Abstract
The effect of extracorporeal blood purification on clinical outcomes in sepsis is assumed to be related to modulation of plasma cytokine concentrations. To test this hypothesis directly, we treated rats that had a cecal ligation followed by puncture (a standard model of sepsis) with a modest dose of extracorporeal blood purification that did not result in acute changes in a panel of common cytokines associated with inflammation (TNF-α, IL-1β, IL-6, and IL-10). Pre- and immediate post-treatment levels of these cytokines were unchanged compared to the sham therapy of extracorporeal circulation without blood purifying sorbent. The overall survival to 7 days, however, was significantly better in animals that received extracorporeal blood purification compared to those with a sham procedure. This panel of common plasma cytokines along with alanine aminotransferase and creatinine was significantly lower 72 h following extracorporeal blood purification compared to sham-treated rats. Thus, the effects of this procedure on organ function and survival do not appear to be due solely to immediate changes in the usual measured circulating cytokines. These results may have important implications for the design and conduct of future trials in sepsis including defining alternative targets for extracorporeal blood purification and other therapies.
Keywords: apheresis, cytokines, inflammation mediators, sepsis, sorbents
Sepsis is the leading cause of death for patients in intensive care units.1,2 The inflammatory response to infection or injury includes the expression of numerous cell-associated and soluble molecules, and it is believed that this systemic inflammation is in large part responsible for the development of shock and subsequent multiorgan injury.3–5 Multiple attempts have been made, and many others are currently underway, to block the specific mediators of coagulation/inflammatory response. However, these attempts have had relatively little impact on overall outcome in the critically ill.6 In a recent large observational study of community-acquired sepsis secondary to pneumonia, we observed the highest risk of death in patients with increased activation of both proinflammatory and anti-inflammatory cytokines.7 Thus, broad-spectrum immune-modulating therapies using drugs or devices have been sought to reduce multiple inflammatory mediators in sepsis.
Extracorporeal blood purification (EBP) techniques such as hemofiltration or apheresis have been proposed for many years as possible strategies to modulate the multiple inflammatory mediators in the same way that hemodialysis is effective in removing multiple uremic toxins.8 However, although preclinical9–12 and even early clinical studies13–15 have shown great promise, multicenter randomized clinical trials have been disappointing.16,17 Importantly, development and testing of EBP for sepsis have been predicated on the assumption that cytokine levels must be altered for the therapy to be effective, and so far clinical trials have failed to result in significant changes in circulating cytokine levels,16,17 whereas preclinical studies have shown robust effects.9–12 This failure has led to the development of novel EBP techniques such as high-volume hemofiltration,18 combined plasma filtration and adsorption,19 and hemoadsorption.20 However, the assumption that EBP works through changes in inflammatory cytokines has never been satisfactorily tested. If this relationship can be proven, it will help guide future work in this area.
We have previously shown that EBP by hemoadsorption using CytoSorb beads (CytoSorbents, Monmouth Junction, NJ) has the capacity to alter circulating cytokine levels and improve survival in experimental endotoxemia10 and cecal ligation puncture (CLP)-induced sepsis12 in animals. However, these studies were carried out in highly lethal models of sepsis (LD90) using very intensive therapy resulting in significant removal of cytokines, and examining only short-term (1 day) survival. Such extreme conditions are rarely encountered clinically21 and may not represent typical human sepsis.
Thus, in this study, we sought to test blood purification in a more realistic setting and evaluate long-term (7 days) survival as an end point. We also scaled our intervention to the point that cytokine levels were no longer reduced, in the short term, by the treatment. Finally, we examined organ function and sought to explore possible mechanisms whereby EBP is effective.
RESULTS
Blood purification did not alter plasma cytokine concentrations acutely
The most commonly measured cytokines in sepsis, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-10, were measured. Differences in these circulating plasma cytokine concentrations for septic rats treated with EBP, sham, or control are shown in Figure 1. Baseline values (18 h after CLP) were not different among the three groups for any cytokine. Plasma cytokine concentrations remained constant immediately after treatment and were not different between groups.
Figure 1. Effects of extracorporeal blood purification (EBP) in cytokine removal.
