for DLPFC in the short-term retention of precise locations in space (e.g. Curtis & D'Esposito, 2004). Can these influential findings be reconciled with the growing consensus from the electrophysiological literature that the DLPFC does not support purely mnemonic functions? The purpose of the present commentary is to introduce to the literature a previously unpublished set of findings that suggests a way that the mnemonic scotoma results might be reconciled with the currently prevailing interpretation of the electrophysiological literature.
The “mnemonic scotoma” revisited
The study of Wajima and Sawaguchi (2004), which produced the data that we present in this commentary, trained two macaque monkeys on an ODR task with 16 possible cue locations (8 polar angles by 2 eccentricities) and a control task in which the cue remained on the screen during the delay and go periods. These tasks were quite similar to those used in the previous neurochemical studies for the DLPFC (Sawaguchi & Goldman-Rakic, 1994; Sawaguchi & Iba, 2001). Briefly, in the ODR task, while the monkey fixated on a central spot (a white square, 0.2 × 0.2°) for 1 s, a visual cue (white square, 0.5 × 0.5°) appeared at one of 16 peripheral locations (8 directions separated by 45° with eccentricities of 10 and 20°) for 0.5 s, followed by a 4 s-delay period during which only the fixation spot was remained on and the monkeys kept fixation on it. After the delay period, the fixation spot turned off (‘go’ signal), which instructed the monkey to make a memory-guided saccade to the cued location. When the first saccade fell inside a circular window of 5° (diameter) around the cued location within 0.7 s, a white square (0.5 × 0.5°), which was the same as the cue, reappeared as a confirmation signal and a drop of water was delivered immediately as a reward. If the first saccade fell into one of the 15 incorrect target windows, the confirmation signal appeared at this spot without delivery of reward. In this case, even if the monkey made a second saccade to the correct target location within 0.7 s from the go signal, this corrective saccade was not rewarded. Thus, to get a reward, the monkey had to acquire the correct target location with the first try. The CON task was exactly the same as the ODR task except that the visual cue remained on during the “delay” and response periods, and the monkey made a visually guided saccade to the visible target.
After the monkeys performed several 5-min blocks of these tasks alternately (12 blocks = 60 min), the experimenters antagonized GABAA receptors in the DLPFC by means of unilateral local injection of bicuculline methiodide (BMI, 2.5–5.0µg/µl, 1µl), an antagonist of GABAA receptor. The postdrug period lasted at least 10 blocks (50 min) (but, in practice, often more than 100 min). The injected sites were located rostral to the frontal eye field, which was identified physiologically by intracortical microstimulation (22 cathodal pulses of 0.3 ms duration at 333 Hz, up to 100 µA). As such, they tested the effect of GABAA receptor blockade on the behavioral performance of the monkeys.
Figure 2A shows the comparison of error rate on the initial saccadic response between pre- and postdrug phases in a typical experimental session, during which BMI (2.5µg, 1µl) was injected into the left DLPFC. In the ODR task, the error rate was significantly increased after the drug injection, with errors coming almost entirely on trials associated with selective cue locations in the right visual field, which were contralateral to the injected site. Figure 2B shows two dimensional eye traces for one animal for trials with three cue locations, separately for pre- and postdrug periods. The number of misdirected saccades was clearly increased after the drug injection for trials with those three target locations. Importantly, the performance of the control task was not impaired by BMI injection (Fig. 2A and B, right panel), which indicates that the deficit on the ODR task was not due to deficits in sensorimotor processes. Thus, the data considered up to this point replicate findings from previous studies of ODR performance after circumscribed, unilateral lesions of the principal sulcus of the DLPFC (Funahashi et al., 1993; Sawaguchi & Goldman-Rakic, 1991, 1994; Sawaguchi & Iba, 2001). Where they offer new insight into the concept of the mnemonic scotoma, however, is in the behavior that followed the misdirected initial saccade.
