Abstract
The astrocyte glutamate transporter, GLT1, is responsible for the vast majority of glutamate uptake in the adult central nervous system (CNS), thereby regulating extracellular glutamate homeostasis and preventing excitotoxicity. Glutamate dysregulation plays a central role in outcome following traumatic spinal cord injury (SCI). To determine the role of GLT1 in secondary cell loss following SCI, mice heterozygous for the GLT1 astrocyte glutamate transporter (GLT1+/−) and wild-type mice received thoracic crush SCI. Compared to wild-type controls, GLT1+/− mice had an attenuated recovery in hindlimb motor function, increased lesion size, and decreased tissue sparing. GLT1+/− mice showed a decrease in intraspinal GLT1 protein and functional glutamate uptake compared to wild-type mice, accompanied by increased apoptosis and neuronal loss following crush injury. These results suggest that astrocyte GLT1 plays a role in limiting secondary cell death following SCI, and also show that compromise of key astrocyte functions has significant effects on outcome following traumatic CNS injury. These findings also suggest that increasing intraspinal GLT1 expression may represent a therapeutically relevant target for SCI treatment.
Keywords: secondary injury, GLT1+/− mice, crush injury, glutamate uptake, excitotoxicity
Introduction
The initial trauma associated with spinal cord injury (SCI) results in cell death and axotomy of passing fibers. Acute damage is followed by a period of secondary injury due to inflammation, vascular insult, depletion of local energy supplies, and alterations in extracellular fluid composition, all of which produce additional cell death and functional deficits (Norenberg et al. 2004).
Glutamate-mediated cell death plays a key role in these secondary events following traumatic central nervous system (CNS) injuries such as SCI (Park et al. 2004; Stys 2004; Yi and Hazell 2006). Exogenous parenchymal administration of glutamate to normal spinal cord results in tissue and functional loss similar to SCI (Xu et al. 2005). Large increases in extracellular excitatory amino acids such as glutamate occur after SCI (Liu et al. 1991; Xu et al. 2004), and can persist for over a week, depending on injury severity (Panter et al. 1990). In addition to focal increases in glutamate, levels can also rise in regions removed from the lesion site, possibly via a spreading mechanism involving activated glial cells (Hulsebosch 2008). NMDA receptor antagonists such as MK-801 produced therapeutic efficacy in various SCI paradigms in only some studies (Agrawal and Fehlings 1997; Gaviria et al. 2000; Holtz and Gerdin 1991; Liu et al. 1997), while AMPA receptor antagonists such as NBQX promoted both gray and white matter tissue sparing, improvement in bladder function, and decreases in allodynia and hyperalgesia in a number of SCI studies (Agrawal and Fehlings 1997; Liu et al. 1997; Mu et al. 2002; Rosenberg et al. 1999; Wrathall et al. 1994). Due to unwanted side effects associated with receptor-acting drugs, it may be advantageous to focally address glutamate toxicity by targeting the normal physiological function of astrocytes, those cells that normally play the central role in regulating CNS glutamate homeostasis.
Astrocytes outnumber their neuronal counterparts approximately ten-fold, and play many crucial roles in adult CNS function, including the vast majority of glutamate uptake (Tanaka et al. 1997). Following SCI, astrocytes undergo a complex and heterogeneous set of morphological, gene expression, and functional changes that depend on factors such as severity, location, and timing relative to insult. These changes can render reactive astrocytes protective and/or harmful via mechanisms (Sofroniew 2009) including: 1) gain of toxic functions, 2) gain of novel neuroprotective functions specific to reactive astrocytes, and 3) maintenance or loss of normal protective homeostatic functions such as glutamate transport.
The major CNS glutamate transporters, GLT1 and GLAST, are expressed almost exclusively by astrocytes in adult mammals (Maragakis and Rothstein 2004); however, GLT1 accounts for the majority of glutamate uptake in most CNS regions, including the spinal cord. By regulating extracellular glutamate homeostasis, GLT1 assures proper synaptic function and prevents excitotoxic injury to neurons, axons and oligodendrocytes. Astrocyte death, altered physiology of astrocytes and their transporters, and/or changes in expression of key astrocyte proteins such as glutamate transporters may result in further susceptibility to secondary excitotoxic injury to neurons, their axonal and dendritic structures, oligodendrocytes and myelin. Few studies have examined the function of astrocyte glutamate transport following SCI. To elucidate the role of GLT1 following SCI, wild-type and mice heterozygous for the glutamate transporter GLT1 (GLT1+/− mice) were subjected to thoracic crush. Histological and functional outcomes were found to be worsened in GLT1+/− mice. These results provide valuable information both for understanding the role that astrocyte glutamate transport plays in the host response to SCI and for designing therapeutic strategies targeting the physiological function of astrocytes.