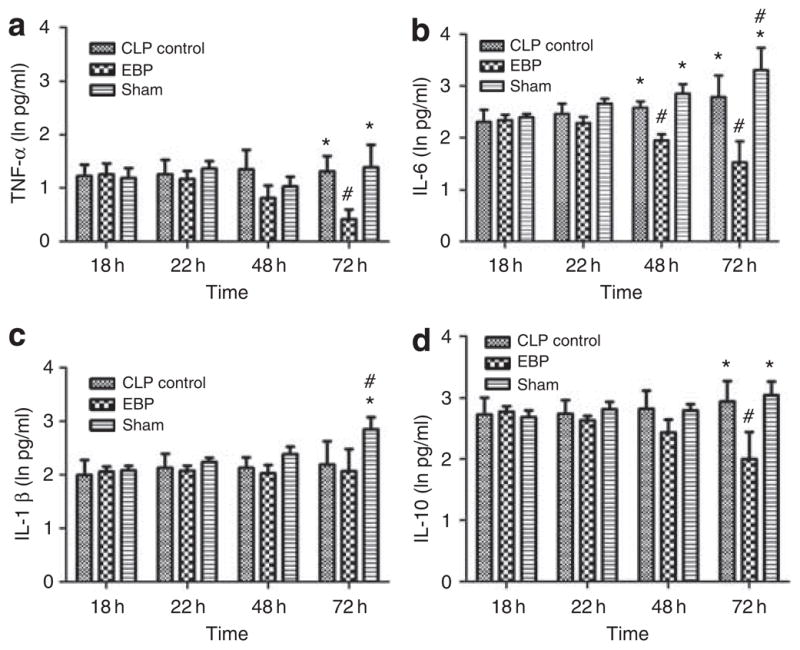
Plasma cytokine levels over time are shown for cecal ligation puncture (CLP) control, EBP, and sham-treated animals. All data were natural log transformed (ln pg/ml) and expressed as mean ± s.e.m. Each panel shows a separate cytokine: (a) tumor necrosis factor-α (TNF-α); (b) interleukin (IL)-6; (c) IL-1β; and (d) IL-10. *P < 0.05, vs. EBP at the same time points; #P < 0.05, vs. the baseline (at 18 h) in the same groups.
However, at later time points (48 and 72 h) and long after intervention, cytokine concentrations were significantly lower in the EBP group (Figure 1). The concentrations of TNF-α, IL-1β, IL-6, and IL-10 were all significantly lower in the EBP group 48 h after treatment (72 h after CLP). Cytokine concentrations in the sham group were not significantly different from controls.
Blood purification improved 1-week survival despite not affecting early cytokine levels
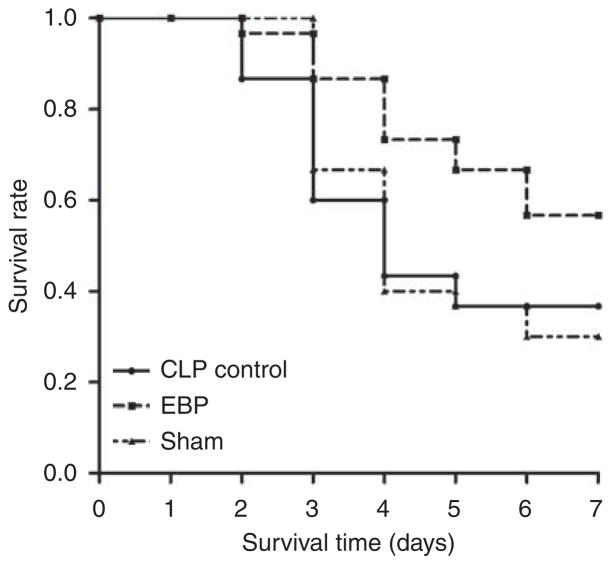
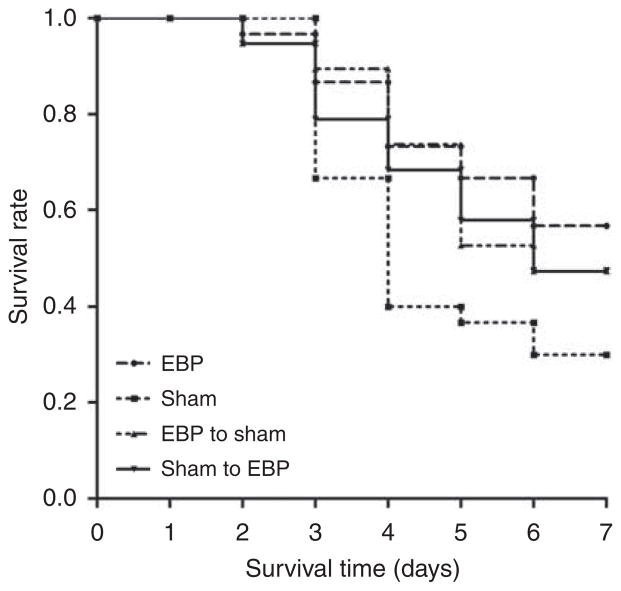
Survival was analyzed until 7 days after CLP. Survival time was greater with EBP compared with sham (hazard ratio: 0.48, P = 0.02) and control (hazard ratio: 0.77, P = 0.04; Figure 2). Survival was not statistically different between sham and control animals.