Figure 2.
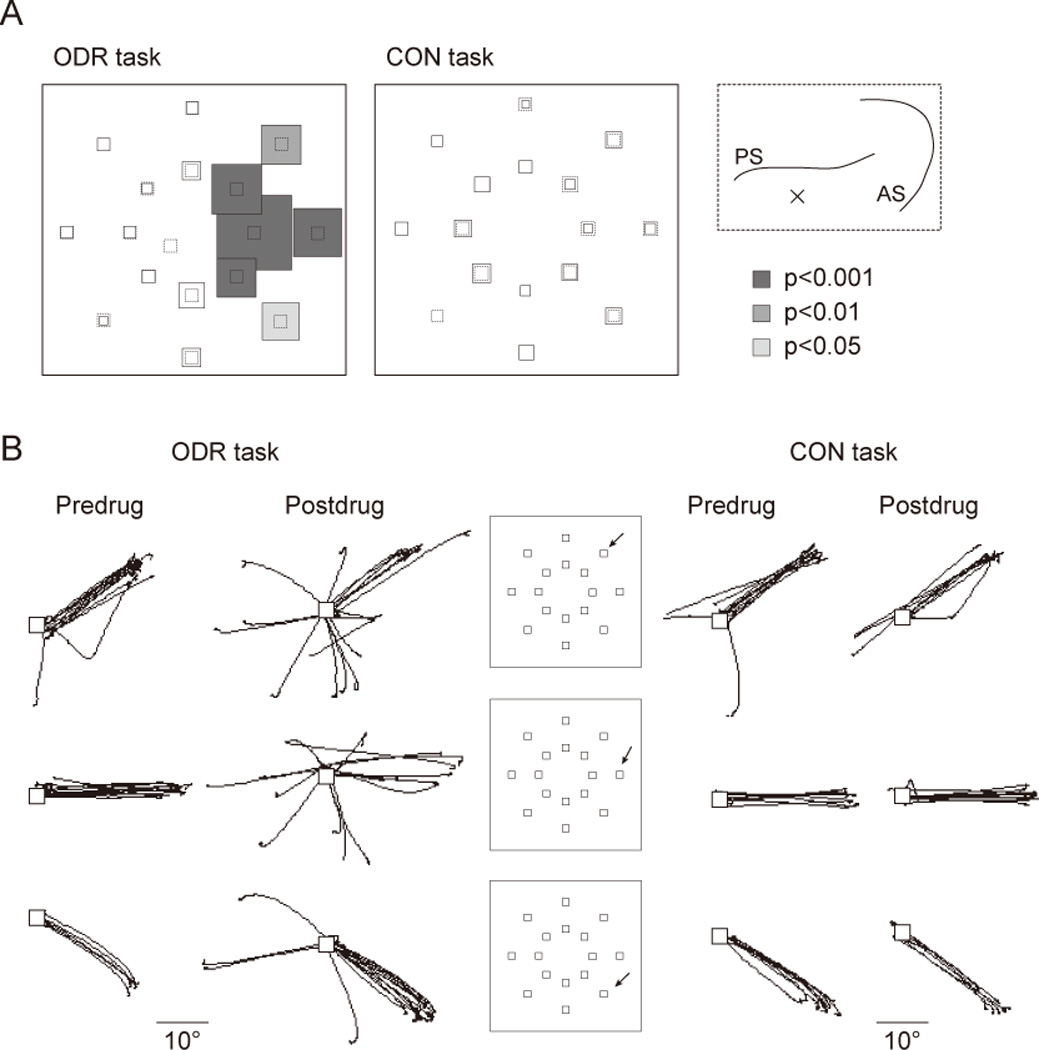
Results from Wajima and Sawaguchi (2004), illustrating the effects of BMI injection in left DLPFC on initial-saccade accuracy in the ODR and control (CON) tasks. Data are from 60 min before injection and from 100 min after injection. A. Percentage change in the discrepancy between the position of the target and the end point of the initial saccades after drug injection, shown separately for each target location. Performance at each location is represented by the size of the boxes centered on each, with dashed lines representing 0% change, and solid lines indicating the proportion change. Shading denotes target locations for which the discrepancy increased significantly after injection (Mann-Whitney U test; p values are represented by the color code indicated to the right). Inset shows the injection site of this session, which was the left DLPFC. B: Superimposed 2-dimensional trajectories of saccades in the ODR and CON task are shown separately for pre- and post-drug periods. Trials with three target locations are shown for each line. The target locations for each line are shown in the insets (arrow). White squares show the fixation point. Abbreviations: Pre, predrug (before injection) period; Post, postdrug (after injection) period.
