Abstract
Modulation of mitochondrial free Ca2+ ([Ca2+]m) is implicated as one of the possible upstream factors that initiates anesthetic-mediated cardioprotection against ischemia-reperfusion (IR) injury. To unravel possible mechanisms by which volatile anesthetics modulate [Ca2+]m and mitochondrial bioenergetics, with implications for cardioprotection, experiments were conducted to spectrofluorometrically measure concentration-dependent effects of isoflurane (0.5, 1, 1.5, 2 mM) on the magnitudes and time-courses of [Ca2+]m and mitochondrial redox state (NADH), membrane potential (ΔΨm), respiration, and matrix volume. Isolated mitochondria from rat hearts were energized with 10 mM Na+- or K+-pyruvate/malate (NaPM or KPM) or Na+-succinate (NaSuc) followed by additions of isoflurane, 0.5 mM CaCl2 (≈200 nM free Ca2+ with 1 mM EGTA buffer), and 250 mM ADP. Isoflurane stepwise: (a) increased [Ca2+]m in state 2 with NaPM, but not with KPM substrate, despite an isoflurane-induced slight fall in ΔΨm and a mild matrix expansion, and (b) decreased NADH oxidation, respiration, ΔΨm, and matrix volume in state 3, while prolonging the duration of state 3 NADH oxidation, respiration, ΔΨm, and matrix contraction with PM substrates. These findings suggest that isoflurane's effects are mediated in part at the mitochondrial level: (1) to enhance the net rate of state 2 Ca2+ uptake by inhibiting the Na+/Ca2+ exchanger (NCE), independent of changes in ΔΨm and matrix volume, and (2) to decrease the rates of state 3 electron transfer and ADP phosphorylation by inhibiting complex I. These direct effects of isoflurane to increase [Ca2+]m, while depressing NCE activity and oxidative phosphorylation, could underlie the mechanisms by which isoflurane provides cardioprotection against IR injury at the mitochondrial level.
Keywords: Cardiac IR injury, Anesthetics, Cardioprotection, Mitochondria, Ca2+ uptake, Ca2+ efflux, Bioenergetics
Introduction
Ischemia-reperfusion (IR) injury results in mitochondrial dysfunction, as shown by impaired mitochondrial ATP production and dysregulation of mitochondrial Ca2+ homeostasis, resulting from reduced membrane potential (ΔΨm), increased matrix swelling, release of cytochrome c, and eventual cell membrane rupture and death [1–4]. An increase in cytosolic free Ca2+ ([Ca2+]c), with a subsequent increase in mitochondrial free Ca2+ ([Ca2+]m), is believed to be a major contributor of mitochondrial dysfunction and mitochondria-mediated cardiac cell injury or death during IR injury [4–6]. Therefore, the use of drugs or cytoprotective strategies that interfere with cytosolic Ca2+ homeostasis could serve as an important strategy in cardioprotection by delaying the onset of IR injury [3, 7, 8].
Anesthetic pre- and post-conditioning (APC and APOC) have emerged as powerful strategies in cardioprotection against IR injury [2, 8–10]. A better understanding of the mechanisms of anesthetics to modify cardiac cell function may provide guidance on how to reduce or delay cardiac cell injury caused by IR. Cardiac mitochondria are believed to play an essential role in mediating cardioprotection by anesthetics [2, 8, 11, 12]. Modulation of [Ca2+]m is believed to be one of the upstream factors that initiate a cascade of protective kinases during cardiac APC or APOC against IR injury [2, 4–7, 13]. In view of the increasingly recognized role of mitochondria as initiators and effectors of various cardioprotective signaling pathways, the effect of isoflurane (a volatile anesthetic) to alter mitochondrial Ca2+ handling and bioenergetics could provide novel insights into the mechanisms by which anesthetics mediate cardioprotection against IR injury.
Ca2+ uptake via the Ca2+ uniporter (CU) is likely the primary mode of Ca2+ entry into respiring mitochondria [14]; the Ca2+ entry is dependent on [Ca2+]c, ΔΨm, and interference by other divalent cations (e.g., Mg2+) that impede Ca2+ entry [15–17]. On the other hand, Na+/Ca2+ exchanger (NCE) serves as a principal Ca2+ efflux pathway for maintaining mitochondrial Ca2+ homeostasis during physiological and pathological conditions. Ca2+ is an integral component of the signaling pathways involved in anesthetic-mediated cardioprotection [2, 4, 6, 13]. However, how volatile anesthetics (e.g., isoflurane) modulate the CU or other Ca2+ transporters (e.g., NCE) to alter mitochondrial Ca2+ transients and bioenergetics that influence cellular function, and how these effects relate to cardioprotection, have not been explored.
The aim of this study was to investigate the direct effects of isoflurane on mitochondrial Ca2+ uptake and release and associated changes in bioenergetics that together, or singly, may underlie the mechanisms of volatile anesthetic-mediated cardioprotection. Our hypothesis was that isoflurane modulates [Ca2+]m, by modulating the CU or NCE, in a ΔΨm- and matrix volume-independent manner, while also altering the bioenergetic state. To test this hypothesis, we used freshly isolated rat heart mitochondria energized with various substrates (Na+- or K+-pyruvate/malate (NaPM or KPM) or Na+-succinate (NaSuc)) and fluorescence techniques to investigate how extra-matrix additions of 0.5 mM CaCl2 ([Ca2+]e ≈ 200 nM with 1 mM EGTA buffer) and 250 mM ADP alter [Ca2+]m, NADH redox state, respiration, ΔΨm, and matrix volume with several concentrations of isoflurane (0, 0.5, 1, 1.5, 2 mM). We found that isoflurane has a concentration-dependent effect to increase state 2 [Ca2+]m with NaPM, but not with KPM substrate, and increase matrix volume and decrease ΔΨm with all substrates, while depressing mitochondrial bioenergetics, i.e., decreasing state 3 NADH oxidation, respiration,ΔΨm, and matrix volume. These results indicate that isoflurane's effects are mediated in part at the mitochondrial level to enhance the net rate of state 2 Ca2+ uptake by attenuating the activity of NCE, independent of ΔΨm and matrix volume, and to inhibit complex I to decrease the rate and prolong the duration of state 3 electron transport and ADP phosphorylation.
Materials and Methods
Mitochondrial isolation
The Medical College of Wisconsin Institutional Animal Care and Use Committee (IACUC) approved the animal use and the experimental protocols of this study. Rat heart mitochondria were isolated by differential centrifugation methods, as described previously for guinea pig hearts [18–20], with some minor modifications. Hearts were isolated from Wistar rats (300–350g), anesthetized with sodium thiobutabarbital (Inactin, Sigma-Aldrich, St. Louis, MO; 150 mg/kg intraperitoneally), and were minced in ice-cold “isolation buffer” containing (in mM) 200 mannitol, 50 sucrose, 5 KH2PO4, 5 3-(N-morpholino) propanesulfonic acid (MOPS), and 1 EGTA, with 0.1% bovine serum albumin (BSA) at pH 7.15 (adjusted with KOH). The minced hearts were suspended in 2.5 ml isolation buffer with 5U/ml protease and homogenized for 30 s. Next, 15 ml isolation buffer was added and the suspension was homogenized further for 60 s. The suspension volume was then adjusted to 25 ml and centrifuged at 8000 g for 10 min. The supernatant was discarded and the pellet was resuspended in 25 ml isolation buffer and centrifuged at 700 g for 10 min. Next, the pellet was discarded and the supernatant was centrifuged at 700 g for 10 min. The supernatant from this step was centrifuged again at 8000 g for 10 min to yield the final mitochondrial pellet, which was suspended in 0.5 ml isolation buffer and kept on ice until used. All isolation procedures were carried out in a cold room (4°C). The mitochondrial protein content was determined using BSA as the standard with Biorad Quick Start Bradford Assay Kit [21]. Depending on the protein content, the suspension volume was adjusted to have a protein concentration of 5 mg protein/ml isolation buffer.
Indo-1 and DMSO loading
For [Ca2+]m measurements, mitochondria (5 mg protein/ml isolation buffer) were incubated in 5 μM indo-1 AM for 20 min at 25°C with constant stirring [18]. Next, the suspension volume was adjusted to 25 ml and recentrifuged at 8000 g for 10 min to remove the residual dye. The dye-loaded mitochondrial pellet was resuspended in 0.5 ml isolation buffer and kept on ice until used. Mitochondrial protein content was redetermined and the suspension volume was readjusted as described above. For bioenergetic measurements (respiration, NADH, ΔΨm, and matrix volume), mitochondria were loaded with DMSO (vehicle for indo-1 AM; 0.1% final concentration) to mimic the experimental conditions of indo-1 loaded mitochondria. All experiments were conducted at room temperature (25°C), unless otherwise stated.
Mitochondrial O2 consumption measurement
To test the functional integrity of mitochondria in day to day experiments and also to examine the effect of isoflurane on states 2, 3 and 4 respiration, the rates of mitochondrial O2 consumption were measured polarographically [18–20] at 30°C with a Clark type electrode system (System S 200A; Strathkelvin Instruments, Glasgow, UK). The DMSO or dye loaded mitochondria were suspended in “respiration buffer” (0.5 mg protein/ml) containing (in mM) 130 KCl, 5 K2HPO4, 20 MOPS, 1 EGTA, and 0.1% BSA at pH 7.15 adjusted with KOH. State 2 respiration was initiated with complex I substrate Na+-pyruvate/malate (NaPM; 10 mM) in the presence of DMSO (vehicle) or different concentrations of isoflurane (0.5, 1, 1.5, 2 mM) given prior to substrate addition. State 3 respiration was measured by adding 250 μM ADP and state 4 respiration was measured after phosphorylation of ADP to ATP. The time-line protocol for the respiration experiments is shown in Fig. 1A. To test the functional integrity of mitochondria on a given experimental day, the respiratory control index (RCI) was calculated from the respiration rate ratio of state 3/state 2 or state 3/state 4. Mitochondrial preparations from hearts with DMSO or dye incubation consistently provided RCIs of about 6 (state 3/state 2) and 4 (state 3/state 4). These mitochondria provided consistent data on bioenergetics (respiration, NADH, Δψm, and matrix volume) and [Ca2+]m in day to day experiments.