Figure 2. Effects of extracorporeal blood purification (EBP) on 1-week survival.
Survival time (days) was observed from the start of cecal ligation puncture (CLP). P = 0.02, EBP vs. sham. P = 0.04, EBP vs. CLP control.
Effects of EBP on high-mobility group box 1 (HMGB-1) and on organ function
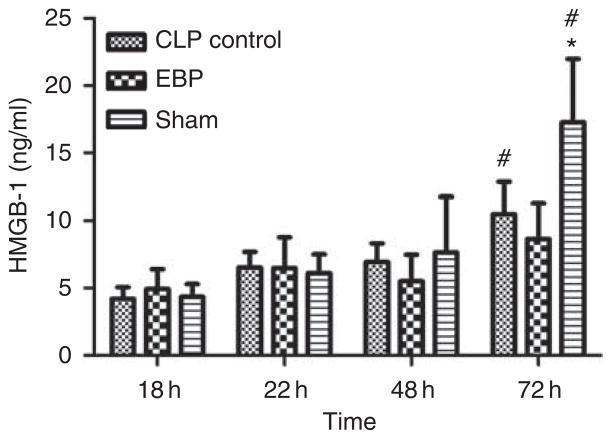
Figure 3 shows the effect of EBP on HMGB-1 in blood. Plasma HMGB-1 increased after CLP (4.34 vs. 17.29 in sham-treated animals, P < 0.05). EBP attenuated this increase. By 48 h after treatment, the difference of HMGB-1 concentrations between the two groups was statistically significant (EBP, 8.65 ng/ml vs. sham, 17.29 ng/ml, P < 0.05).
Figure 3. Effects of extracorporeal blood purification (EBP) on high-mobility group box 1 (HMGB-1).
Plasma HMGB-1 levels are shown over time (mean ± s.e.m., ng/ml). *P < 0.05, vs. EBP at the same time points; #P < 0.05, vs. the baseline (at 18 h) in the same groups.
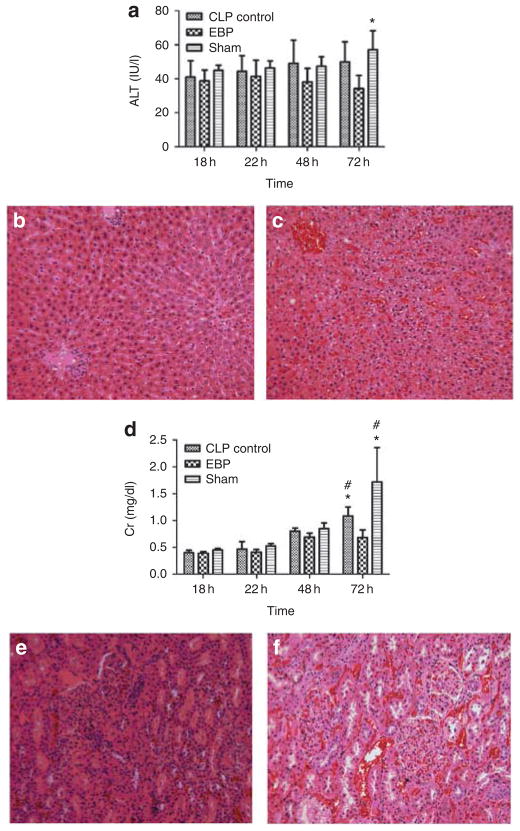
We monitored liver function using alanine aminotransferase (ALT) and used serum creatinine to monitor renal function. Figure 4 demonstrates the effects of EBP on ALT and creatinine. Before treatment (18 h after CLP), mean ALT values were similar in both groups. However, by 48 h after treatment, the ALT was significantly lower in the EBP-treated rats (23.13 vs. 57.07 IU/l, P < 0.05). Changes in serum creatinine were similar (0.68 vs. 1.72 mg/dl, 48 h after treatment, P < 0.05). Histopathology (liver and kidney) was consistent with the biochemical changes (Figure 4).
Figure 4. Effects of extracorporeal blood purification (EBP) on organ function.
Shown are plasma alanine aminotransferase (ALT; IU/l) and creatinine (Cr; mean ± s.e.m., mg/dl). *P < 0.05, vs. EBP at the same time points; #P < 0.05, vs. the baseline (at 18 h) in the same groups. (a) Plasma ALT. (b) Liver histology from EBP showing mild swelling of hepatocytes. (c) Liver histology slice from sham showing moderate to severe swelling of hepatocytes with focal piecemeal necrosis. (d) Plasma creatinine. (e) Kidney histology from EBP showing vacuolization in tubules; however, these changes are milder compared with sham (f). (f) Kidney histology from sham showing significant vacuolization in tubules.