Materials and Methods
GLT1 Heterozygous (GLT1+/−) Mice
GLT1+/− mice (Tanaka et al. 1997) allow for examination of the role of GLT1 on outcomes following SCI. GLT1 knockout mice were not used because the majority of these animals die postnatally due to seizures. Previous work demonstrated that, compared to wild-type littermates, these GLT1+/− mice retain approximately half of the intraspinal GLT1 protein levels and GLT1-based functional glutamate uptake (Pardo et al. 2006).
Thoracic Crush Injury
The care and treatment of animals (wild-type mice: n = 23; GLT1+/− mice: n = 23) in all procedures was conducted in strict accordance with the guidelines set by the European Communities Counsel Directive (November 24th, 1986), the NIH Guide for the Care and Use of Laboratory Animals, the Guidelines for the Use of Animals in Neuroscience Research and the Johns Hopkins University IACUC, and measures were taken to minimize any potential pain or animal discomfort. Mice (approximately 20–30 grams) received i.p. injections of anesthetic cocktail [acepromazine maleate (0.7mg/kg; Fermenta Animal Health, Kansas City, MO), ketamine (95.0mg/kg; Fort Dodge Animal Health; Fort Dodge, IA), and xylazine (10.0mg/kg; Bayer, Shawnee Mission, KS)]. The back musculature was excised, and a laminectomy was performed above the T8–T10 levels of the spinal cord. Adult wild-type (Jackson) and GLT1+/− mice received a 6mm thoracic (T9) forceps crush SCI, as described previously (Plemel et al. 2008). Uninjured control animals underwent the same surgery, including laminectomy, but did not receive crush injury. After surgical procedures, animals were allowed to recover on a circulating warm water heating pad until awake, and then returned to their home cages, usually requiring a continuous observations period of 2–4 hours. Animals were monitored on a daily basis thereafter. Antibiotic powder was applied to the closed wounds to prevent infection. Bladders were manually expressed twice-daily for the first week post-injury.
Tissue processing
Euthanasia was performed by inhalation anesthesia to minimize pain and distress. This method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Animals (both uninjured control and crush mice) were sacrificed at 2, 4, and 14 days post-injury by transcardial perfusion with 0.3% saline, followed by ice-cold 4% paraformaldehyde (Fisher Scientific; Pittsburgh, PA). The entire spinal cord was removed. The thoracic region was isolated and post-fixed in 4.0% paraformaldehyde, followed by cryoprotection in 30% sucrose (Fisher)/.1 M phosphate buffer at 4°C for 3 days. Tissue surrounding the lesion site was embedded in OCT (Fisher), fast frozen with dry ice, and stored at −80°C until processed. Spinal cord tissue blocks were cut in the transverse plane at 30μm thickness. Sections were collected on glass slides and stored at −20°C until analyzed.
GLT1 Western Analysis
Aliquots of homogenized cord samples from thoracic level 9 were subjected to SDS-polyacrylamide gel electrophoresis. Blots were probed with antibody specific for GLT-1 (1:5,000) (Lepore et al. 2008). Immunoreactivity was visualized by enhanced chemiluminescence, quantified densitometrically using Quantity One v4.0 software (BioRad), and normalized to actin.
Glutamate Uptake
Fresh micro-dissected spinal cord (from thoracic level 9) was homogenized in 5.0 mM Tris/320.0 mM sucrose with Complete protease inhibitor cocktail (Roche, Indianapolis, IN), using a hand held pellet pistol. Samples were then washed three times in ice-cold PBS (pH 7.4), before they were re-suspended in either Na+ containing Krebs buffer (120.0 mM NaCl, 25.0 mM Tris–HCl pH 7.4, 5.0 mM KCl, 2.0 mM CaCl2, 1.0 mM KH2PO4, 1.0 mM MgSO4, 10% glucose), or Na+ free Krebs (120.0 mM choline: substituted for NaCl). Samples were incubated with 10.0 μM 3H-glutamate for 4 min at 37°C, then harvested and washed three times (Tris buffer, pH 7.4) on Whatman GF/B paper (fired) (#FPD-100), using a tissue harvester (Brandel, Gaithersburg, MD). Radioactivity was determined by standard scintillation counting procedure. Mean Na+-dependent uptake was determined in duplicates, subtracting the mean uptake in Na+-free duplicates. In order to determine the contribution of GLT1-mediated transport, a separate set of samples was also incubated with 500.0 μM dihydrokainate (DHK) along with 10.0 μM 3H-glutamate for 4 min at 37°C, then harvested and washed three times on Whatman GF/B paper, using a tissue harvester. Radioactivity was determined by standard scintillation counting procedure. (Pardo et al. 2006)
Histological Analysis
Cresyl Violet Staining and Lesion Identification
Sections were stained by 0.5% Cresyl-violet acetate and imaged using a Zeiss Imager Z1 microscope at 10X magnification (Paul et al. 2009). Using Zeiss Axiovision Rel. 4.6 software, lesion area and spared tissue area were outlined and quantified. Specifically, lesion area and tissue sparing area were determined on every 10th section by tracing the entire area of the cord and the injury area to obtain 2-dimensional values, Injury was defined as areas including both lost tissue (actual cavity formation) and surrounding damaged tissue in which the normal anatomical structure of the spinal was lost. Spared tissue was defined as the remaining areas where the normal anatomical structure of the spinal cord was preserved. Lesion volume was determined using the Cavalieri estimator of volume (V = [Σ(A1+A2+…An) × D] − [Amax × Y], where D = Distance between measurements and Y = thickness of each section). Ventral horn neuronal cell bodies were outlined in these same Cresyl-violet stained sections using the Axiovision software, and neuronal cell body areas were quantified. Ventral horn was defined as gray matter ventral to the central canal. Neuron counts were separated into small (75–250 μm2) and large (> 250 μm2) ventral horn neurons. Lesion / spared tissue area and ventral horn neuronal counts were determined in serial sections spaced at successive distances of 180 μm.