Although the experimental procedure of Wajima and Sawaguchi (2004) largely replicated that of previous ODR tasks, a critical difference was the inclusion in their analyses of data acquired after the registration of the initial response. Critically, when considering the time window following erroneous initial saccades, it was observed that on most of these trials the next saccade was made to the correct target location for that trial (Fig. 3B). That is, the corrective saccade was made into the putative mnemonic scotoma. This corrective saccade usually occurred soon after the first saccade, although by design neither first, erroneous saccades nor second, corrective saccades were rewarded. The implication of this finding is that the errors on the initial saccades can not have been due to a failure of memory, per se. Rather, a more accurate characterization of behavior on error trials is that the monkey maintained an intact memory of the cued location, but, for its initial response, selected a target that was not the remembered target.
Figure 3.
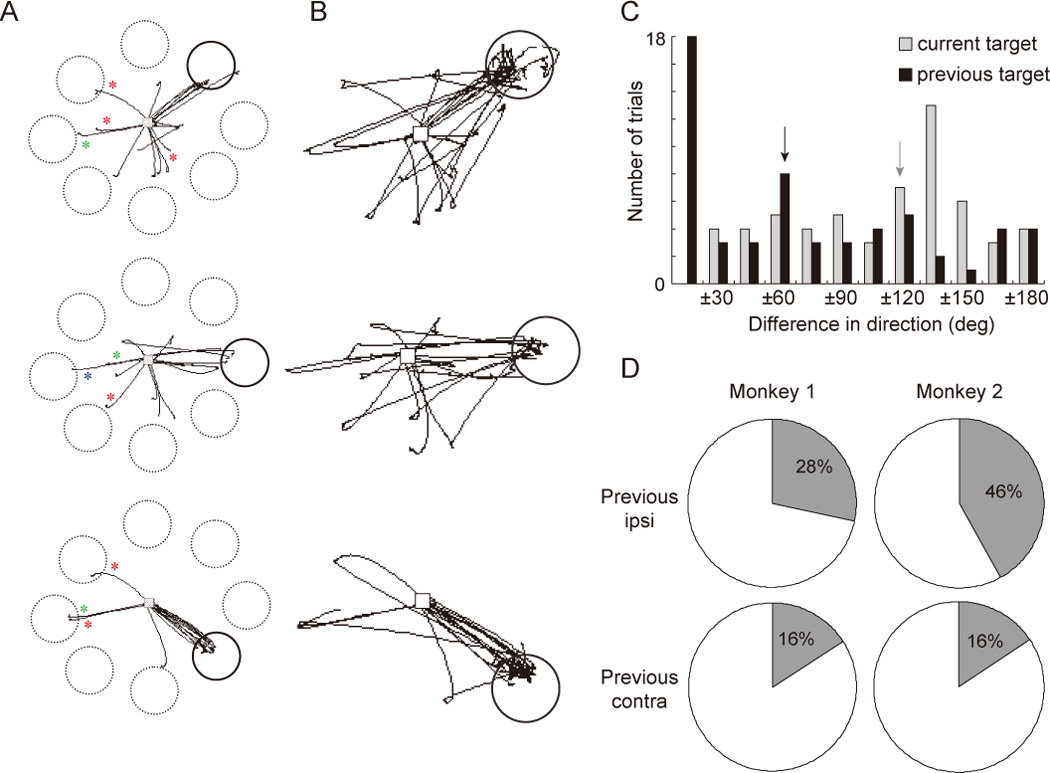
A. 2-dimensional trajectories of initial saccades in the ODR task, incorporating the same data from Fig. 2B, but with the actual (solid circle) or potential (dashed circles) target windows. Saccades falling within the solid circle correspond to correct trials. Traces with red asterisks show (erroneous) saccades that were directed toward the location that had been cued and captured in the previous trial (i.e., the previous trial was correctly performed). Traces with green and blue asterisks correspond to saccades for which the previous trial was an error trial, with green indicating saccades to the location cued on the previous trial and blue indicating saccades to the location acquired by the initial saccade on the previous trial. B. Incorporates data from A, but with the time window extended to include the saccade that followed the initial saccade. Note that most of these second saccades were directed to the location that was to be chosen by the first saccade (i.e., most of these are “corrective saccades”). C. Distribution of erroneous initial saccades on all error trials from both monkeys, illustrating quantitatively that errors showed a greater influence of information from the previous, rather than the current, trial. Each trial is scored in two ways: the difference of direction (in degrees) between the initial saccade and the location cued on the current trial (gray); and the difference of direction between the initial saccades and the location cued on the previous trial (black). Arrows indicate the bar containing the median value for each scoring procedure. (Note that this analysis does not take into account whether the previous trial was correct, and