HMGB-1 levels and organ function in control animals were not different from sham-treated animals at any time point.
Effects of exchange transfusions between EBP and sham-treated rats
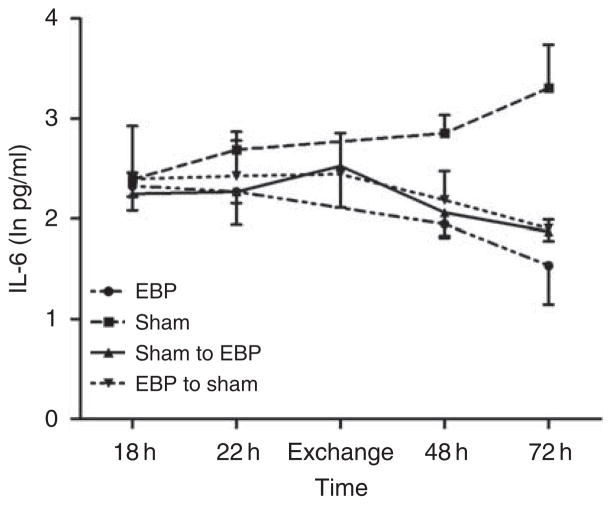
To help elucidate the mechanism whereby EBP improved survival, we carried out a separate set of experiments in which we performed exchange transfusions between EBP and sham-treated rats immediately after treatment. Our results showed a near matching of IL-6 levels (Figure 5) and survival (Figure 6) between the two groups.
Figure 5. Effects of exchange transfusion on interleukin-6 (IL-6).
Shown are plasma IL-6 levels over time for each of the four groups. Data were natural log transformed (ln pg/ml) and expressed as mean ± s.e.m. EBP, extracorporeal blood purification group; EBP to sham, sham animals received blood from the EBP animals; Sham, sham group; Sham to EBP, EBP animals received blood from the sham animals. IL-6 at 72 h in sham group was significantly higher than at baseline (18 h), and IL-6 at 72 h in the other three groups was lower than their baseline levels (P < 0.05).
Figure 6. Effects of exchange transfusion on survival.
Survival time (day) was observed from the start of cecal ligation puncture (CLP) to 7 days. EBP, extracorporeal blood purification group; EBP to sham, sham animals received blood from the EBP animals; Sham, sham group; Sham to EBP, EBP animals received blood from the sham animals. P = 0.02, EBP vs. Sham; P = 0.045, Sham to EBP vs. Sham; P = 0.047, EBP to sham vs. Sham.
Effects of EBP on nuclear factor-κB (NF-κB) activation in circulating leukocytes
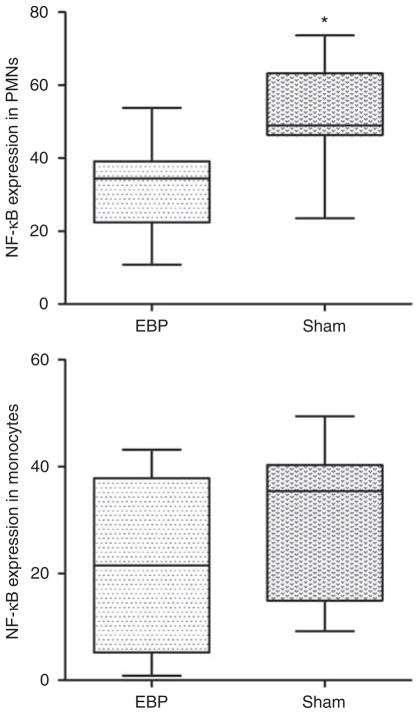
To further explore whether EBP affects key cellular elements in the production of cytokines, we measured NF-κB activation in circulating leukocytes. NF-κB activation in both polymorphonuclear neutrophils (PMNs) and monocytes was reduced with EBP compared with sham treatment. However, only the NF-κB decrease in PMNs was statistically significant (Figure 7).
Figure 7. Effects of extracorporeal blood purification (EBP) on nuclear factor-κB (NF-κB) activation in circulating leukocytes.
Data are expressed as median (range, n = 7). EBP, extracorporeal blood purification group; PMN, polymorphonuclear neutrophil; Sham, sham group. *P < 0.05, EBP vs. Sham.