TUNEL
Apoptotic cells in the spinal cord were identified and quantified using the Promega Dead End TUNEL System (Promega, Madison, WI) combined with streptavidin conjugated to Alexa Fluor 555 (1:500; Molecular Probes). Controls in which biotinylated nucleotide was omitted were conducted, and staining was found to be specific. Samples were counterstained with DAPI (1:1000; Sigma) to identify nuclei, and coverslipped with anti-fade mounting media (Fluorosave, CN Biosciences; La Jolla, CA). Slides were subsequently stored at 4°C. Images were acquired on either Zeiss Imager Z1 microscope (Roper Scientific; Trenton, NJ) or on a Zeiss laser confocal microscope. Images were analyzed using either Metamorph or Zeiss confocal software. Adobe Photoshop CS (Adobe, San Jose, CA) was used to prepare figures.
Behavioral Analysis
All behavioral measurements were obtained one day prior to crush injury, once per day on days 1–7 post-crush, and on day 14 post-crush. Hindlimb motor function was assessed using the hindlimb BMS (Basso Mouse Scale) scale, as previously described (Basso et al. 2006). Hindlimb grip strength was assessed according to previous studies (Lepore et al. 2008).
Statistical Analysis
Hindlimb BBB, grip strength, lesion size, tissue sparing and neuron counts were analyzed via ANOVA using the statistical software Sigmastat (SAS Software). Lesion volume was determined using Cavalieri’s Method. In some cases, Student t-test was performed to compare data between groups. All data are presented as mean ± S.E.M., and significance level was set at p ≤ 0.05.
Results
Recovery in Hindlimb Motor Performance Was Reduced in GLT1+/− Mice Following Thoracic Crush SCI
To evaluate the effects of reduced astrocyte GLT1 levels on functional and histological outcomes following SCI, wild-type and GLT1+/− mice received a 6.0 mm forceps crush at thoracic level 9 (n = 18/group for all studies). Both groups showed a drop in hindlimb BMS score (Fig 1A) and hindlimb grip strength (Fig 1B) at 1 day post-injury (n = 8/group for behavioral analyses). The difference between groups in BMS was significant at 2 day post-injury. Both groups also showed progressive recovery in these two functional measurements during the first 14 days post-injury; however, recovery was significantly slowed in GLT1+/− mice, with these mice never recovering to the level of wild-type mice by 14 days post-injury. No differences were observed in weight (Fig 1C), suggesting that a systemic effect of GLT1 loss did not occur.
Figure 1. Recovery in Hindlimb Motor Performance Was Reduced in GLT1+/− Mice Following Thoracic Crush SCI.
Following 6.0 mm forceps crush at thoracic level 9, both wild-type and GLT1+/− mice showed a drop in hindlimb BMS score, which was exaggerated in GLT1+/− mice at 2 days post-injury (A). Hindlimb grip strength at 1 day post-injury was similar between the groups (B). Both groups also showed progressive recovery in these two functional measurements during the first 14 days post-injury; however, recovery was significantly slowed in GLT1+/− mice, with the heterozygous mice never recovering to the level of wild-type mice by 14 days post-injury. No differences were observed in weight (C). * p < 0.05.