thus does not reflect the additional factor of the magnitude of the error relative to the initial saccade on the previous trial. I.e., unlike panel B, this analysis does not distinguish “proactive interference” from “perseverative” errors.) D. Percentages of erroneous saccades that were directed to the previous cue or target locations are shown separately for the target location of the previous trial (ipsi vs. contralateral visual fields to the injected site). Errors were observed more often when the previous cue had been located in the ipsilateral visual fields than when that had been presented in the contralateral visual field.
To explore why, on error trials, the initial saccade selected a target different from the remembered target, the authors investigated whether there was any systematicity to the direction of these initial saccades. The first observation was that erroneous saccades tended to select one of the 15 alternative target locations that had not been cued on that trial (Fig. 2B). Thus, the initial saccade was to a learned target location, not just a haphazard movement. Secondly, and strikingly, the target selected by erroneous initial saccades tended to be related to a location that had been relevant on the previous trial, in that it had been the cued location and/or the target acquired by the initial saccade on the immediately preceding trial. This is illustrated for the three locations from Fig. 2 in Fig. 3A, and for all error trials in Fig. 3C. To quantify this pattern, the discrepancy in degrees between the direction that would be required to acquire the current target (i.e., to respond correctly) and the direction of the actual saccadic response was computed for each error trial (gray bars in Fig. 3C), and this was compared with the discrepancy between the direction that had been required on the previous trial and that of the actual saccadic response (black bars in Fig. 3C). Interestingly, the value was smaller in the latter case, reflecting the fact that, on error trials, the monkey tended to choose the target that had been cued in the previous trial more often than the actual target location of the current trial (Fig. 3C). This kind of error occurred more often when the previous trial’s target had been located in the visual field ipsilateral to the injected hemisphere (i.e., in the “good” visual field) than when it had been in the contralateral field (Fig. 3D).
The effect of BMI was tested in a total of 47 injection sites. Of them, 36 sites yielded a significant deficit in the ODR task. In separate experimental sessions, saline was injected into 12 of the 36 effective sites, and it was confirmed that the saline injection had no significant effects either on the ODR or on the CON tasks relative to predrug performance.