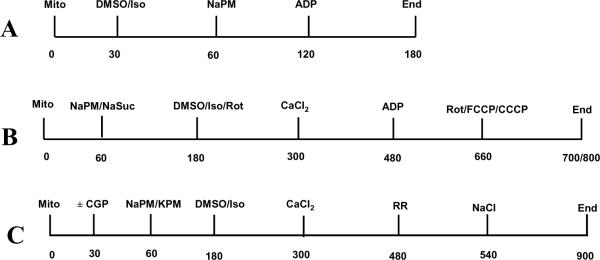
Figure 1.
Time-line protocols (in s) for isolated mitochondrial experiments. (A) Respiration experiments, (B) [Ca2+]m, NADH, Δψm and matrix volume experiments, and (C) Extended time-line protocol for [Ca2+]m measurements involving NCE kinetics. Mito: mitochondria; PM: pyruvate/malate; DMSO: dimethyl sulfoxide; Iso: isoflurane; CaCl2: calcium chloride; ADP: aden-osine diphosphate; FCCP: carbonyl cyanide p-trifluoromethoxyphenylhydrazone; CCCP: carbonylcyanide m-chlorophenylhydrazone; Rot: rotenone; NCE: Na+/Ca2+ exchanger.
Mitochondrial redox state (NADH) measurement
The DMSO loaded mitochondria at a concentration of 0.5 mg protein/ml respiration buffer were used to spectrophotometrically (PTI: Photon Technology International, Birmingham, NJ) measure the time-courses of NADH during states 2, 3 and 4 respiration in the presence of DMSO (vehicle) or isoflurane (0.5, 1, 1.5, 2 mM), given after attaining state 2 respiration with NaPM, followed by 0.5 mM CaCl2 (≈200 nM free Ca2+) and 250 μM ADP. The time-courses of NADH during states 2, 3 and 4 respiration were also assessed to compare effects of complex I substrate (NaPM) and complex II substrate (NaSuc) in the presence of DMSO, isoflurane (1 mM) or rotenone (30 nM), with and without 0.5 mM added CaCl2; 30 nM rotenone was used to mimic the response of 1 mM isoflurane.
The time-line protocol for the NADH measurements is shown in Fig. 1B. NADH was measured as autofluorescence [18, 19] by exciting the mitochondrial sample at 350 nm (λex) and collecting the emission signals (F) at 456 nm and 395 nm (λem). The λem's were switched between two photon counters with a time resolution of 1 s. The F395 signals were less sensitive to the NADH changes, but account for light scattering associated with matrix volume changes and/or conformational changes, which were proportionally present in the F456 signals that are sensitive to the NADH changes. Therefore, the fluorescence ratio (R =F456/F395) is interpreted as a measure of NADH changes that corrects for artifacts due to changes in the light scattering. Rotenone (1 μM) and FCCP (20 μM) were added in state 4 to obtain the end points for calibration of the NADH time-course between maximally reduced and maximally oxidized states. The NADH autofluorescence signals at the two respective wavelengths were also used as background fluorescence signals for analyzing the indo-1 AM fluorescence signals for [Ca2+]m measurements.
Mitochondrial free Ca2+ ([Ca2+]m) measurement
The indo-1 loaded mitochondria (0.5 mg protein/ml respiration buffer) were used in the same cuvette system (PTI) to spectrophotometrically measure the time-courses of [Ca2+]m during states 2, 3 and 4 respiration in the absence and presence of isoflurane (0, 0.5, 1, 1.5, 2 mM), with the same time-line protocol as described for NADH (Fig. 1B). In another set of experiments, the time-line protocol for [Ca2+]m measurements was extended (Fig. 1C) to evaluate the effects of 1 mM isoflurane on both Ca2+ uptake via the CU and Ca2+ efflux via the NCE, using the substrate KPM or NaPM with CGP-37157 (an inhibitor of NCE) present. Fluorescence signals with the DMSO (for background autofluorescence, NADH) or indo-1 (for [Ca2+]m) loaded mitochondria were obtained at the same excitation (λex=350 nm) and emission (λem=395 and 456 nm) wavelengths. The signals from the respective mitochondria were subtracted, time point by time point, to correct for the effect of background autofluorescence (NADH) on indo-1 fluorescence. The ratio of the two corrected signals (R =F395/F456) accounted for individual signal variations as well as for the differences in the amount of indo-1 taken up into the matrix in a given experiment. The Rmax signal (maximum Ca2+ load that leads to saturation of indo-1 dye with Ca2+) was determined by adding 0.5 μM cyclosporine A (CsA, mPTP: mitochondrial permeability transition pore blocker) and 5 mM CaCl2 to the respiring mitochondria; the Rmin signal (zero Ca2+ load where all indo-1 dye become free of Ca2+) was monitored with A23187 (a Ca2+ ionophore) that was added to the mitochondria during state 2 respiration [18]. [Ca2+]m was calculated using the standard calibration formula [22]: [Ca2+]m (nM) =Kd,app · Sf2/Sb2 · (R–Rmin)/ (Rmax–R); Kd,app (apparent dissociation constant for dye-Ca2+ binding) was determined to be 330 nM at pH 7.15; Sf2 is the signal intensity of free indo-1 measured at 456 nm and Sb2 is the signal intensity of Ca2+-saturated indo-1 measured at 456 nm. The ratio Sf2/Sb2, Rmax, and Rmin was measured independently for each day's experiments.
For [Ca2+]m calibration, the measured Kd,app (330 nM) was further corrected to account for pH changes that occur due to release of protons from 1 mM EGTA present in the buffer with addition of 0.5 mM CaCl2. This correction was made using the formula Kd,app =Kd (1+[H+/]/KH), where KH is the dissociation constant for binding of protons to indo-1, which is about 10−6.5 M or 315 nM, and Kd is the true dissociation constant for binding of Ca2+ to indo-1, which is about 265 nM. Matrix pH was measured spectrofluorometrically in the same PTI system using 2'7'-bis-(2-carboxyethyl)-5'(and 6-) carboxy-fluroescein (BCECF) AM loaded mitochondria that leads to a pH decrease of 0.07 units (data not shown) upon addition of 0.5 mM CaCl2 to 1 mM EGTA buffer. Accounting for this pH change, the Kd,app increases from 330 nM to 344 nM.
Although matrix indo-1 fluorescence was measured during states 2, 3 and 4 respiration, we show here only the [Ca2+]m time-course during state 2 respiration. In a recent study [18], we observed that matrix indo-1 fluorescence increases when ADP is added to the buffer, and that fluorescence returns to the baseline (state 2) level after ADP is phosphorylated to ATP; this results in an apparent increase in [Ca2+]m during state 3 respiration [18]. However, the mechanism of this increase in matrix indo-1 fluorescence, leading to the apparent increase in [Ca2+]m, during state 3 respiration is unclear. Therefore, we do not have an accurate measure of [Ca2+]m after ADP addition (states 3 and 4).
Mitochondrial membrane potential (Δψm) measurement
Δψm was measured during states 2, 3 and 4 respiration in the same PTI system using the fluorescent dye rhodamine 123 (Rh123; 50 nM; Calbiochem, San Diego, CA) in the buffer with λex =503 nm and λem =527 nm [23]. Rh123 is a monovalent cation that is taken up into the matrix of charged mitochondria where it accumulates due to a large electronegative potential. An increase in Δψm is associated with a decrease in fluorescence intensity. The DMSO loaded mitochondria were suspended in respiration buffer (0.5 mg protein/ml) and the time-course of Δψm was measured under various experimental conditions with the same time-line protocol as for NADH and [Ca2+]m (Fig. 1B). The uncoupler CCCP (4 μM) was added to maximally (100 %) depolarize the mitochondria; Δψm at any time point was expressed as a percentage of the fluorescence with maximal depolarization.
Mitochondrial matrix volume measurement
Changes in matrix volume as a consequence of changes in the water and solute contents of the matrix after adding substrate, DMSO or different concentrations of isoflurane, CaCl2, and ADP was measured spectrofluorometrically in the same PTI system using light-scattering technique [24, 25]. This was carried out by exciting the DMSO loaded mitochondrial sample (0.5 mg protein/ml) at a particular wavelength and collecting the emitted signals (photon counts) at the same wavelength (e.g., λex = λem=520 nm). A decrease and increase in photon counts due to light scattering is interpreted as the expansion and contraction of matrix volume, respectively. The light scattering signals were normalized with respect to their baseline levels (prior to substrate addition). Because the signals at all wavelengths were proportionally scattered (data not shown), we corrected the indo-1 fluorescence signals for NADH autofluorescence to accurately estimate both NADH and [Ca2+]m.