GLT1 Modifies Lesion Size Following Thoracic Crush SCI
Cresyl violet stained sections of thoracic spinal cord from mice that received a crush SCI were analyzed for lesion size and spared tissue at 14 days post-injury (n = 7/group for histological analyses) (Fig 2A). This time was chosen because secondary cell death has been reported to be mostly over by this point (Okano et al. 2003). The lesion was larger in GLT1+/− mice not only near the crush epicenter (Fig 2A–B), but also in regions extending away from the epicenter in both rostral (Fig 2C–D) and caudal directions. Compared to wild-type mice, GLT1+/− mice had significantly larger lesion areas (Fig 2E) and significantly smaller spared tissue areas (Fig 2F) at multiple distances from the lesion epicenter, both in rostral and caudal directions. Lesioned tissue was observed in wild-type mice extending up to ~1.0 mm away from the crush epicenter in both directions, while lesions in GLT1+/− mice extended to approximately ~2.0 mm from the epicenter. When lesion areas at regions extending through the thoracic spinal cord were analyzed using Cavalieri’s Method (Paul et al. 2009), overall lesion volume in GLT1+/− mice was found to be significantly larger than wild-type controls (Fig 2G).
Figure 2. Lesion Size Was Increased and Tissue Sparing Was Decreased in GLT1+/− Mice Following Thoracic Crush SCI.
Cresyl violet stained sections were analyzed for lesion size and spared tissue at 14 days post-injury, as demarcated by the dotted line (A). The lesion site was larger in GLT1+/− mice not only near the crush epicenter (A: wild-type, B: GLT1+/−), but also in regions extending away from the epicenter in both rostral (C: wild-type, D: GLT1+/−) and caudal directions. Compared to wild-type mice, GLT1+/− mice had significantly larger lesion areas (E) and significantly smaller spared tissue areas (F) at multiple distances from the lesion epicenter, both in rostral and caudal directions. Overall lesion volume in GLT1+/− mice was found to be significantly larger than wild-type controls using Cavalieri’s Method (G). At 14 days post-laminectomy (n = 5 mice/group), no differences were found in tissue area between uninjured wild-type and GLT1+/− groups at any point along the thoracic spinal cord (H). * p < 0.05.
To evaluate whether GLT1 heterozygous mice had smaller spinal cord tissue areas even prior to injury, uninjured wild-type and GLT1+/− mice were sacrificed at 14 days post-laminectomy (n = 5 mice/group). No differences were found in tissue area between wild-type and GLT1+/− groups at any point along the thoracic spinal cord (Fig 2H).
Intraspinal GLT1 Protein Levels and GLT1 Mediated Glutamate Uptake Were Significantly Reduced in GLT1+/− Mice
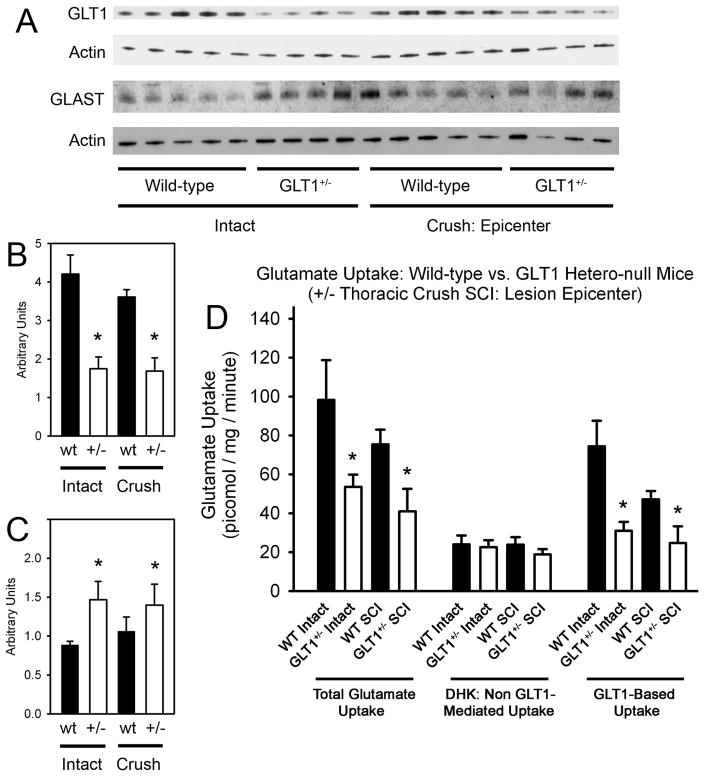
To correlate functional and histological outcomes with GLT1 levels, intraspinal GLT1 protein levels were quantified by immunoblotting of whole spinal cord tissue from thoracic spinal cord level 9 (level of crush) in uninjured and injured mice at 4 days post-surgery (n = 4–5 mice/group) (Fig 3A). In both the injured and uninjured spinal cord, GLT1 protein levels were reduced ~50–60% in GLT1+/− mice compared to wild-type controls (Fig 3B). Interestingly, overall GLT1 protein levels were not significantly changed in either wild-type or GLT1+/− mice following crush SCI when compared to injured wild-type and GLT1+/− animals, respectively.
Figure 3. Intraspinal GLT1 Protein Levels and Functional GLT1 Mediated Glutamate Uptake Were Reduced in GLT1+/− Mice.