Implications
The importance of these results is that they suggest an alternative explanation for the oft-replicated finding that unilateral dysfunction of the DLPFC produces systematic errors in DR performance. Instead of resulting from a “mnemonic scotoma”, they may reflect a higher-order deficit, one including elements of susceptibility to proactive interference and of perseveration. This, in turn, invites a reconsideration of functional interpretations for delay-period activity of the DLPFC. This is particularly the case because there are data, from Rao, Williams, & Goldman-Rakic (2000), of the effects of BMI on neuronal activity during the ODR task. In this experiment, BMI was iontophoresed near the tip of the recording electrode. The effect of BMI was to change the delay-period activity of many neurons, most notably a loss of spatial tuning. Can we reconcile the fact that blockade of GABAA receptors in the DLPFC alters delay-period neuronal activity in the ODR with the possibility that the corresponding behavioral deficit may not be mnemonic in character? If so, this would necessarily require the interpretation of sustained delay-period activity of the DLPFC to emphasize a function other than short-term memory.
The dependence of ODR errors on information from the previous trial points to a computation that spans a temporal interval longer than the brief delay period of a single trial. One possibility is an updating operation that integrates information about recent behaviors and outcomes with current goals and, therefore, future behaviors. This interpretation fits with the combined iontophoresis and electrophysiology findings, in that they revealed that the effects of BMI on neuronal activity are not limited to the delay period of the ODR task, but are also seen in other periods, including the post-response period (Rao et al., 2000). Further, although systematic analysis of neither intertrial interval nor pre-cue fixation period is reported in this paper, inspection of Figures 3 and 6 suggest that activity of those between-trial periods was also modulated by BMI. Thus, both the present behavioral results and previous electrophysiological results are consistent with the suggestion that the ODR deficit may reflect impaired goal selection, perhaps due to a misrepresentation of an already accomplished goal as pending.
An alternative, although less specific, explanation of the ODR deficit may simply be that the addition of the “memory delay” to an oculomotor task increases the ambiguity about what is the appropriate response to the ‘go’ signal. It is well established that the PFC supports behavior when choice is ambiguous. For example, DLPFC is shown to be more active when the human subjects respond with low-confidence than when they do with high-confidence (Kim & Cabeza, 2009), and it is thought to be engaged when cues from the environment are ambiguous and/or there is a prepotent, but inappropriate, response that is cued (Miller and Cohen, 2001). We note, however, that on the basis of this assumption one might expect a large proportion of erroneous saccades to be directed to locations near the actual target. This follows from the fact that the delay would introduce spatial ambiguity, to which information from the current trial would contribute, as well as temporal ambiguity (i.e., present vs. current cue/response). In the current experiment, however, such errors were not increased with BMI infusion.
As reviewed in the Introduction, many contemporary accounts of DLPFC function emphasize a role that is not simply mnemonic. This reflects the fact that they have used tasks that require more than (or other than) a transient sensory memory. For example, the studies of Lebedev et al. (2004) and of Tsujimoto and Sawaguchi (2004) required the withholding of a response to a visible stimulus during the delay period. The studies of Takeda and Funahashi (2002, 2004, 2007) required the dynamic transformation of information from that of the cue location to that of the appropriate saccadic target. Lee and colleagues (Barraclough, Conroy, & Lee, 2004; Seo, Barraclough, & Lee, 2007) and Miller and colleagues (Histed, Pasupathy, & Miller, 2009) required the monkeys to adjust their behavior dynamically based on the history of their choices and outcomes and have shown a cross-trial effect of the previous choices and outcomes on the activity of DLPFC neurons. Finally and relatedly, several studies by Genovesio, Wise, and colleagues can be summarized as requiring the segregation of information about previous vs. future events. In these studies, monkeys had to choose the target of a future saccade (“future goal”) with information about the previously chosen target (“previous goal”) conditioned on the current visual instruction. This design thereby enabled a discrimination of the neuronal representation of previous goals from that for future goals. Genovesio et al. (2006) found two separate populations of neurons: one encoded previous goal location and the other encoded the future goal. Interestingly, the activity of neurons with tuning to the previous target tended to be correlated with that of future-tuning neurons (Tsujimoto, Genovesio, & Wise, 2008), which may reflect the selection of a future goal based on information from previous trials.