DISCUSSION
This study demonstrates first and foremost that the effects of EBP are not explained solely by removal of common sepsis cytokines from the plasma space. CytoSorb, an adsorbent polymer, was found to attenuate late increases in inflammatory mediators (cytokines and HMGB-1), improve organ (liver and renal) function, and improve long-term (1 week) survival in this rodent model of CLP-induced sepsis despite not having significant effects on early cytokine levels. These results are important because current efforts to develop and test blood purification strategies for the treatment of sepsis are predicated on the removal of cytokines. Our results indicate that clinical effects of blood purification are not limited to and do not require short-term changes in circulating levels of the most commonly measured inflammatory cytokines. This finding has important implications for the design of clinical trials of EBP for treatment of patients with severe sepsis.
Despite failing to modulate cytokines acutely, our results are consistent with those of our previous studies in acute lethal sepsis models using endotoxin injection10 and CLP.12 However, this study is significantly closer to the clinical scenario because it was designed to evaluate long-term (1 week) survival in a model of sepsis that resulted in a mortality rate similar to that observed clinically. We also began therapy only after animals began to manifest sepsis as evidenced by clinical signs and inflammatory mediator levels. Finally, we used a smaller device (1 ml) in comparison with our previous studies. On the basis of body weight, this 1 ml device in a 500 g rat would translate to a 140 ml device in a 70 kg man, which is smaller than a standard hemodialysis filter.
We used CLP-induced sepsis to test our device because it resembles clinical sepsis21 where the infection spreads from a local focus to generalized septic shock.21–23 The peritonitis that ensues is polymicrobial (for example, Escherichia coli, Proteus mirabilis, Enterococcus, Bacteroides fragilis, and so on) and resembles human disease. Animals appear normal for ~10 h after CLP, and then begin to demonstrate a hyperdynamic, hyperinsulinemic, hypermetabolic state along with high blood lactate. Later, CLP-induced sepsis may become hypodynamic24 and the cytokine response is fully activated by 16–18 h. The mortality of CLP can also be adjusted by varying the length of the cecum ligation and the size and number of puncture sites.23–25 It was established that the percentage of cecum ligated was the principal determinant of mortality, with 90% mortality in 2 days if > 33% of the cecum was ligated with three punctures using a 20-gauge needle in our previous model.12,24 In this study, we ligated 25% of the cecum and punctured two times with a 20-gauge needle. In our experience, using older rats, this model resulted in a 50–60% mortality at 1 week with renal and liver injury. We chose IL-1β, TNF-α, IL-6, and IL-10 as the target markers, as these markers represent common pro- and anti-inflammatory mediators. These markers are easily detected in this model. Moreover, changes of these markers are related to the prognosis in septic patients.7 We started treatment at 18 h after CLP as the concentrations of most mediators reach peak levels and the ligated cecum begins to heal after 24 h.26
Given that the measured cytokine levels were not decreased acutely, the exact mechanisms by which the EBP resulted in late changes in these mediators, reduced organ injury, and improved survival remain unclear. In our previous studies, the improved survival after endotoxic injection10 and severe CLP12 with treatment using a larger adsorptive device were associated with the removal of inflammatory cytokines. However, associations cannot establish causality. In contrast, in this study, we were able to demonstrate clinical effects despite the absence of early cytokine changes, and therefore we can accept the null hypothesis that clinical effects are not dependent on altering cytokine levels. Similar to the effect of EBP on cytokines, changes in markers of organ function were not apparent until 24–48 h after treatment. This was not due to differential censoring as virtually no animals died before day 3. The finding suggests that the effect of EBP by this scaled-down device was not merely to remove cytokines for 4 h but to influence downstream events.