Intraspinal GLT1 and GLAST protein levels were quantified by Western blotting of whole spinal cord tissue from thoracic level 9 (level of crush) in uninjured and injured mice at 4 days surgery (A). In both the injured and uninjured spinal cord, GLT1 protein levels were reduced ~50–60% in GLT1+/− mice compared to wild-type controls (B). Intraspinal GLAST levels were elevated in both injured and uninjured GLT1+/− mice compared to wild-type controls (C), but the injury itself had no effect on GLAST levels in either wild-type or GLT1+/− mice when compared to the respective uninjured controls (C). Actin served as the loading control. Functional intraspinal glutamate uptake was measured in whole spinal cord tissue from thoracic level 9 (level of crush). (D). In both the injured and uninjured spinal cord, both total Na+-dependent glutamate uptake and GLT1 mediated glutamate uptake were significantly reduced in GLT1 hetero-null mice compared to wild-type controls, while non-GLT1 mediated glutamate uptake was not different between GLT1+/− and wild-type groups. In wild-type mice, GLT1 mediated uptake also showed a trend toward reduction following injury compared to uninjured controls. * p < 0.05.
Intraspinal levels of the other major glial glutamate transporter, GLAST, were also assessed at thoracic spinal cord level 9 via immunoblotting at 4 days post-surgery (n = 4–5 mice/group) (Fig 3A). GLAST levels were modestly elevated in GLT1+/− mice compared to wild-type controls, both in uninjured mice and following crush SCI (Fig 3C). However, injury itself had no effect on GLAST levels in either wild-type or GLT1+/− mice when compared to the respective uninjured controls when the whole spinal cord segment was analyzed (Fig 3C).
Similarly, functional intraspinal glutamate transport was measured (n = 5–9 mice/group) in whole spinal cord tissue from thoracic level 9 (level of crush). In both the injured and uninjured spinal cord, both total Na+-dependent glutamate uptake (which includes uptake via GLT1 and other Na+-dependent glutamate transporters) and GLT1 mediated glutamate uptake (Na+-dependent uptake not blocked by the GLT1-specific inhibitor, DHK) were significantly reduced in GLT1 hetero-null mice compared to wild-type controls, while non-GLT1 mediated glutamate uptake (after DHK inhibition) was not different between GLT1+/− and wild-type groups (Fig 3D). In wild-type mice, GLT1 mediated uptake also showed a trend toward reduction following injury compared to uninjured controls, demonstrating that while total GLT1 protein was not reduced following crush injury (at least when assessed at the level of whole spinal cord segment) functional uptake was partially lost.
Reduced GLT1 Expression Results in Neuronal Loss and Apoptotic Cell Death Following Thoracic Crush SCI
Cresyl violet stained sections (Fig 4A–C) of thoracic spinal cord from mice that received a crush SCI were analyzed for counts of small and large ventral horn neurons at 14 days post-injury. Compared to wild-type mice, GLT1+/− mice had significantly greater losses of both small (Fig 4D: 75–250 μm2) and large (Fig 4E: > 250 μm2) ventral horn neurons at multiple distances from the lesion epicenter. Neuronal loss was greater in GLT1+/− mice not only near the crush epicenter, but also in regions extending away from the epicenter, particularly in the rostral direction.
Figure 4. Neuronal Loss Was Increased in GLT1+/− Mice Following Thoracic Crush SCI.
Spinal cord sections were stained by cresyl violet (A–C) to quantify neurons in the ventral horn (A: outlined, B–C). Compared to wild-type mice, GLT1+/− mice had significantly greater losses of both small (D; 75–250 μm2) and large (E; > 250 μm2) ventral horn neurons at multiple distances from the crush epicenter. In uninjured wild-type and GLT1+/−mice sacrificed at 14 days post-laminectomy, no differences were found in small (F) or large (G) ventral horn neuron counts between wild-type and GLT1+/− groups at any point along the thoracic spinal cord. * p < 0.05. Scale bars: 50μm (A–C).
To assess whether the reduction of both small and large neurons in GLT1 heterozygous mice was not the result of reduced numbers prior to injury, uninjured wild-type and GLT1+/− mice were sacrificed at 14 days post-laminectomy (n = 5 mice/group). No differences were found in small (Fig 4F) and large (Fig 4G) ventral horn neuron counts between wild-type and GLT1+/− groups at any point along the thoracic spinal cord.
Apoptotic cell death was examined using the apoptotic marker, TUNEL (Fig 5A–B). Quantification of apoptosis at 2 and 14 days post-injury (n = 5 mice/group/time point) was conducted in sections at 0.5 mm rostral from the lesion epicenter. Compared to wild-type mice, there was significantly greater gray matter, white matter and total spinal cord apoptosis in GLT1+/− spinal cord at both 2 and 14 days post-injury (Fig 5C). These findings demonstrate that loss of GLT1 exacerbates secondary neuronal cell death following SCI and that glutamate-mediated cell death persists (albeit at a lower level) up to at least two weeks post-injury.