It is important to note that the misrepresentation of an already accomplished goal as still pending can also produce errors on tasks and behaviors that, unlike the ODR, do not require the short-term retention of trial-specific information. Two examples are cardinal clinical signs of patients with PFC damage -- perseveration and environmental dependency syndrome. The former is classically seen in the Wisconsin Card Sorting Task, when a previously appropriate response is reexecuted even though a change of the sorting rule means that a different response is now needed to achieve the goal (Barcelo & Knight, 2002; Milner, 1963). The latter (exemplified by, say, using a toothbrush encountered in the bathroom of a friend’s house) can result if a habitual goal state (e.g., maintaining good oral hygene) is activated in an inappropriate context (Knight & D'Esposito, 2003).
Caveats
There are important limitations to be born in mind when considering the findings presented here. First, because the authors of this commentary did not generate these data and do not have access to the original raw data, the standard assumption of authors taking responsibility for the integrity of the data can not be made. From this perspective, the skeptical reader may want to consider the ideas presented here as just that – ideas about a possible alternative explanation for “mnemonic scotoma” findings that require empirical validation. Even if taken at face value, these results should be interpreted carefully, because GABA is an inhibitory transmitter, and so the selective blockade of its receptors could potentially produce effects that differ qualitatively from those produced by a gross surgical lesion. We emphasize that BMI does not inactivate the affected area, but rather blocks the inhibitory action of GABA receptors. It is also notable that we do not know the exact boundaries of the affected area. A possible resultant effect could be the unmasking (or alteration) of activity in cells within the affected area (Sawaguchi, 2001), as well as the excitatory modulation of activity in task-related cells (Rao et al., 2000). It is also likely that BMI injection has behavioral effects in addition to those that were detailed here. For example, many researchers have noted anecdotally that BMI can result in a marked increase in the number of breaks of fixation that occur across a testing session. Finally, because we have drawn on the physiological data from a previous BMI iontophoresis experiment (Rao et al., 2000) to help us interpret the behavioral findings presented here, it should be noted that these speculations await confirmation from a study in which the effects of BMI on physiology and on behavior are measured from the same animals, from the same experimental session.
Conclusion
It is our hope that the data and ideas presented here will contribute to ongoing efforts to understand the functions of the PFC. One domain where they may have implications is in understanding psychiatric and neurological disorders. For example, inhibitory mechanisms in the DLPFC mediated by GABAergic neurons are thought to be involved in schizophrenia, and cognitive deficits in schizophrenia have been attributed to deficits of working memory (Lewis, Hashimoto, & Volk, 2005). However, the clinical syndrome of schizophrenia patients is not well characterized as a simple impairment of maintenance functions. It may be that the function that we have considered here -- selecting appropriate future responses according to the preceding context through segregated representation of previous and future events – could have relevance for understanding this disorder (e.g. Elvevag, Maylor, & Gilbert, 2003). Considering this or related dysfunction might also have relevance for understanding normal aging (Einstein, McDaniel, Manzi, Cochran, & Baker, 2000; Kliegel, McDaniel, & Einstein, 2000) and dementia (Maylor, Smith, Della Sala, & Logie, 2002).
In conclusion, the heretofore unpublished findings that we present in this commentary suggest a way to reconcile the literature on the mnemonic scotoma with the findings from more recent electrophysiological studies. At the very least, they illustrate the fact that a deficit on a test of working memory need not imply a working memory deficit. It is our hope that the publication of these data contribute to continuing efforts to achieve an integrated understanding of the function and physiology of the PFC.