Time-line protocols for experiments
Fig. 1A shows the time-line protocol for respiration measurements, whereas Fig. 1B depicts the time-line protocol used for NADH, [Ca2+]m, Δψm, and matrix volume measurements; Fig. 1C shows the extended time-line protocol for [Ca2+m measurements involving NCE kinetics. In all cases, the experiments were initiated at t =0 with isolated mitochondria suspended in the respiration buffer (0.5 mg protein/ml). The time-course of states 2, 3 and 4 respiration was monitored by adding to the mitochondrial sample DMSO (vehicle) or isoflurane at t =30 s, followed by substrate (NaPM; 10 mM) at t =60 s and ADP (250 μM) at t =120 s, which initiates state 3 respiration proceeding to state 4 respiration after the conversion of ADP to ATP (Fig. 1A). On the other hand, the time-courses of NADH, [Ca2+]m, Δψm, and matrix volume were measured during each respiration state by adding the substrate (NaPM; 10 mM or NaSuc; 10mM) at t =60 s, followed by DMSO/isoflurane or rotenone (30 nM) at t =180 s, CaCl2 (0 or 0.5 mM) at t =300 s, and ADP (250 μM) at t =480 s (Fig. 1B). In the extended time-line protocol for [Ca2+]m measurements for inferring NCE kinetics (Fig. 1C), instead of ADP, the CU inhibitor ruthenium red (RR; 25 μM) was added at t =480 s followed by the addition of NaCl (10 mM) at t =540 s. In NADH measurements, rotenone (1 μM) or FCCP (20 μM) was added at t =660 s (state 4) to achieve maximally reduced and maximally oxidized autofluorescence signals, respectively, as the end points for NADH calibration. Similarly, in Δψm measurements, CCCP (4 μM) was added at t =660 s to maximally (100 %) depolarize the mitochondria, as the end point for Δψm calibration. To account for the effects of DMSO alone, experiments were conducted with a control group (no treatment) and the data were compared to isoflurane and DMSO group data.
Isoflurane was dissolved in DMSO to achieve concentrations of 0.5, 1, 1.5 and 2 mM that correspond to 1, 2, 3 and 4 minimal alveolar concentration (MAC), respectively, which are values commonly used for surgical anesthesia. The actual isoflurane concentrations at the beginning and end of each day's experiments were verified by gas chromatography (GC). Average concentrations of isoflurane by GC varied within ±10% of their initially prepared values of 0.5, 1, 1.5 and 2 mM.
Data analysis and statistics
All data were analyzed using computer programs written in MATLAB that access the raw data of specific variables (NADH, [Ca2+]m, Δψm, and matrix volume) from Excel® spread sheets of day to day experiments generated from the PTI software. The programs performed the necessary calculations to calibrate absolute values and changes in variables from one state to the other, including statistical analyses (i.e., computations of mean, standard deviation, and standard error of a particular variable over multiple day experiments). The program for [Ca2+]m calculations utilized both indo-1 fluorescence signals and NADH autofluorescence signals for background and light scattering corrections. The final data of a particular variable were expressed as mean ± standard error (SE) over at least 4 replicates of the same variable (n =4). Comparisons within and between groups were performed by linear regression analysis, or by one-way ANOVA (analysis of variance) with Tukey's post-hoc test for significance of means. Differences with P < 0.05 (two-tailed) were considered significant.
Results
Isoflurane –induced decreases in mitochondrial respiration
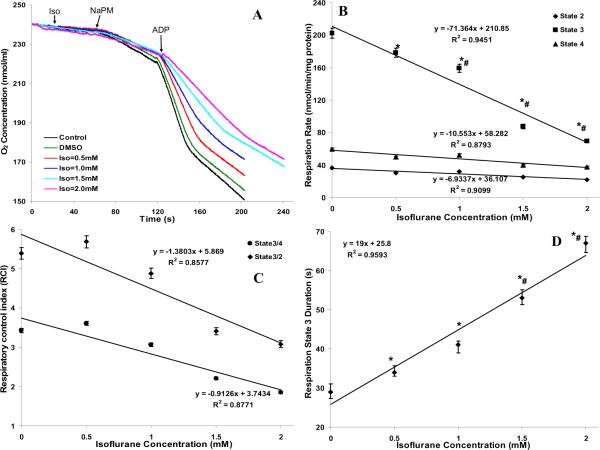
The concentration-dependent effect of isoflurane (0.5, 1, 1.5, 2 mM) on the time-courses of mitochondrial O2 concentration during states 2, 3 and 4 respiration with NaPM is shown in Fig. 2A; the corresponding rates of respiration (O2 consumption), RCIs (state 3/state 4 and state 3/state 2) that are measures of mitochondrial integrity, and duration of state 3 respiration are summarized in Fig. 2B,C,D. Isoflurane significantly decreased state 3 respiration in a concentration-dependent manner: 178 ±2.5, 159 ±2.3, 87 ±1.7 and 70 ±1.1 nmol mg−1 min−1 at 0.5, 1, 1.5 and 2 mM isoflurane, respectively, when compared to the control (192 ±3) and DMSO (202 ±3.1) groups, without appreciably changing state 2 or state 4 respiration among all groups (Fig. 2B). The effects of isoflurane on state 3 respiration was also manifested in the computed RCIs (Fig. 2C) and observed duration of state 3 respiration: 34 ±1 s, 41 ±2.1 s, 53 ±1.7 s and 67 ±2.4 s at 0.5, 1, 1.5 and 2 mM isoflurane, respectively, compared to the control (27 ±2 s) and DMSO (29 ±1.7 s) groups (Fig. 2D). This slowed rate and prolonged duration of state 3 respiration with NaPM and increasing isoflurane concentration indicates a slower rates of electron transport and ADP phosphorylation. However, the amount of O2 consumed during state 3 respiration (ADP phosphorylation) remained approximately constant, suggesting that the P/O ratio remained approximately constant, ≈2.5.
Figure 2.
(A) Representative traces of time-course of mitochondrial O2 concentration during states 2, 3 and 4 respiration with isoflurane (0.5, 1, 1.5 and 2 mM) compared to control (no treatment) and vehicle (DMSO) groups. (B, C, D) Summary of the effect of isoflurane, compared to DMSO (Iso = 0 mM), on the rates of states 2, 3 and 4 O2 consumption, respiratory control indices (RCIs), and duration of state 3 O2 consumption. Note that isoflurane caused a stepwise decrease in the rate of state 3 O2 consumption (B), decreased the RCI that was defined as state 3/4 respiration and state 3/2 respiration to assess mitochondrial functional integrity by isoflurane (C), and increased the duration of state 3 -induced O2 consumption (D), consistent with an ADP-induced decrease in NADH oxidation and decline in Δψm (see Figs. 3 and 5). The amount of O2 consumed during state 3 respiration (ADP phosphorylation) remained approximately constant, suggesting that the P/O ratio remained approximately constant (~2.5). Data are means ±SE of 3 replicates.
Isoflurane –induced changes in mitochondrial redox state (NADH)
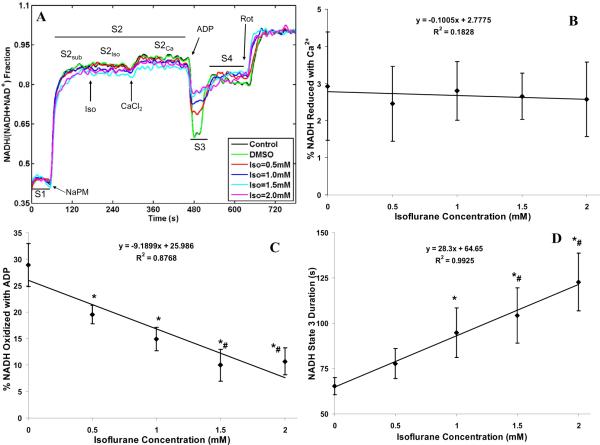
The concentration-dependent effect of isoflurane and 0.5 mM CaCl2 (≈200 nM free Ca2+) on the time-courses of mitochondrial NADH during states 2, 3 and 4 respiration with NaPM is shown in Fig. 3A. The corresponding % NAD+ reduced with Ca2+, % NADH oxidized with ADP, and duration of state 3 NADH oxidation are summarized in Fig. 3B,C,D. Isoflurane had little effect on NADH levels during state 2 or state 4 respiration as shown in the time-course tracings (Fig. 3A). Adding 0.5 mM CaCl2 caused a slight additional increase in state 2 NADH levels (about 3%), independent of isoflurane concentration (Fig. 3B). Adding ADP caused a significant oxidation of NADH in control and DMSO groups; however, in the presence of isoflurane, ADP promoted a pronounced isoflurane concentration-dependent decrease in state 3 NADH oxidation: 20 ±1.8, 15 ±2, 10 ±3 and 10 ±3% at 0.5, 1, 1.5 and 2 mM isoflurane, respectively, when compared to the control (29 ±3%) or DMSO (29 ±4%) group (Fig. 3C). In addition, isoflurane also significantly increased the duration of state 3 NADH oxidation: 78 ±8.2 s, 95 ±13.8 s, 104 ±15.3 s and 123 ±16 s at 0.5, 1, 1.5 and 2 mM isoflurane, respectively, compared to the control (63 ±4.2 s) and DMSO (65 ±4.8 s) group (Fig. 3D). This ADP-induced decrease in the rate and increase in the duration of NADH oxidation with NaPM and increased isoflurane concentration is consistent with the depressed state 3 respiration due, in part, to slowed rates of electron transfer and ADP phosphorylation; this is likely attributable to complex I inhibition by isoflurane.
Figure 3.