It must be emphasized that the overall net effect on survival in this study could be attributable to removal of other mediators besides those that we measured. Indeed, we cannot rule out simultaneous removal of different mediators, which could possibly prevent the formation of other biologically active substances, such as prostaglandins, leukotrienes, chemokines, or other cytokines, molecules that up- or down-regulate membrane receptors, selectins, and adhesion molecules.27 We have previously shown that EBP results in reduced NF-κB DNA binding,10 and thus attenuation of cytokines may have been because of reduced production, as NF-κB DNA binding is a key intermediary step leading to gene expression of several inflammatory cytokines, including TNF and IL-6. Furthermore, patients with sepsis not only face increased proinflammatory cytokines, but also exhibit leukocyte hyporesponsiveness to inflammatory stimuli. The general picture of the clinical disorder is therefore better characterized by an immune dysregulation than by a simple pro- or anti-inflammatory disorder.28 Ronco et al.29 have suggested a peak concentration hypothesis in which therapies may be effective if they can cut the peaks of the concentrations of both pro- and anti-inflammatory mediators, leading to the restoration of immune homeostasis. It has been reported that some blood purification techniques may regulate neutrophil function30 and improve monocyte function.31
To better elucidate the mechanism whereby EBP improved organ function and survival, we conducted a second set of experiments in which we performed exchange transfusions between EBP and sham-treated rats immediately after treatment. Our hypothesis was that benefits incurred by EBP would be transferred to sham-treated animals, whereas the ‘residual toxicity’ of the septic blood taken from sham-treated animals would be transferred to the EBP-treated animals. Our results were not as expected. Rather than ‘exchanging harm/benefit’, there was an ‘averaging’ of effect with a near matching of late IL-6 levels (Figure 5) and survival (Figure 6) between the two groups. We interpret these findings to indicate that cytokine modulation per se was only responsible for a portion of the benefit seen. In other words, exchanging blood between EBP and sham-treated animals resulted in plasma IL-6 concentrations only slightly higher than animals treated with EBP and not exchanged, yet survival was essentially midway between EBP and sham. Conversely, sham-treated animals given exchange transfusions with EBP-treated animals showed benefit in terms of IL-6 levels and survival compared with sham treatment alone. Taken together with the delayed effect on plasma cytokines, HMGB-1, ALT, and creatinine, these results suggest that EBP had effects other than cytokine modulation. We also carried out additional studies to determine whether direct effects on circulating immune effector cells are responsible for the effects on survival seen in this model and found that NF-κB activation in circulating PMNs was decreased with EBP. NF-κB DNA binding is a crucial first step in the release of multiple inflammatory cytokines.32 These results suggest that EBP can function at the level of the circulating immune effector cell and result in subsequent decreased cytokine production. We speculate that this effect may be due to changes in local cytokine concentrations within the device, which are not manifested systemically.
Although the current sets of experiments more closely represent human sepsis, there are important limitations. First, we did not administer antibiotics to the animals in this study because these therapies alter the inflammatory response and may well have obscured any ‘signal’. In addition, the administration of bactericidal antibiotics such as β-lactam drugs may promote the release of lipopolysaccharide from Gram-negative bacteria.33 Second, we recognize that rodent sepsis is only a crude model of human sepsis, and that multiple interventions effective in lower animals have not translated into treatments for humans.21
In summary, EBP with CytoSorb titrated to a level below which acute changes in cytokines are observed, and in a clinically realistic model of human sepsis, attenuated late inflammatory mediator activation induced by CLP sepsis and improved organ function and 1-week survival. Exchange transfusions between treated animals and shams transferred part of the benefit/harm. This study suggests that survival does not rely solely on changes in the usually measured circulating cytokines. Thus, further study is needed to better understand the mechanisms responsible for the effects of EBP in sepsis and clinical trials, including seeking alternative targets that need to be designed with these findings in mind.
MATERIALS AND METHODS
Cecal ligation and puncture
Following approval by the Animal Care and Use Committee of the University of Pittsburgh, we anesthetized 90 adult (24–28 weeks old, weight 450–550 g) male Sprague–Dawley rats with pentobarbital sodium (40 mg/kg intraperitoneally). Our CLP procedure was modified (25% ligated length of cecum and 20-gauge needle, two-puncture) in rats to induce less lethal sepsis compared with what we have described previously.12 The abdomen was closed and 20 ml/kg lactated ringers were given subcutaneously as fluid resuscitation. Topical anesthetic was applied to the surgical wound. Rats were returned back to their cages and allowed food and water ad libitum. At 18 h after CLP, the animals were re-anesthetized with pentobarbital sodium. The femoral vein and the internal jugular vein were isolated by dissection and cannulated with 1.27 mm polyethylene-90 tubing for use of extracorporeal circulation.
Experimental protocol
At 18 h after CLP, these animals were randomly assigned to receive either EBP (n = 30) or sham treatment (Sham, n = 30) for 4 h. The extracorporeal circulation was driven by a mini pump (Fisher Scientific, Pittsburgh, PA) from internal jugular vein to femoral vein at a blood flow rate of 0.8–1.0 ml/min. In the EBP group, the extracorporeal circulation passed through a 1 ml cartridge containing CytoSorb polymer beads (CytoSorbents). In the sham group, the blood was passed through tubing with the same dead space as the column. A volume of 20 unit/ml of heparin was used to prevent coagulation in this circuit. After a 4-h intervention, the treatment was stopped and the rats were observed for recovery, returned to the animal facility, and given access to food and water. Survival time was assessed up to 7 days. Another group (n =30) with the same CLP procedure but without extracorporeal circulation was used as the control.