Figure 5. Apoptotic Cell Death Was Increased in GLT1+/− Mice Following Thoracic Crush SCI.
Apoptotic cell death was examined using the apoptotic marker, TUNEL, at 2 (A) and 14 days post-injury (B). Quantification of apoptosis at 2 and 14 days post-injury (n = 5 mice/group/time point) was conducted in sections at 0.5 mm rostral from the lesion epicenter. Compared to wild-type mice, there was significantly greater gray matter, white matter and total spinal cord apoptosis in GLT1+/− spinal cord at both 2 and 14 days post-injury (C). * p < 0.05. Scale bar: 500μm (A).
Discussion
Glutamate-mediated excitotoxicity has been implicated in secondary degenerative events following SCI (Park et al. 2004), including findings of significantly increased intraspinal glutamate levels (Liu et al. 1991; Xu et al. 2004) and neuroprotection mediated by administration of glutamate receptor antagonists, particular those targeting the AMPA receptor subtype (Agrawal and Fehlings 1997; Liu et al. 1997; Mu et al. 2002; Rosenberg et al. 1999; Wrathall et al. 1994). However, few studies have examined changes in astrocyte glutamate transporter expression and function following traumatic CNS injury. For example, increased levels of GLT1 and a second glial glutamate transporter, GLAST, following thoracic contusion have been reported (Vera-Portocarrero et al. 2002); however, expression changes were only examined during the first 24 hours. The present study examined whether in vivo reduction in the levels of the major astrocyte glutamate transporter, GLT1, following SCI resulted in significant alterations in injury.
This transporter accounts for approximately 95% of astrocytic glutamate transport in the CNS (Tanaka et al. 1997), demonstrating its central role in maintenance of extracellular glutamate homeostasis. We observed that lesion size was increased, tissue sparing was decreased and motor dysfunction was worsened in GLT1+/− mice, and these changes were accompanied by increased loss of spinal cord neurons and apoptosis. While neuronal loss and overall tissue damage was significantly elevated in GLT1 heterozygous mice, we have not shown that this exacerbated injury was directly due to excitotoxic stimulation of glutamate receptors located on vulnerable cell types. There is a possibility that enhanced neuronal death, for example, occurred secondarily to other events such as increased microglial activation (Cao and Zhang 2008) resulting from increased intraspinal glutamate levels in GLT1+/− mice. However, our data support that large reductions in both intraspinal GLT1 protein levels and functional glutamate uptake in spinal cord regions are anatomically localized in regions of cell loss.
Manipulation of the GLT1 transporter using this model has been previously reported in the examination of other types of injury. Lesion size was significantly increased in GLT1−/− mice following cold probe injury to neonatal cortex (Tanaka et al. 1997). Due to the early lethality of complete knockout animals, GLT1 heterozygous mice were used in the present study because SCI was conducted on adult mice, and there was still a significant reduction in intraspinal GLT1 protein and functional transport. Interestingly, reduction in GLT1 expression levels by approximately 50% was sufficient to result in significantly worsened histological and functional outcomes.
When entire spinal cord at crush epicenter was analyzed, GLT1 protein was not reduced in wild-type mice after injury compared to uninjured controls, while functional GLT1-mediated glutamate uptake levels showed a trend toward reduction, at least in this thoracic crush model. In contrast, others have shown that GLT1 protein levels are significantly reduced following crush SCI in the rat, even out to 4 months post-injury (Olsen et al. 2010). We have previously reported (Lepore et al. 2011) differential changes in GLT1 expression levels at various anatomical regions following thoracic contusion SCI. Specifically, total numbers of GLT1-expressing astrocytes and total regional GLT1 expression were reduced in white matter regions, while we found loss of total numbers of GLT1-expressing astrocytes but no decrease in total regional GLT1 expression in gray matter regions. In our current GLT1 Western analysis, significant changes may not have been observed because GLT1 protein levels were measured across the entire cord segment and therefore may be missing these important spatial differences. However, loss in GLT1-mediated uptake was observed following injury. The use of GLT1 heterozygous mice would therefore model the loss of GLT1 levels occurring in white matter regions and the loss of functional uptake throughout the spinal cord segment following SCI, and would also assess the normal neuroprotective role of GLT1-based glutamate uptake in both white and gray matter.