(A) Time-course of mitochondrial redox state (NADH) during states 2, 3 and 4 respiration with isoflurane (0.5, 1, 1.5 and 2 mM) compared to control (no treatment) and vehicle (DMSO) groups. NADH state in each group was normalized on a scale of 0 (fully oxidized with FCCP) to 1 (fully reduced with rotenone) to express NADH state as a % total pool of NAD + NADH. State 2 was subdivided into substrate (S2sub), isoflurane (S2iso), and CaCl2 (S2Ca) effects. Adding ADP (250 μM) caused NADH oxidation (state 3; S3) followed by recovery to state 4 (S4). Traces represent means of 4 replicates in each group. (B, C, D) Summary of effects of 0. 5 mM added CaCl2 (≈200 nM free Ca2+) on state 2 NADH reduction in the presence of isoflurane, of isoflurane+ADP on state 3 NADH oxidation, and of isoflurane on duration of state 3 NADH oxidation; Isoflurane (0.5, 1, 1.5 and 2 mM) effect is compared to the DMSO (Iso = 0 mM) effect. Note that adding CaCl2 caused further NADH reduction for all isoflurane levels (B), ADP transiently oxidized NADH while isoflurane stepwise decreased this level of oxidation (C), and that isoflurane stepwise increased the duration of the state 3 -induced NADH oxidation (D). Data are means ±SE of 4 replicates. * indicates significant differences exist between isoflurane and control/DMSO groups, while # indicates significant differences exist between high and low isoflurane groups (p < 0.05).
To further evaluate that isoflurane inhibits complex I in our model, the time courses of mitochondrial redox state (NADH) during states 2, 3 and 4 respiration were measured and compared with different substrates (NaPM, Fig. 4A,B; NaSuc, Fig. 4C,D), with 0.5 mM added CaCl2 (≈200 nM free Ca2+) vs. no added CaCl2 (Fig. 4A,C vs. Fig. 4B,D), and with isoflurane (1 mM), rotenone (30 nM), and vehicle (DMSO). These data show that 1 mM isoflurane could mimic the inhibition of complex I by 30 nM rotenone with both substrates and extra-matrix CaCl2 perturbations. Furthermore, with NaSuc substrate, the duration of state 3 NADH oxidation was longer with the DMSO control than with either isoflurane (1 mM) or rotenone (30 nM) due to inhibition of complex I.
Figure 4.
Time-courses of mitochondrial redox state (NADH) during states 2, 3 and 4 respiration comparing: effects of different substrates (Na+-pyruvate/malate (NaPM) (A,B) and Na+-succinate (NaSucc) (C, D)); effects of 0.5 mM added CaCl2 (≈200 nM free Ca2+) (A,C) vs. no added CaCl2 (B,D); and effects of isoflurane (1 mM) vs. rotenone (30 nM) vs. vehicle (DMSO). NADH redox state was normalized in each group as described in Fig. 3. Traces represent means of 4 replicates in each group. These data demonstrate similarities in the effects of isoflurane and rotenone (a known inhibitor of complex I activity), with different substrates and at different extra-matrix and matrix Ca2+ ([Ca2+]e, [Ca2+]m).
Isoflurane –induced decreases in mitochondrial membrane potential (Δψm)
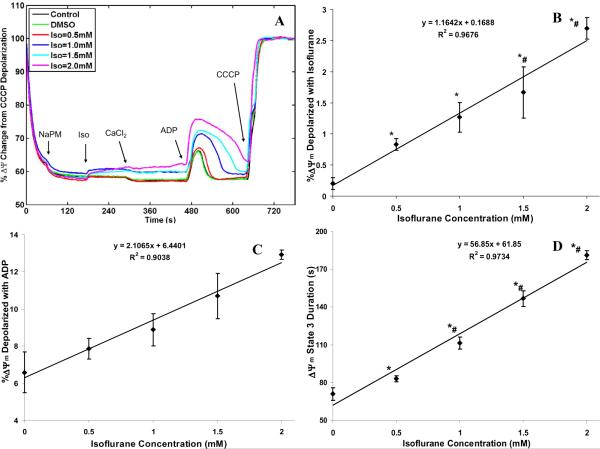
The concentration-dependent effect of isoflurane and 0.5 mM CaCl2 (≈200 nM free Ca2+) on the transients of Δψm during states 2, 3 and 4 respiration with NaPM is shown in Fig. 5A. The corresponding % depolarization in Δψm with added isoflurane and ADP and duration of state 3 Δψm depolarization are summarized in Fig. 5(B,C,D). Isoflurane lowered Δψm during states 2, 3 and 4 respiration as shown in the time-dependent traces (Fig. 5A). Isoflurane caused a slight concentration-dependent fall in Δψm in state 2 up to 2.7%, with negligible changes in control and DMSO groups (Fig. 5B); adding 0.5 mM CaCl2 did not alter Δψm appreciably beyond the light scattering effects due to matrix volume expansion. Addition of ADP caused a transient and reversible phosphorylation-dependent depolarization of mitochondria in all groups. Isoflurane increased the transient decrease in Δψm by ADP (Fig. 5C) in a concentration-dependent manner, as well as prolonged the duration of depolarization (Fig. 5D): from 71 ±5 s (control) and 71 ±5 s (DMSO) to 83 ±2 s (Iso =0.5 mM), 112 ±5 s (Iso =1 mM), 147 ±6 s (Iso =1.5 mM), and 181 ±4 s (Iso =2 mM). This observation of isoflurane on ADP-induced falls in Δψm and rise in the duration of Δψm depolarization with NaPM with increasing isoflurane concentration is corroborated by the decreased rate and increased duration of state 3 respiration and NADH oxidation, which together reflects the effect of isoflurane to slow the rates of electron transfer and ADP phosphorylation.
Figure 5.
(A) Time-course of mitochondrial membrane potential (ΔΨm) during states 2, 3 and 4 respiration with isoflurane (0.5, 1, 1.5 and 2 mM) compared to control (no treatment) and vehicle (DMSO) groups. ΔΨm was normalized with respect to maximal (100%) depolarization obtained with CCCP. Traces represent means of 4 replicates in each group. (B, C, D) Summary of effects of isoflurane, compared to DMSO (Iso = 0 mM), on state 2 ΔΨm, of isoflurane+ADP on state 3 ΔΨm, and of isoflurane on state 3 duration of ΔΨm. Note that isoflurane caused a small stepwise decrease in state 2 ΔΨm (B), a stepwise decrease in state 3 ΔΨm (C), and a stepwise increase in duration of the state 3 -induced decrease in ΔΨm (D). Data are means ±SE of 4 replicates. * indicates significant differences exist between isoflurane and control/DMSO groups, while # indicates significant differences exist between high and low isoflurane groups (p < 0.05).
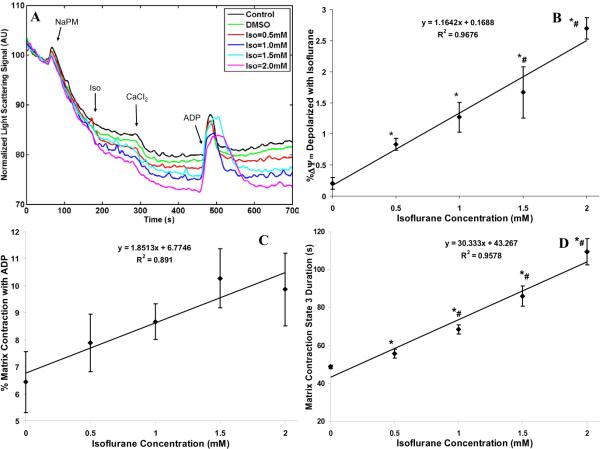
Isoflurane –induced changes in mitochondrial matrix volume
The changes in matrix volume with additions of substrate (NaPM; 10 mM), CaCl2 (0.5 mM) and ADP (250 μM) in control, DMSO and isoflurane (0.5, 1, 1.5, 2 mM) groups are shown in Fig. 6A. The corresponding % changes in matrix volume from baselines with added CaCl2 and ADP and duration of state 3 volume change are summarized in Fig. 6B,C,D. Isoflurane and CaCl2 each caused changes in matrix volume during states 2, 3 and 4 respiration as shown in the time-dependent traces (Fig. 6A); in general, isoflurane and CaCl2 caused volume expansion, whereas ADP caused volume contraction. Isoflurane induced a small dose-dependent volume expansion in state 2 compared to the control and DMSO groups; adding CaCl2 led to further volume expansion in all groups (≈ 4–5%), nearly independent of isoflurane concentration (Fig. 6B). Adding ADP caused volume contraction that was dependent on isoflurane concentration (%): 6.7 ±0.8 (control), 6.4 ±1.1 (DMSO), 7.9 ±1.1 (Iso =0.5 mM), 8.7 ±0.7 (Iso =1 mM), 10.3 ±1.1 (Iso =1.5 mM), and 9.9 ±1.3 (Iso =2 mM) (Fig. 6C). Isoflurane increased the duration of ADP-induced volume contraction from 48 ±0.3 s (control) and 49 ±0.9 s (DMSO) to 56 ±3 s (Iso =0.5 mM), 68 ±2 s (Iso =1 mM), 86 ±5 s (Iso =1.5 mM), and 109 ±7 s (Iso =2 mM) (Fig. 6D). This increase in the duration of ADP-induced volume contraction with NaPM and increasing isoflurane concentration is consistent with the increased durations of state 3 respiration (Fig. 2D), NADH oxidation (Fig. 3D), and Δψm depolarization (Fig. 5D), each reflecting slower rates of electron flow and ADP phosphorylation in the presence of isoflurane.
Figure 6.