Acknowledgments
The authors of this document thank Ms. Kayo Wajima for granting permission for the results to be published in this form, for generously providing figures, and for valuable discussions of this work. S. Wise and E. Murray also contributed helpful discussions. The preparation of this document was supported in part by a Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (22700340) and Intramural Research Grant (22-6) for Neurological and Psychiatric Disorders of NCNP to S.T., and by National Institutes of Health (U.S.A.) MH064498 to B.R.P.
Footnotes
The empirical data presented here were conceived, generated, and analyzed by K. Wajima under the supervision of T. Sawaguchi, and presented at the 27th Annual Meeting of the Japan Neuroscience Society/3rd Joint Meeting of the Japan Neuroscience Society and the Japanese Society for Neurochemistry, Osaka, Japan, 21–23 September 2004.
Although there is also a large literature on the long-term memory functions of the PFC (reviewed, e.g., in Tulving & Craik, 2000), this paper is limited to short-term and working memory.
References
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–356. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nature Neuroscience. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. Journal of Comparative and Physiological Psychology. 1976;90:293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya D. Retention of delayed-alternation: effect of selective lesions of sulcus principalis. Science. 1969;165:1271–1273. doi: 10.1126/science.165.3899.1271. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. Journal of Neurophysiology. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nature Neuroscience. 2001;4:311–316. doi: 10.1038/85179. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. The effects of prefrontal lesions on working memory performance and theory. Cogntive Affective and Behavioral Neuroscience. 2004;4:528–539. doi: 10.3758/cabn.4.4.528. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Manzi M, Cochran B, Baker M. Prospective memory and aging: forgetting intentions over short delays. Psychology of Aging. 2000;15:671–683. doi: 10.1037//0882-7974.15.4.671. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Maylor EA, Gilbert AL. Habitual prospective memory in schizophrenia. BMC Psychiatry. 2003;3:9. doi: 10.1186/1471-244X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. Journal of Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic "scotomas". Journal of Neuroscience. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. Journal of Neurocytology. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. Fourth ed. London: Academic Press; 2008. [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Wise SP. Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. Journal of Neuroscience. 2006;26:7305–7316. doi: 10.1523/JNEUROSCI.0699-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesio A, Tsujimoto S, Wise SP. Prefrontal cortex activity during the discrimination of relative distance. Journal of Neuroscience. 2011;31:3968–3980. doi: 10.1523/JNEUROSCI.5373-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology: The nervous system. Vol. V. Bethesda: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. Localization of function within the dorsolateral prefrontal cortex of the rhesus monkey. Experimental Neurology. 1970;27:291–304. doi: 10.1016/0014-4886(70)90222-0. [DOI] [PubMed] [Google Scholar]
- Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63:244–253. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar CR, Pasternak T. Flexibility of sensory representations in prefrontal cortex depends on cell type. Neuron. 2009;64:730–743. doi: 10.1016/j.neuron.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF. The functions of the frontal association areas in monkeys. Comparative Psychology Monographs. 1936;13:1–60. [Google Scholar]
- Kim H, Cabeza R. Common and specific brain regions in high- versus low-confidence recognition memory. Brain Research. 2009;1282:103–113. doi: 10.1016/j.brainres.2009.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MA, Einstein GO. Plan formation, retention, and execution in prospective memory: a new approach and age-related effects. Memory and Cognition. 2000;28:1041–1049. doi: 10.3758/bf03209352. [DOI] [PubMed] [Google Scholar]
- Knight RT, D'Esposito M. Lateral prefrontal syndrome: a disorder of executive control. In: D'Esposito M, editor. Neurological fundations of cognitive neuroscience. Cambridge, MA: MIT Press; 2003. pp. 259–279. [Google Scholar]