Because the present studies were not conducted with conditional knockouts (these mice do not exist), reduced levels of GLT1 in heterozygous mice during development could, as one example, increase the intrinsic vulnerability of various CNS cell types to glutamate toxicity in the adult CNS following SCI or even the vulnerability to other non-glutamate based mechanisms. However, no studies using these GLT1 heterozygous mice have suggested this type of increased cell autonomous vulnerability of various CNS cell types. We show significant reductions in both GLT1 protein and functional glutamate uptake in GLT1 heterozygous mice at the site of injury, suggesting that this loss represents at least one primary mechanism for worsened outcome. With respect to developmental compensatory changes, we observed increased GLAST expression in GLT1 heterozygous mice (which could be postulated to compensate for loss of GLT1), yet increased tissue and function loss were still seen in these mice, suggesting a central role for GLT1 in extracellular glutamate regulation following SCI. To address these possible issues, future work could test the effects of focal and/or temporally acute loss of GLT1 in the diseased CNS using oligonucleotide-based strategies. Previous studies using isolated spinal cord white matter models of anoxic or traumatic injuries demonstrated that glutamate transport inhibition actually decreased oligodendrocyte, myelin and axonal conduction loss (Li et al. 1999). In an in vivo ischemia model in the hippocampal CA1 region, glutamate-mediated toxicity was increased at early time points and decreased at later time points in GLT1 null mice following ischemic insult (Mitani and Tanaka 2003). In line with the latter study, we report early worsening of neurotoxicity with GLT1+/− mice by day 2 post-crush, possibly during the time frame when glutamate elevation is at its highest. While ischemia is one component of the complex panorama of events following SCI (Baptiste and Fehlings 2006), many additional events are occurring that make this SCI model different from a pure ischemia model. Nevertheless, targeted local inhibition of glutamate transport in the contusion model of SCI was shown to reduce the early rise in extracellular glutamate levels following injury (McAdoo et al. 2000).
We also previously examined the role of GLT1 reduction in the mutant human SOD1-expressing model of ALS, a slowly progressing neurodegenerative disease, by crossing GLT1 heterozygous mice with SOD1G93A mice (Pardo et al. 2006). The reduction of GLT1 accelerated motor neuron loss, decline in motor function and the progression of disease following symptomatic onset. Interestingly, in that study we also noted an increase in the expression of the other major glial glutamate transporter, GLAST, in GLT1+/− mice, which was evident primarily at endstage of disease. A similar increase in intraspinal GLAST expression was observed in the current study, suggesting that upregulation of this second glial transporter was not sufficient to compensate for decreased GLT1 expression following crush SCI.
Compared to wild-type animals, the lesion in GLT1+/− mice in the present study was not only larger close to the crush epicenter, but also expanded beyond the rostral and caudal bounds of the lesion observed in wild-type controls. This finding suggests that: 1) GLT1 plays a key role in minimizing glutamate-mediated cell loss at these more distant locations, 2) GLT1 may also be playing a crucial homeostatic role in even more distant locations, particularly considering that a focal injury to the spinal cord can have far-reaching effects in areas such as the location of axotomized cell bodies in the brain (McDonald and Becker 2003), and 3) therapeutically targeting GLT1 is needed not only in the immediate vicinity of the lesion, but also more broadly. The effects at locations away from the crush epicenter were not uniform. For example, increased neuronal loss observed in GLT1 heterozygous mice was more pronounced and extended over a greater distance in the rostral direction from the crush epicenter. One possible explanation for this phenomenon may be the large amount of descending glutamatergic input that would be significantly interrupted caudal to the crush site.
Reactive astrocytes have traditionally been viewed as problematic for neuroregeneration strategies because of their role in axonal growth inhibition, including cell surface expression of growth inhibitory molecules and the hypertrophy and interdigitation of astrocytes to form a dense reactive scar that acts as a physical barrier to axonal extension. However, previous studies have also shown that astrocytes play crucial roles in minimizing secondary injury and consequent loss of function following SCI (Faulkner et al. 2004). The response of astrocytes following trauma should therefore be viewed as a heterogeneous process that varies with the type and severity of insult, as well as with the timing and location from the insult (Sofroniew 2009). Furthermore, this response cannot be viewed as a singular response of all astrocyte functions in all astrocytes. Instead, individual proteins and corresponding function need to be addressed on an individual basis in the context of this complex heterogeneous cell response.
The present work suggests that maintaining physiological functions of astrocytes such as glutamate transport may be a therapeutically relevant target for SCI treatment. Indeed, studies in animal models of other CNS diseases associated with glutamate transporter dysregulation have shown that pharmacological (Rothstein et al. 2005) or cell-based increases (Lepore et al. 2008) in GLT1 expression can be neuroprotective. Collectively, these results suggest that GLT1 might play a changing role in regulating glutamate homeostasis following SCI and other CNS insults and that the role of GLT1, as well as the way it should be therapeutically targeted both spatially and temporally, differs for various CNS diseases associated with glutamate dysregulation.
Acknowledgments
We thank: all members of the Maragakis lab for discussion; the NIH (grant F32-NS059155 to A.C.L.), the Paralyzed Veterans of America (grant 160837 to A.C.L.), and the Craig H. Neilsen Foundation (grant 190140 to A.C.L.) for funding.