(A) Time-course of matrix volume changes during states 2, 3 and 4 respiration with isoflurane (0.5, 1, 1.5 and 2 mM) compared to control (no treatment) and vehicle (DMSO) groups. A decrease in light scattering photon counts signifies volume expansion, while an increase signifies volume contraction. Light scattering signals were normalized with respect to their baseline levels. Traces represent means of 4 replicates in each group. Note that adding 0.5 mM CaCl2 (≈200 nM free Ca2+) had only a slight effect to further promote volume expansion after isoflurane. (B,C,D) Summary of effects of added CaCl2 on state 2 volume expansion in the presence of isoflurane, of isoflurane+ADP on state 3 volume contraction, and of isoflurane on state 3 -induced volume contraction; Isoflurane (0.5, 1, 1.5 and 2 mM) effect is compared to the DMSO (Iso = 0 mM) effect. Note that isoflurane caused further slight volume expansion in state 2 after adding CaCl2 (B), isoflurane stepwise reduced the state 3 -induced volume contraction (C), and isoflurane stepwise increased the state 3 -induced duration of matrix contraction (D). Data are plotted as means ±SE of 4 replicates. * indicates significant differences exist between isoflurane and control/DMSO groups, while # indicates significant differences exist between high and low isoflurane groups (p < 0.05).
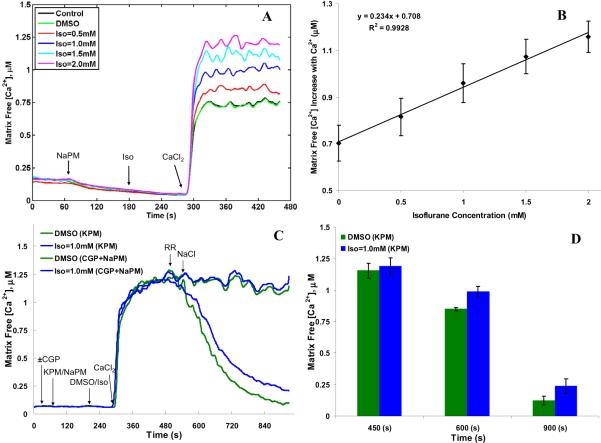
Isoflurane –induced changes in mitochondrial free Ca2+ ([Ca2+]m)
The concentration-dependent effect of isoflurane and 0.5 mM CaCl2 (≈200 nM free Ca2+) on the dynamics of [Ca2+]m during states 2 respiration with NaPM is shown in Fig. 7A. The corresponding increases in state 2 [Ca2+]m with added CaCl2 are summarized in Fig. 7B. Isoflurane increased [Ca2+]m in a concentration-dependent manner after adding 0.5 mM CaCl2 as shown in the time-dependent traces (Fig. 7A). The Na+-dependent substrate NaPM caused an initial small decrease in [Ca2+]m from ~150 nM to almost 0 nM due to activation of the NCE (Fig. 7A). Adding 0.5 mM CaCl2 during state 2 respiration caused an increase in [Ca2+]m to 707 ±80 nM and 702 ±70 nM in the control and DMSO groups, respectively, indicating that DMSO did not itself alter [Ca2+]m (Fig. 7B). In the presence of isoflurane, [Ca2+]m increased significantly over the control and DMSO values in a concentration-dependent manner to 815 ±80 nM (Iso =0.5 mM), 960 ±80 nM (Iso =1 mM), 1074 ±70 nM (Iso =1.5 mM), and 1158 ±70 nM (Iso =2 mM) (Fig. 7B), despite the isoflurane-induced slight Δψm depolarization (Fig. 5B) and mild matrix expansion (Fig. 6B) during state 2 respiration.
Figure 7.
(A) Time-course of mitochondrial free Ca2+ ([Ca2+]m) with 0.5 mM added CaCl2 (≈200 nM free Ca2+) during state 2 respiration with Na+-pyruvate/malate (NaPM) and isoflurane (0.5, 1, 1.5 and 2 mM) compared to control (no treatment) and vehicle (DMSO) groups. (B) Summary of the effects of adding CaCl2 on [Ca2+]m during state 2 respiration with NaPM. Note that isoflurane had no effect on altering basal [Ca2+]m but that [Ca2+]m was markedly elevated after adding CaCl2. Moreover, isoflurane stepwise increased state 2 [Ca2+]m, compared to DMSO (Iso = 0 mM), suggesting a net increase in Ca2+ uptake. (C) Time-course of [Ca2+]m with 0.5 mM added CaCl2 (≈200 nM free Ca2+) and RR+10 mM added NaCl during state 2 respiration with substrates NaPM+CGP or KPM and isoflurane (1mM) compared to DMSO. (D) Summary effect of adding CaCl2 and RR+NaCl on [Ca2+]m during state 2 respiration with KPM. Note that isoflurane did not induce any further increase in [Ca2+]m, compared to DMSO, after adding CaCl2 with either substrates; however, isoflurane attenuated matrix Ca2+ efflux, as assessed by decreased [Ca2+]m with NaCl addition after blocking the CU with RR when using KPM substrate, indicating isoflurane -induced attenuation of NCE activity. Traces represent means of 4 replicates in each group and summary data are plotted as means ±SE of 4 replicates. * indicates significant differences exist between isoflurane and control/DMSO groups, while # indicates significant differences exist between high and low isoflurane groups (p < 0.05).
Isoflurane may differentially alter the activity of the mitochondrial CU or NCE as a cause for the increased [Ca2+]m after adding CaCl2 using NaPM substrate. To test this, [Ca2+]m was measured with KPM vs. NaPM in the presence of a NCE inhibitor (CGP-37157) at a single concentration of isoflurane (1 mM) vs. DMSO with 0.5 mM added CaCl2 (≈200 nM free Ca2+) followed by additions of RR (25 μM) and NaCl (10 mM) (Fig. 7C,D). These data show that isoflurane did not induce any further increase in [Ca2+]m (1153 ±58 nM) compared to DMSO (1189 ±66 nM) with the substrate KPM or NaPM +CGP during the [Ca2+] uptake phase (t =450 s). However, isoflurane attenuated matrix Ca2+ efflux, as assessed by decreased [Ca2+]m, after blocking the CU with RR and then adding NaCl in the KPM substrate experiments ([Ca2+]m =848 ±13 nM at t =600 s and 121 ±34 nM at t =900 s with DMSO vs. 987 ±41 nM at t =600 s and 237 ±57 nM at t =900 s with isoflurane) (Fig. 7D). The decrease in [Ca2+]m induced by NaCl addition was totally negated when CGP was used with the Na+-containing substrate (NaPM). These data thus indicate that the isoflurane-mediated increases in [Ca2+]m with the Na+-based substrate (NaPM) is a direct result of attenuated NCE activity.
Discussion
The mitochondrion is recognized as an upstream target for cardiac IR injury and a downstream effector of cardioprotection during volatile anesthetic-induced pre- and post-conditioning (APC, APOC) [2–4, 6, 8]. The precise mechanisms of triggering, signaling and mediation of the cardioprotective action of volatile anesthetics (e.g., isoflurane) involving mitochondria remain elusive. The direct effects of isoflurane on mitochondrial bioenergetics and Ca2+ homeostasis was the focus of this study with the aim to better understand the mechanisms underlying the cardioprotective effect of volatile anesthetics.
We used isolated cardiac mitochondria (respiring with different substrates: NaPM, KPM and NaSuc) and fluorescence techniques to study the effects of extra-matrix additions of 0.5 mM CaCl2 ([Ca2+]e ≈200 nM with 1 mM EGTA buffer) and 250 mM ADP on [Ca2+]m, NADH, respiration, ΔΨm, and matrix volume at several concentrations of isoflurane (0, 0.5, 1, 1.5, 2 mM). We found that isoflurane has several direct and interrelated effects on mitochondrial function. Fore-most, isoflurane exerts a concentration-dependent effect to reduce and slow the rates of state 3 respiration and NADH oxidation, to further expand state 2 and contract state 3 matrix volume, and to partially depress states 2 and 3 ΔΨm. Moreover, isoflurane concentration-dependently increases state 2 [Ca2+]m resulting from added CaCl2 with NaPM, but not with KPM, substrate. The isoflurane-induced increases in state 2 [Ca2+]m with NaPM substrate, despite increasingly lower ΔΨm and higher matrix volume, indicate that the apparent rise in Ca2+ influx, or decline in Ca2+ efflux, across the inner mitochondrial membrane (IMM) is more dependent on altered Ca2+ binding affinities of the two principal Ca2+ transporters CU and NCE, and less dependent on electrochemical gradient of Ca2+ across the (IMM) at increasing isoflurane concentrations. Isoflurane is a very lipid soluble compound that can modify protein structure. The use of KPM as substrate and an NCE inhibitor demonstrate that isoflurane's effect to increase [Ca2+]m is totally blocked, which strongly suggests that isoflurane decreases the net efflux of Ca2+ by directly or allosterically attenuating NCE activity. Our bioenergetic experiments support the notion that isoflurane also inhibits complex I to decrease the rate and prolong the duration of state 3 electron transfer and ADP phosphorylation, despite the lower ΔΨm which would tend to enhance respiration.
In the context of cardioprotection, the isoflurane-mediated attenuation of NCE activity leading to reduced net Ca2+ efflux (i.e. increased [Ca2+]m) could be an initiating factor in the sequence of events leading to protection against IR injury. On the other hand, isoflurane may mediate cardioprotection in large part by depressing mitochondrial bioenergetics before, during, or after IR injury. It is not clear from this study if isoflurane's effects to enhance [Ca2+]m while depressing mitochondrial NCE activity and bioenergetics singularly or together mediate cardioprotection. What appears evident from the data is that isoflurane's effect to enhance [Ca2+]m exacerbates the depression of the bioenergetic state by its influence to reduce ΔΨm.