To explore the mechanism of the EBP, we conducted a separate set of experiments involving another 40 animals. These animals were exposed to the same CLP procedure and were randomized to receive either EBP or sham treatment for 4 h just as before. At the end of treatment, however, an exchange transfusion was carried out between the EBP and sham-treated rats. This was accomplished by crossing the circulation and pumping blood from each animal to the other at a rate of 0.8–1.0 ml/min for 30–45 min. The maximum exchange (~50%) would be reached within this time period based on the following calculation: Cdonor = 0.5 × [1 +exp(−2Q/V × t)]; Crecep = 0.5 × [1−exp(−2Q/V × t)], where Cdonor and Crecep are the exchange percentage of donor and recipient, respectively, Q (ml/min) is the flow rate, V (ml) is the effective circulating volume, and t (min) is the exchange time. One animal died in the process of setting up the extracorporeal circulation, and thus this pair of animals was excluded. The remaining 38 animals were observed for 1 week.
An additional study was carried out to determine whether the direct effects of EBP on circulating immune effector cells could be demonstrated. A total of 14 rats (n = 7 each) were exposed to the same CLP procedure and were randomized to receive either EBP or sham treatment for 4 h. Blood was collected for NF-κB nuclear binding in circulatory PMNs and monocytes. Liver and kidney were harvested for histological observations.
Measurements and calculations
Blood (0.8 ml) was drawn from the femoral line at 18 h after CLP (immediately before treatment), after treatment, at 48 h, and at 72 h after CLP. Blood samples were also obtained immediately after exchange transfusion. The maximum blood loss for each animal was < 20% of total blood volume. We measured a panel of common plasma cytokines (TNF-α, IL-1β, IL-6, and IL-10), and HMGB-1, ALT, and creatinine.
Cytokines were measured with the multiplex bead-based Luminex assays (Invitrogen, Camarillo, CA). These assays are solid-phase protein assays that use spectrally encoded antibody-conjugated beads as the solid support.34 Our assays were performed in a 96-well plate format and analyzed with a Bio-Rad Bio-Plex 200 instrument, and the data were analyzed with the Bio-Plex Manager 4.0 software (Hercules, CA).
HMGB-1 was measured using an enzyme-linked immunosorbent assay (Shino-Test Corporation, Chiba, Japan, measurement range 0–80 ng/ml).35 ALT was determined using a LDH–NADH coupled assay (Pointe Scientific, Canton, MI). Plasma creatinine was measured with a creatinine enzymatic assay kit (BioVision Technologies, Mountain View, CA).
The NF-κB DNA binding in circulating PMNs and monocytes was measured with flow cytometry using a Cycletest Plus DNA Reagent Kit (Becton Dickinson, Franklin Lakes, NJ) and anti–NF-κB p65 antibodies and subtracting nonspecific binding using Isotype control antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Data were analyzed on the FCS Express software (De Novo Software, Los Angeles, CA).
Liver and kidney sections were fixed in 10% neutral-buffered formalin, dehydrated in graded anhydrous absolute ethanol, and embedded in paraffin. Histological sections (5 μm) were stained with hematoxylin, eosin, and periodic acid-Schiff.
Statistical analysis
All numerical data were expressed as mean ± standard error (s.e.m.). Normal distribution of the data was confirmed by visual inspection of result histograms, and all analyses were repeated after the data were natural log (ln) transformed. Our primary analysis was between CytoSorb and sham-treated animals and was based on survival time as assessed by Kaplan–Meier, and OS in each group was compared using Fisher’s exact (SPSS11, Chicago, IL). Plasma cytokine concentrations, HMGB-1, ALT, and creatinine were compared by examining the mean differences among and within groups and analyzed by analysis of variance for repeated measures. Data for NF-κB were expressed as median (ranges) and compared with the Wilcoxon rank test. P < 0.05 was considered to be of significant difference.
Acknowledgments
This study was supported by a grant from the National Heart Lung and Blood Institute (NHLBI) R01HL080926. The content is solely the responsibility of the authors and does not necessarily represent the official views of NHLBI, or the National Institutes of Health. CytoSorb polymer was generously provided by CytoSorbents.