- Kojima S, Goldman-rakic PS. Delay-related activity of prefrontal neurons in rhesus-monkeys performing delayed-response. Brain Research. 1982;248:43–49. doi: 10.1016/0006-8993(82)91145-3. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. Journal of Neurophysiology. 1971;34:337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biology. 2004;2:e365. doi: 10.1371/journal.pbio.0020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Malmo RB. Interference factors in delayed response in monkey after removal of the frontal lobes. Journal of Neurophysiology. 1942;5:295–308. [Google Scholar]
- Maylor EA, Smith G, Della Sala S, Logie RH. Prospective and retrospective memory in normal aging and dementia: an experimental study. Memory and Cognition. 2002;30:871–884. doi: 10.3758/bf03195773. [DOI] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Constantinidis C. Persistent discharges in the prefrontal cortex of monkeys naive to working memory tasks. Cerebral Cortex. 2007;17 Suppl 1:i70–i76. doi: 10.1093/cercor/bhm063. [DOI] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Stanford TR, Constantinidis C. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. Journal of Neuroscience. 2011;31:6266–6276. doi: 10.1523/JNEUROSCI.6798-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting - role of frontal lobes. Archives of Neurology. 1963;9 90-&. [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Mishkin M, Rosvold HE, Kaplan SJ. Effects on delayed-response performance of lesions of dorsolateral and ventromedial frontal cortex of baboons. Journal of Compapative and Physiological Psychology. 1952;45:565–575. doi: 10.1037/h0061240. [DOI] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C. Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. Journal of Neuroscience. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T. Unmasking of silent "task-related" neuronal activity in the monkey prefrontal cortex by a GABA(A) antagonist. Neuroscience Research. 2001;39:123–131. doi: 10.1016/s0168-0102(00)00204-2. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Iba M. Prefrontal cortical representation of visuospatial working memory in monkeys examined by local inactivation with muscimol. Journal of Neurophysiology. 2001;86:2041–2053. doi: 10.1152/jn.2001.86.4.2041. [DOI] [PubMed] [Google Scholar]
- Seo H, Barraclough DJ, Lee D. Dynamic signals related to choices and outcomes in the dorsolateral prefrontal cortex. Cerebral Cortex. 2007;17 Suppl 1:i110–i117. doi: 10.1093/cercor/bhm064. [DOI] [PubMed] [Google Scholar]
- Stamm JS. Electrical stimulation of monkeys' prefrontal cortex during delayed-response performance. Journal of Comparative and Physiological Psychology. 1969;67:535–546. doi: 10.1037/h0027294. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. Journal of Neurophysiology. 2002;87:567–588. doi: 10.1152/jn.00249.2001. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Population vector analysis of primate prefrontal activity during spatial working memory. Cerebral Cortex. 2004;14:1328–1339. doi: 10.1093/cercor/bhh093. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Relationship between prefrontal task-related activity and information flow during spatial working memory performance. Cortex. 2007;43:38–52. doi: 10.1016/s0010-9452(08)70444-1. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Transient neuronal correlations underlying goal selection and maintenance in prefrontal cortex. Cerebral Cortex. 2008;18:2748–2761. doi: 10.1093/cercor/bhn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T. Properties of delay-period neuronal activity in the primate prefrontal cortex during memory- and sensory-guided saccade tasks. European Journal of Neuroscience. 2004;19:447–457. doi: 10.1111/j.0953-816x.2003.03130.x. [DOI] [PubMed] [Google Scholar]
- Tulving E, Craik FIM. The oxford handbook of memory. New York: Oxford University Press; 2000. [Google Scholar]
- Wajima K, Sawaguchi T. The role of GABAergic inhibiton in suppressing perseverative responses in the monkey prefrontal cortex. Neuroscience Research. 2004;50 Supplement 1:P3–P317. [Google Scholar]
- Warden MR, Miller EK. Task-dependent changes in short-term memory in the prefrontal cortex. Journal of Neuroscience. 2010;30:15801–15810. doi: 10.1523/JNEUROSCI.1569-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM, Akert K, editors. The frontal granular cortex and behavior. New York: McGraw-Hill Book Company; 1964. [Google Scholar]