Isoflurane depresses mitochondrial bioenergetics –implications for cardioprotection
Altered mitochondrial function with APC or APOC has largely been characterized by monitoring changes in NADH levels or transporter/enzyme activities (e.g., complex I activity) that link to other effectors being affected in triggering the protective mechanism against myocardial IR injury. This notion is consistent with the observation of a small sevoflurane-induced increase in NADH level in isolated perfused guinea pig hearts [26]. In our isolated mitochondrial study, isoflurane itself caused little change in state 2 NADH level, whereas it greatly attenuated the extent of NADH oxidation during state 3 respiration. This is most likely due to the slowed rates of electron transfer and ADP phosphorylation so that the slower rate of H+ pumping by NADH-linked intermediates is better matched to the slower rate of H+ entering the matrix via complex V (F1F0-ATPase). This would prolong the duration of state 3 ADP phosphorylation, which is consistently reflected in the measured durations of state 3 NADH oxidation, respiration, ΔΨm, and matrix contraction with complex I substrates (NaPM or KPM).
Previous studies in isolated perfused hearts show that volatile anesthetics attenuate electron transport chain (ETC) activity at the level of complex I based on decreased NADH oxidation. This in turn is thought to stimulate a small amount of ROS production and/or activation of mitochondrial ATP-sensitive potassium channels (mKATP) and mitochondrial calcium-sensitive potassium channels (mKCa) as effectors in cardioprotection induced by anesthetics or other compounds [27, 28]. Moreover, inhibition of complex I has been suggested as a likely target of volatile anesthetics in isolated cardiac mitochondria [29–31] and in other experimental models [32–34]. Our results on isoflurane-induced decreases in state 3 NADH oxidation and respiration, and the accentuated fall in state 3 ΔΨm, indicate attenuation of electron transfer and H+ pumping by ETC, especially at complex I. This notion is substantiated by the measured time-courses of matrix pH with BCECF-AM dye that showed no change in matrix pH at different concentrations of isoflurane compared to the control and DMSO groups (data not shown). This appears to rule out the possibility that isoflurane has a protonophoric effect to allow H+ leak through the IMM [35], which would itself enhance the respiratory rate, but which is the opposite of what was observed.
To substantiate our view that isoflurane decreases state 3 electron transfer and ADP phosphorylation by inhibiting complex I, we conducted bioenergetic experiments with both complex I substrate (NaPM) and complex II substrate (NaSuc) with 0.5 mM added CaCl2 or no added CaCl2 (Fig. 4A–D). Specifically, we compared the change in NADH redox state with titrated concentrations of the complex I inhibitor rotenone and found that 1 mM isoflurane mimicked inhibition of complex I equivalent to 30 nM rotenone. These results demonstrate similar effects of isoflurane (1 mM) and rotenone (30 nM) using different substrates and at different [Ca2+]e and ([Ca2+]m.
Taken together, the available data support our observations that isoflurane reduced mitochondrial bioenergetic activity by decreasing NADH oxidation and ADP phosphorylation. In turn, this may ultimately preserve the mitochondrial redox state needed for effective mitochondrial ATP production during reperfusion [26]. The strategy of targeting ETC complexes to modify electron transfer [34], and in part ROS production from ETC complexes [1, 36], can also provide an alternative strategy for cardioprotection by isoflurane.
Although not probed in this study, the possibility of a moderate increase in ROS production while attenuating complex I activity cannot be excluded, as isoflurane is shown here to moderately reduce O2 consumption in isolated mitochondria. Prior studies suggest a dual effect of isoflurane on the ETC: mild uncoupling combined with attenuated complex I activity [34, 37], and further effects on complexes downstream of complex I, predominantly, complex III [38]; the latter was suggested to trigger ROS production as a mechanism of isoflurane-induced cardioprotection [4, 36]. A high NADH/NAD+ ratio in the matrix is favorable to induce an increase in electron leak and ROS production if electrons are blocked in the FeS clusters of the FMN complex as a result of reduced NADH pool [39–41]. The small decrease in ΔΨm induced by isoflurane implies that this may be a direct or indirect factor in anesthetic-mediated cardioprotection [11, 12], at least in part by inhibiting the ROS production that can occur in a highly reduced state [20, 42]. Attenuation of mitochondrial respiration with isoflurane by inhibiting complex I may exert cardioprotection [37, 43] by attenuating both ΔΨm and ROS production [12, 44]. Thus, if isoflurane preserves the NADH pool by attenuating complex I activity and stimulating NADH-induced ROS production, then this could be an important trigger of cardioprotective pathways that lead to protection against IR injury [45]. These direct effects may contribute to the overall signaling cascade involved in APC and other anesthetic-mediated cardioprotective strategies in which mitochondrial ROS has been implicated as the critical trigger [8, 46]. Indeed, mitochondrial ROS generation is influenced by [Ca2+]m, ΔΨm, and redox state [1], and knowledge of how isoflurane directly modulates mitochondrial function should provide novel insights into the mechanisms of anesthetic-mediated cardioprotection [4].
Isoflurane enhances mitochondrial free Ca2+ ([Ca2+]m) despite a decrease in membrane potential (Δψm) and increase in matrix volume
Our study shows that isoflurane causes a slight concentration-dependent decrease in Δψm during state 2 respiration (Fig. 5). This indicates that the observed increase in state 2 [Ca2+]m with increasing isoflurane concentration in the NaPM substrate experiments (Fig. 7A,B) is not solely dependent on Δψm for the electrochemical gradient to promote enhanced Ca2+ influx into the matrix via the CU. In general, Ca2+ uptake via the CU occurs at the expense of Δψm, i.e., it utilizes the energy of Δψm to transport extra-matrix free Ca2+ across the IMM [3, 47]. This is consistent with our previous study that showed a CaCl2 concentration-dependent increase in Ca2+ uptake, through the ruthenium red-sensitive CU, in freshly isolated cardiac mitochondria at a high Δψm [18]. Thus, one would expect that a decrease in Ca2+ uptake would occur when Δψm is decreased, contrary to what we observed in this study, indicating other possible mechanisms associated with this increase in state 2 [Ca2+]m.
To further confirm that isoflurane increases state 2 [Ca2+]m with NaPM substrate and is independent of Δψm, we added 0.5 mM CaCl2 and measured [Ca2+]m in the presence of an uncoupler, dinitrophenol (DNP; 20 μM), and then added DMSO or 1 mM isoflurane. This data showed that DNP, which induced partial Δψm depolarization decreased [Ca2+]m; in contrast, isoflurane in the presence of DNP increased [Ca2+]m despite partial Δψm depolarization (data not shown).
Since isoflurane and CaCl2 both led to matrix volume expansion during state 2 respiration (Fig. 6), the increase in state 2 [Ca2+]m that we observed with increasing isoflurane concentration in the NaPM substrate experiments (Fig. 7A,B) may be somewhat underestimated due to apparent decrease in indo-1 AM fluorescence by light scattering. This indicates that the increase in state 2 [Ca2+]m is not due to matrix volume contraction.
We examined other possibilities for increased state 2 [Ca2+]m with isoflurane using NaPM as substrate. For example, there could occur in an apparent increase in indo-1 AM fluorescence via an indo-1 and isoflurane interaction, via matrix Ca2+ buffering proteins, and/or from decreased matrix pH via a possible protonophoric effect of isoflurane. To test these possibilities, experiments were conducted in triton-x treated indo-1 AM loaded mitochondria and BCECF-AM loaded mitochondria in the presence of DMSO and isoflurane. Because we found no change in indo-1 AM fluorescence as well as no change in matrix pH at different concentrations of isoflurane compared to the control and DMSO groups (data not shown), this ruled out these possibilities. Also, with the minimal effect of isoflurane on state 2 Δψm depolarization (2.7%), we would not expect substantial change in pH as proton pumps must be working fast to maintain the same O2 consumption level. This was further confirmed by the observation of very little change in state 2 respiration at different isoflurane concentrations (Fig. 2A).
We also examined [Ca2+]m dynamics after adding CaCl2 using the CU inhibitor RR, which was added before adding NaPM. This data (not displayed) did not demonstrate any increase in [Ca2+]m in control (DMSO) or in isoflurane-treated mitochondria, thus excluding another mechanism, i.e. isoflurane-induced alteration of indo-1 fluorescence, as a cause for the increased [Ca2+]m in the presence of isoflurane.
Another possibility for increased state 2 [Ca2+]m with NaPM substrate could arise from an effect of isoflurane to reduce matrix Ca2+ buffering by Ca2+ binding proteins. It is conceivable that isoflurane may intercalate into the IMM and block Ca2+ binding proteins (e.g., annexin) to interact with the phospholipid bilayer through phosphorylation [48, 49]. Though, we can not dismiss this possibility, when isoflurane was added after CaCl2 in selected control and DMSO experiments we did not see any further increase in [Ca2+]m (data not shown). It is also unlikely that isoflurane might affect matrix Ca2+-phosphate precipitate formation because the solubility product constants for CaHPO4 and Ca3(PO4)2 formation are Ksp =10− and 3*10−30, respectively. With a matrix pH of around 7.2–7.3 and the above Ksp values, [Ca2+]m would need to exceed 2 μM for any appreciable amount of Ca2+-phosphate formation [50, 51].