Footnotes
DISCLOSURE
JAK is a paid consultant for CytoSorbents. All the other authors declared no competing interests.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2002;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–467. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 4.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 5.van der Poll T, Van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:413–426. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 6.Singer M. The key advance in the treatment of sepsis in the last 10 years… doing less. Crit Care. 2006;10:122. doi: 10.1186/cc4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellum JA, Kong L, Fink MP, et al. GenIMS Investigators. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the GenIMS study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkataraman R, Subramanian S, Kellum JA. Extracorporeal blood purification in severe sepsis. Crit Care. 2003;7:139–145. doi: 10.1186/cc1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo R, Kellum JA, Gandhi CR, et al. The effect of intensive plasma water exchange by hemofiltration on hemodynamics and soluble mediators in canine endotoxemia. Am J Respir Crit Care Med. 2000;161:1429–1436. doi: 10.1164/ajrccm.161.5.9809127. [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA, Song M, Venkataraman R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit Care Med. 2004;32:801–905. doi: 10.1097/01.ccm.0000114997.39857.69. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Kase H, Shimoyama O, et al. Effects of polymyxin B-immobilized fiber using a rat cecal ligation and perforation model. ASAIO J. 2009;55:246–250. doi: 10.1097/MAT.0b013e31819434ab. [DOI] [PubMed] [Google Scholar]
- 12.Peng ZY, Carter MJ, Kellum JA. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit Care Med. 2008;36:1573–1577. doi: 10.1097/CCM.0b013e318170b9a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honore PM, Jamez J, Wauthier M, et al. Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med. 2000;28:3581–3587. doi: 10.1097/00003246-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ratanarat R, Brendolan A, Piccinni P, et al. Pulse high-volume haemofiltration for treatment of severe sepsis: effects on hemodynamics and survival. Crit Care. 2005;9:R294–R302. doi: 10.1186/cc3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL, Laterre PF, Cohen J, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005;23:400–405. doi: 10.1097/01.shk.0000159930.87737.8a. [DOI] [PubMed] [Google Scholar]
- 16.Cole L, Bellomo R, Hart G, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30:100–106. doi: 10.1097/00003246-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37:803–810. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 18.Honoré PM, Joannes-Boyau O, Collin V, et al. Continuous hemofiltration in 2009: what is new for clinicians regarding pathophysiology, preferred technique and recommended dose? Blood Purif. 2009;28:135–143. doi: 10.1159/000227282. [DOI] [PubMed] [Google Scholar]
- 19.Ronco C, Brendolan A, Lonnemann G, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30:1250–1255. doi: 10.1097/00003246-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Kellum JA. Hemoadsorption therapy for sepsis syndromes. Crit Care Med. 2003;31:323–324. doi: 10.1097/00003246-200301000-00060. [DOI] [PubMed] [Google Scholar]
- 21.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 22.Neilson D, Kavanagh JP, Rao PN. Kinetics of circulating TNF alpha and TNF soluble receptors following surgery in a clinical model of sepsis. Cytokine. 1996;8:938–943. doi: 10.1006/cyto.1996.0126. [DOI] [PubMed] [Google Scholar]
- 23.Walley KR, Lukacs NW, Standiford TJ, et al. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard WJ, Choudhry M, Schwacha MG, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 25.Singleton KD, Wischmeyer PE. Distance of cecum ligated influences mortality, tumor necrosis factor-α and interleukin-6 expression following cecal ligation and puncture in the rat. Eur Surg Res. 2003;35:486–491. doi: 10.1159/000073387. [DOI] [PubMed] [Google Scholar]
- 26.Maier S, Traeger T, Entleutner M, et al. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock. 2004;21:505–511. doi: 10.1097/01.shk.0000126906.52367.dd. [DOI] [PubMed] [Google Scholar]
- 27.Peng Z, Singbartl K, Simon P, et al. Blood purification in sepsis: a new paradigm. Contrib Nephrol. 2010;165:322–328. doi: 10.1159/000313773. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, Bonello M, Bordoni V, et al. Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif. 2004;22:164–174. doi: 10.1159/000074937. [DOI] [PubMed] [Google Scholar]
- 30.Olsson J, Paulsson J, Dadfar E, et al. Monocyte and neutrophil chemotactic activity at the site of interstitial inflammation in patients on high-flux hemodialysis or hemodiafiltration. Blood Purif. 2009;28:47–52. doi: 10.1159/000210037. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Liu ZH, Chen ZH, et al. Improvement of monocyte function and immune homeostasis by high volume continuous venovenous hemofiltration in patients with severe acute pancreatitis. Int J Artif Organs. 2008;31:882–890. doi: 10.1177/039139880803101004. [DOI] [PubMed] [Google Scholar]
- 32.Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Leeson MC, Fujihara Y, Morrison DC. Evidence for lipopolysaccharide as the predominant proinflammatory mediator in supernatants of antibiotic-treated bacteria. Infect Immun. 1994;62:4975–4980. doi: 10.1128/iai.62.11.4975-4980.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szodoray P, Alex P, Brun JG, et al. Circulating cytokines in primary Sjogren’s syndrome determined by a multiplex cytokine array system. Scand J Immunol. 2004;59:592–599. doi: 10.1111/j.0300-9475.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamada S, Yakabe K, Ishii J, et al. New high mobility group box 1 assay system. Clin Chim Acta. 2006;372:173–178. doi: 10.1016/j.cca.2006.04.016. [DOI] [PubMed] [Google Scholar]