Footnotes
Author Contributions
A.C.L. conceived, designed and conducted the experiments, analyzed the data, prepared the figures, wrote the manuscript, and supervised the project. N.J.M. supervised and participated in writing the manuscript. J.O. designed and conducted experiments and analyzed the data. A.S.K., E.J.Y., A.T., A.H. and C.P.O. conducted experiments.
Contributor Information
Angelo C. Lepore, Email: alepore3@jhmi.edu.
John O’Donnell, Email: jodonnell@jhu.edu.
Andrew S. Kim, Email: akim41@jhmu.edu.
Eun Ju Yang, Email: eyang14@jhmi.edu.
Alisha Tuteja, Email: atuteja1@jhu.edu.
Amanda Haidet-Phillips, Email: ahaidet1@jhmi.edu.
Colin P. O’Banion, Email: cobanio1@jhmi.edu.
Nicholas J. Maragakis, Email: nmaragak@jhmi.edu.
Literature Cited
- Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17(3):1055–63. doi: 10.1523/JNEUROSCI.17-03-01055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23(3–4):318–34. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23(5):635–59. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32(5):972–83. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaviria M, Privat A, d'Arbigny P, Kamenka J, Haton H, Ohanna F. Neuroprotective effects of a novel NMDA antagonist, Gacyclidine, after experimental contusive spinal cord injury in adult rats. Brain Res. 2000;874(2):200–9. doi: 10.1016/s0006-8993(00)02581-6. [DOI] [PubMed] [Google Scholar]
- Holtz A, Gerdin B. MK 801, an OBS N-methyl-D-aspartate channel blocker, does not improve the functional recovery nor spinal cord blood flow after spinal cord compression in rats. Acta Neurol Scand. 1991;84(4):334–8. doi: 10.1111/j.1600-0404.1991.tb04964.x. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294–301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, O'Donnell J, Bonner JF, Paul C, Miller ME, Rauck B, Kushner RA, Rothstein JD, Fischer I, Maragakis NJ. Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury. J Neurosci Res. 2011;89(7):1001–17. doi: 10.1002/jnr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19(14):RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547(2):344–8. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- Liu S, Ruenes GL, Yezierski RP. NMDA and non-NMDA receptor antagonists protect against excitotoxic injury in the rat spinal cord. Brain Res. 1997;756(1–2):160–7. doi: 10.1016/s0006-8993(97)00137-6. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15(3):461–73. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Xu G, Robak G, Hughes MG, Price EM. Evidence that reversed glutamate uptake contributes significantly to glutamate release following experimental injury to the rat spinal cord. Brain Res. 2000;865(2):283–5. doi: 10.1016/s0006-8993(00)02296-4. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Becker D. Spinal cord injury: promising interventions and realistic goals. Am J Phys Med Rehabil. 2003;82(10 Suppl):S38–49. doi: 10.1097/01.PHM.0000086994.53716.17. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23(18):7176–82. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Azbill RD, Springer JE. NBQX treatment improves mitochondrial function and reduces oxidative events after spinal cord injury. J Neurotrauma. 2002;19(8):917–27. doi: 10.1089/089771502320317078. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21(4):429–40. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y. Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol. 2003;14(3):191–8. doi: 10.1016/s1084-9521(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment. Brain. 2010;133(4):1013–25. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter SS, Yum SW, Faden AI. Alteration in extracellular amino acids after traumatic spinal cord injury. Ann Neurol. 1990;27(1):96–9. doi: 10.1002/ana.410270115. [DOI] [PubMed] [Google Scholar]
- Pardo AC, Wong V, Benson LM, Dykes M, Tanaka K, Rothstein JD, Maragakis NJ. Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754–74. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine. 2009;34(4):328–34. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel JR, Duncan G, Chen KW, Shannon C, Park S, Sparling JS, Tetzlaff W. A graded forceps crush spinal cord injury model in mice. J Neurotrauma. 2008;25(4):350–70. doi: 10.1089/neu.2007.0426. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19(1):464–75. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4(2):113–30. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Mills CD, Ye Z, Fullwood SD, McAdoo DJ, Hulsebosch CE, Westlund KN. Rapid changes in expression of glutamate transporters after spinal cord injury. Brain Res. 2002;927(1):104–10. doi: 10.1016/s0006-8993(01)03329-7. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci. 1994;14(11 Pt 1):6598–607. doi: 10.1523/JNEUROSCI.14-11-06598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Ye Z, Hulsebosch CE, McAdoo DJ. Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Exp Neurol. 2004;187(2):329–36. doi: 10.1016/j.expneurol.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Zhang L, Cain L, McAdoo DJ. Administration of glutamate into the spinal cord at extracellular concentrations reached post-injury causes functional impairments. Neurosci Lett. 2005;384(3):271–6. doi: 10.1016/j.neulet.2005.04.100. [DOI] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48(5):394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]