From all the possibilities, we first concluded that the increases in state 2 [Ca2+]m with increasing isoflurane concentration in the NaPM substrate experiments (Fig. 7A,B) was most likely due to increased influx or decreased efflux of Ca2+ across the IMM that involves direct or allosteric activation of either the CU or inhibition of the NCE by isoflurane. Because of isoflurane's high lipophilicity, it can directly or indirectly (allosterically) interact with the lipids around the Ca2+ transporters or with their hydrophobic peptides to alter their binding affinities of Ca2+. For example, we thought that isoflurane might decrease the Km of Ca2+ for the CU and/or increase the Vmax of the CU to increase binding of Ca2+ to the CU and/or enhance maximal activity of the CU to promote enhanced Ca2+m uptake. Indeed, it was reported that isoflurane activates mKATP channels reconstituted in lipid bilayers [52] and that the mKATP channel blocker, 5-hydroxydecanoate (5-HD), attenuates the cardioprotective effects of isoflurane in an isolated perfused rabbit heart model [53]. In preliminary experiments we found that the mKCa channel inhibitor paxilline had no effect on the isoflurane-mediated rise in [Ca2+]m (data not shown).
To distinguish if isoflurane acts on the CU and/or the NCE to increase [Ca2+]m after adding CaCl2 using the substrate NaPM, we also measured [Ca2+]m using substrate PMK, or NaPM in the presence of NCE inhibitor CGP-37157, with an extended time-line protocol (Fig. 1C). After energizing mitochondria with KPM or NaPM + CGP, the CU inhibitor RR and then NaCl were added in the presence of DMSO or 1 mM isoflurane (Fig. 7C,D). In this protocol, isoflurane, like DMSO, did not induce any further increase in [Ca2+]m after adding CaCl2; moreover, NaCl had no effect to activate Ca2+ efflux as shown by no further change in [Ca2+]m when using NaPM + CGP. When using KPM, and after blocking the CU with RR, isoflurane attenuated the fall in [Ca2+]m, i.e., decreased Ca2+m efflux, after adding NaCl to activate NCE. These data showed clearly that the isoflurane-enhanced, CaCl2 induced, increase in state 2 [Ca2+]m with Na+-containing substrate NaPM occurs primarily by attenuating the activity of the NCE. The mechanism how isoflurane attenuates NCE activity (direct or indirect; change in protein configuration; post-translational modification) is not known at this time. However, this is the first report demonstrating that a volatile anesthetic can attenuate matrix Ca2+ efflux, and explains in part why we observed an increased [Ca2+]m despite a decrease in Δψm.
To provide additional evidence that the isoflurane-induced increase in state 2 [Ca2+]m with NaPM as substrate results from inhibited NCE activity, we monitored [Ca2+]e under the extended time-line protocol (Fig. 1C) to examine NCE kinetics under the conditions of reduced buffer EGTA (40 μM) and added CaCl2 (30 μM) to elicit [Ca2+]e responses comparable to the presented studies. Without the extensive buffering of Ca2+ in the extra-matrix buffer, we could observe changes in [Ca2+]e as Ca2+enters or exits the matrix. We found that isoflurane did not alter the gradual fall in [Ca2+]e due to uptake via CU after the fast initial rise due to adding CaCl2 but did attenuate the gradual rise in [Ca2+]e after adding RR and then NaCl to stimulate NCE (data not shown).
Implication of isoflurane-induced increased [Ca2+]m to bioenergetics
[Ca2+]m is believed to enhance/stimulate mitochondrial dehydrogenase enzymes, TCA cycle, and NADH production, and hence to increase state 3 respiration rate. Thus, it could be expected that the CaCl2 –induced enhancement of [Ca2+]m by isoflurane would enhance respiration and NADH, which it did not (Figs. 2–4). This discrepancy may be attributed to several factors. First, 10 mM of substrate (NaPM or KPM) to energize mitochondria may saturate the ADP-independent TCA cycle flux. In a recent study [54], it was reported that to observe an effect of increased Ca2+ to stimulate state 3 NADH production and respiration, a low substrate concentration is necessary. Second, the substrate glutamate +malate provides a greater NADH response to increasing extra-matrix [Ca2+]. Third, state 2 [Ca2+]m after adding CaCl2 in our study is about 700 nM with DMSO and rises to over 1000 nM after adding isoflurane (Figs. 7A,C), which may be high enough to saturate the mitochondrial dehydrogenase enzymes [55], and hence the TCA cycle flux that produce NADH for the ETC and OxPhos. Fourth, isoflurane has another effect to attenuate complex I activity, which itself leads to decreased electron flow and ADP phosphorylation during state 3 respiration. Therefore, the increased state 2 [Ca2+]m that we observe with isoflurane with saturating NaPM substrate concentration (10 mM) would not be expected to increase state 3 respiration and NADH oxidation. However, when we used 10 mM NaSuc as the substrate we found that the duration of state 3 NADH oxidation was shorter (faster respiration) after adding 1 mM isoflurane with or without added CaCl2 (Fig. 4A–D).
Implication of isoflurane-induced increased [Ca2+]m to cardioprotection
One might expect that a decrease in mitochondrial Ca2+ uptake would occur when Δψm is decreased. Interestingly, in our study, a decline in state 2 Δψm in the presence of isoflurane was associated with a greater accumulation of Ca2+ in the matrix with NaPM substrate. This suggests that isoflurane's cardioprotective effect is a result of an indirect interplay between a mild decrease in Δψm due to slower respiration and an enhanced net Ca2+ uptake, which tends to further reduce Δψm. This notion is supported by our mitochondrial Ca2+ uptake measurement in the presence of an uncoupler (DNP; 20 μM) and in the presence of DMSO and isoflurane (1 mM), since DNP was suggested to be cardioprotective [12]. Thus, a partial decrease in Δψm by isoflurane during ischemia could preserve the H+ gradient necessary for efficient ATP synthesis to meet the energy demand of cardiomyocytes during reperfusion to reduce IR injury.
Previous studies of volatile anesthetic-preconditioning of isolated cardiomyocytes [11, 12, 56] and isolated perfused hearts [57] demonstrate this therapy attenuates Ca2+m overload. In this case the protective effects are measured after washout of the volatile anesthetic that characterizes the memory phase of preconditioning. In contrast, we focused here on the direct effects of a volatile anesthetic on mitochondrial bioenergetics and Ca2+ handling, which showed an increase in state 2 [Ca2+]m in the presence of isoflurane with NaPM as substrate due to an attenuation of the NCE activity. This anesthetic-induced increase in [Ca2+]m we observed may be part of a triggering mechanism to initiate signaling pathways for preconditioning that may translate into reduced Ca2+m uptake due to attenuated cytosolic Ca2+ overload and/or CU activity.
A recent study in isolated cardiomyocytes [12] showed that APC affords protection against oxidative stress by attenuating Δψm that leads to decreased ROS generation and attenuation of Ca2+m accumulation; in contrast, in the present study in isolated cardiac mitochondria, a similar decrease in Δψm resulted in an increase in [Ca2+]m. The mechanism for an APC-mediated decrease in Δψm is not clear. A large increase in [Ca2+]m is known to be deleterious as it leads to mPTP opening during cardiac IR injury; paradoxically our study implies that the small increases in [Ca2+]m elicited by isoflurane could play a significant role in volatile anesthetic-mediated cardioprotection by reducing electron flow and proton pumping, and thereby reducing state 3 respiration, NADH oxidation, ADP phosphorylation, and Δψm.
Our finding that exposure of mitochondria to isoflurane showed enhanced [Ca2+]m may seem to be anti-protective. Although Ca2+ overload initiates mPTP opening [58], the enhanced Ca2+ uptake with isoflurane did not induce mPTP opening in this study as observed by the return of the measured indices to state 4. However, isoflurane, by depressing the bioenergetic state of mitochondria with the assistance of a small increase in Ca2+ uptake, may activate signaling pathways that reduce Ca2+ overload on reperfusion after ischemia [57] and reduce activation of mPTP [57, 59]. Permanent opening of the mPTP is thought to be induced or exacerbated by excess ROS emission [60] and/or excess Ca2+ loading. Opening of the mKATP channel may modify Δψm [61] or matrix volume and pH [24, 62] to reduce the deleterious Ca2+ overload.
Taking together, the mechanism(s) of cardiac protection afforded by volatile anesthetics involves the interplay of [Ca2+]m regulation, bioenergetic control, and possibly ROS emission. Our study suggests that a moderate increase in [Ca2+]m coupled to depressed mitochondrial bioenergetics may together be important factors in the triggering phase of volatile anesthetic-mediated pre- and post-conditioning, or in cardioprotection in general.
Highlights
-
>
We study mechanisms of isoflurane modulating mitochondrial bioenergetics and [Ca2+]m.
-
>
Isoflurane increases state 2 [Ca2+]m independent of changes in ΔΨm and matrix volume.
-
>
Increased [Ca2+]m is due to inhibition of mitochondrial Na+/Ca2+ exchanger (NCE).
-
>
Isoflurane decreases NADH oxidation, respiration, ΔΨm, and matrix volume in state 3.
-
>
Isoflurane delays state 3 duration of bioenergetic variables by complex I inhibition.
Acknowledgement
This work was supported by the National Institute of Health grants P01-GM066730-08 (ZJB) and R01-HL095122-02 (RKD and AKSC). The authors are thankful to Drs. Beard and Vinnakota for participating in the discussion and providing many helpful suggestions. The authors are also thankful to Dr. AD Bolens for providing the measurements of Kd of indo-1 and Ca2+ binding used in this study, and to M Herrera and JL Vega for assistant in the early phases of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: Portions of this work were presented at the Experimental Biology Meeting (FASEB J 1048.7, 2010) and the Biophysical Society Meeting (Biophys J 100:81a, 2011).
References
- [1].Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- [2].Zaugg M, Schaub MC. Signaling and cellular mechanisms in cardiac protection by ischemic and pharmacological preconditioning. J Muscle Res Cell Motil. 2003;24:219–249. doi: 10.1023/a:1026021430091. [DOI] [PubMed] [Google Scholar]
- [3].Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemi-areperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steenbergen C, Murphy E, Watts JA, London RE. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circ Res. 1990;66:135–146. doi: 10.1161/01.res.66.1.135. [DOI] [PubMed] [Google Scholar]
- [6].Namekata I, Shimada H, Kawanishi T, Tanaka H, Shigenobu K. Reduction by SEA0400 of myocardial ischemia-induced cytoplasmic and mitochondrial Ca2+ overload. Eur J Pharmacol. 2006;543:108–115. doi: 10.1016/j.ejphar.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [7].Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- [8].Camara AK, Lesnefsky EJ, Stowe DF. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal. 2010;13:279–347. doi: 10.1089/ars.2009.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- [10].Pagel PS. Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth. 2008;22:753–765. doi: 10.1053/j.jvca.2008.03.005. [DOI] [PubMed] [Google Scholar]
- [11].Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- [12].Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+ Am J Physiol Cell Physiol. 2010;299:C506–515. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- [14].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- [15].Dash RK, Qi F, Beard DA. A biophysically based mathematical model for the kinetics of mitochondrial calcium uniporter. Biophys J. 2009;96:1318–1332. doi: 10.1016/j.bpj.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pradhan RK, Qi F, Beard DA, Dash RK. Characterization of membrane potential dependency of mitochondrial Ca2+ uptake by an improved biophysical model of mitochondrial Ca2+ uniporter. PLoS One. 2010;5:e13278. doi: 10.1371/journal.pone.0013278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pradhan RK, Qi F, Beard DA, Dash RK. Characterization of Mg2+ inhibition of mitochondrial Ca2+ uptake by a mechanistic model of mitochondrial Ca2+ uniporter. Biophys J. 2011;101:2071–2081. doi: 10.1016/j.bpj.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haumann J, Dash RK, Stowe DF, Boelens AD, Beard DA, Camara AK. Mitochondrial free [Ca2+] increases during ATP/ADP antiport and ADP phosphorylation: exploration of mechanisms. Biophys J. 2010;99:997–1006. doi: 10.1016/j.bpj.2010.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- [20].Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol. 2007;292:C148–156. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- [21].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [22].Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- [23].Huang M, Camara AK, Stowe DF, Qi F, Beard DA. Mitochondrial inner membrane electrophysiology assessed by rhodamine-123 transport and fluorescence. Ann Biomed Eng. 2007;35:1276–1285. doi: 10.1007/s10439-007-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–415. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- [25].Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AK. Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening. Am J Physiol Cell Physiol. 2010;298:C530–541. doi: 10.1152/ajpcell.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- [27].Riess ML, Kevin LG, McCormick J, Jiang MT, Rhodes SS, Stowe DF. Anesthetic preconditioning: the role of free radicals in sevoflurane-induced attenuation of mitochondrial electron transport in Guinea pig isolated hearts. Anesth Analg. 2005;100:46–53. doi: 10.1213/01.ANE.0000139346.76784.72. [DOI] [PubMed] [Google Scholar]
- [28].Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AKS. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [29].Hall GM, Kirtland SJ, Baum H. The inhibition of mitochondrial respiration by inhalational anaesthetic agents. Br J Anaesth. 1973;45:1005–1009. doi: 10.1093/bja/45.10.1005. [DOI] [PubMed] [Google Scholar]
- [30].Merin RG. Inhalation anesthetics and myocardial metabolism: possible mechanisms of functional effects. Anesthesiology. 1973;39:216–255. doi: 10.1097/00000542-197308000-00012. [DOI] [PubMed] [Google Scholar]
- [31].Rusy BF, Komai H. Anesthetic depression of myocardial contractility: a review of possible mechanisms. Anesthesiology. 1987;67:745–766. doi: 10.1097/00000542-198711000-00020. [DOI] [PubMed] [Google Scholar]
- [32].Kissin I, Aultman DF, Smith LR. Effects of volatile anesthetics on myocardial oxidation-reduction status assessed by NADH fluorometry. Anesthesiology. 1983;59:447–452. doi: 10.1097/00000542-198311000-00016. [DOI] [PubMed] [Google Scholar]
- [33].Hanley PJ, Loiselle DS. Mechanisms of force inhibition by halothane and isoflurane in intact rat cardiac muscle. J Physiol. 1998;506(Pt 1):231–244. doi: 10.1111/j.1469-7793.1998.231bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–693. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pravdic D, Mio Y, Sedlic F, Pratt PF, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane protects cardiomyocytes and mitochondria by immediate and cytosol-independent action at reperfusion. Br J Pharmacol. 2010;160:220–232. doi: 10.1111/j.1476-5381.2010.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- [37].Sedlic F, Pravdic D, Hirata N, Mio Y, Sepac A, Camara AK, Wakatsuki T, Bosnjak ZJ, Bienengraeber M. Monitoring mitochondrial electron fluxes using NAD(P)H-flavoprotein fluorometry reveals complex action of isoflurane on cardiomyocytes. Biochim Biophys Acta. 2010;1797:1749–1758. doi: 10.1016/j.bbabio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- [39].Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- [40].Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- [43].Abdel-Rahman U, Risteski P, Tizi K, Kerscher S, Behjati S, Zwicker K, Scholz M, Brandt U, Moritz A. Hypoxic reoxygenation during initial reperfusion attenuates cardiac dysfunction and limits ischemia-reperfusion injury after cardioplegic arrest in a porcine model. J Thorac Cardiovasc Surg. 2009;137:978–982. doi: 10.1016/j.jtcvs.2008.09.025. [DOI] [PubMed] [Google Scholar]
- [44].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [45].Hanukoglu I, Rapoport R. Routes and regulation of NADPH production in steroidogenic mitochondria. Endocr Res. 1995;21:231–241. doi: 10.3109/07435809509030439. [DOI] [PubMed] [Google Scholar]
- [46].Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96:949–955. doi: 10.1213/01.ANE.0000052515.25465.35. table of contents. [DOI] [PubMed] [Google Scholar]
- [47].Saotome M, Katoh H, Satoh H, Nagasaka S, Yoshihara S, Terada H, Hayashi H. Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;288:H1820–1828. doi: 10.1152/ajpheart.00589.2004. [DOI] [PubMed] [Google Scholar]
- [48].Megli FM, Mattiazzi M, Di Tullio T, Quagliariello E. Annexin V binding perturbs the cardiolipin fluidity gradient in isolated mitochondria. Can it affect mitochondrial function? Biochemistry. 2000;39:5534–5542. doi: 10.1021/bi992779z. [DOI] [PubMed] [Google Scholar]
- [49].Hawkins TE, Das D, Young B, Moss SE. DT40 cells lacking the Ca2+-binding protein annexin 5 are resistant to Ca2+-dependent apoptosis. Proc Natl Acad Sci U S A. 2002;99:8054–8059. doi: 10.1073/pnas.132598099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- [51].Nicholls DG, Chalmers S. The integration of mitochondrial calcium transport and storage. J Bioenerg Biomembr. 2004;36:277–281. doi: 10.1023/B:JOBB.0000041753.52832.f3. [DOI] [PubMed] [Google Scholar]
- [52].Nakae Y, Kwok WM, Bosnjak ZJ, Jiang MT. Isoflurane activates rat mitochondrial ATP-sensitive K+ channels reconstituted in lipid bilayers. Am J Physiol Heart Circ Physiol. 2003;284:H1865–1871. doi: 10.1152/ajpheart.01031.2002. [DOI] [PubMed] [Google Scholar]
- [53].Tonkovic-Capin M, Gross GJ, Bosnjak ZJ, Tweddell JS, Fitzpatrick CM, Baker JE. Delayed cardioprotection by isoflurane: role of K(ATP) channels. Am J Physiol Heart Circ Physiol. 2002;283:H61–68. doi: 10.1152/ajpheart.01040.2001. [DOI] [PubMed] [Google Scholar]
- [54].Vinnakota KC, Dash RK, Beard DA. Stimulatory effects of calcium on respiration and NAD(P)H synthesis in intact rat heart mitochondria utilizing physiological substrates cannot explain respiratory control in vivo. J Biol Chem. 2011;286:30816–30822. doi: 10.1074/jbc.M111.242529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Qi F, Pradhan RK, Dash RK, Beard DA. Detailed kinetics and regulation of mammalian 2-oxoglutarate dehydrogenase. BMC biochemistry. 2011;12:53. doi: 10.1186/1471-2091-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu H, Wang L, Eaton M, Schaefer S. Sevoflurane preconditioning limits intracellular/mitochondrial Ca2+ in ischemic newborn myocardium. Anesth Analg. 2005;101:349–355. doi: 10.1213/01.ANE.0000154197.24763.EC. table of contents. [DOI] [PubMed] [Google Scholar]
- [57].Riess ML, Camara AK, Novalija E, Chen Q, Rhodes SS, Stowe DF. Anesthetic preconditioning attenuates mitochondrial Ca2+ overload during ischemia in Guinea pig intact hearts: reversal by 5-hydroxydecanoic acid. Anesth Analg. 2002;95:1540–1546. doi: 10.1097/00000539-200212000-00013. table of contents. [DOI] [PubMed] [Google Scholar]
- [58].Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- [59].Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology. 2010;113:906–916. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;290:H2024–2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- [61].Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol. 1999;519(Pt 2):347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP-sensitive K+ channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002;283:H284–295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]