Abstract
Objective
SirT1 has been previously implicated in the regulation of human cartilage homeostasis and chondrocyte survival. Exposing human osteoarthritic chondrocytes to TNFα generates a stable and enzymatically inactive 75kDa form of SirT1 (75SirT1) via Cathepsin B-mediated cleavage. Because 75SirT1 is resistant to further degradation, we assumed it has a distinct role in osteoarthritis (OA) pathology, which we sought out to identify in this study.
Methods
OA and normal human chondrocytes were analyzed for the presence of Cathepsin B and 75SirT1. Confocal imaging of SirT1 monitored its subcellular trafficking following TNFα stimulation. Co-immunofluorescent staining was carried out for Cathepsin B, mitochondrial Cox IV and Lysosome-associated membrane protein I (LAMP-I) together with SirT1. Human chondrocyte were tested for apoptosis via FACS analysis and immunoblotting for caspase 3 and 8. Human chondrocyte mitochondrial extracts were obtained and analyzed for 75SirT1/Cytochrome C association.
Results
Confocal imaging and immunoblot analyses following TNFα challenge of human chondrocytes, demonstrated that 75SirT1 was exported to the cytoplasm and colocalized with the mitochondrial membrane. Consistently, immunoprecipitation and immunoblot analyses revealed that 75SirT1 is enriched in mitochondrial extracts and associates with Cytochrome C, following TNFα stimulation. Preventing nuclear export of 75SirT1 or reducing levels of FLSirT1 and 75SirT1 augmented chondrocyte apoptosis in the presence of TNFα Cathepsin B and 75SirT1 were elevated in OA vs. normal chondrocytes. Additional analyses shows that human chondrocytes exposed to OA-derived synovial fluid generate the 75SirT1 fragment.
Conclusion
These data suggest that 75SirT1 promotes chondrocyte survival following exposure to proinflammatory cytokines.
INTRODUCTION
Articular cartilage (AC) inflammation has often been attributed as a secondary affect of OA development, since increased matrix degradation occurs under these conditions (1–6). Specifically, synovial TNFα and IL-1β have been shown to drive matrix breakdown through their ability to upregulate cartilage catabolic enzymes as matrix metalloproteinases (MMPs) and downregulate anabolic matrices components (5,6,7).
The NAD-dependant protein deacetylase silent mating type information regulation 2 homolog 1 (SirT1) positively mediates the expression of major cartilage anabolic components (namely collagen 2α1 and aggrecan) and has been associated with OA pathology (8,9). Recent findings reveal that SirT1 is cleaved and inactivated under proinflammatory stimuli of TNFα or IL-1β, resulting in abrogated gene expression of collagen 2α1 and aggrecan, both of which are essential components of AC (10). Cathepsin B, which has been previously associated with OA pathology (11,12), was found to mediate site-specific cleavage of full-length SirT1 (FLSirT1) at a.a. 533 to generate an inactive 75kDa fragment (i.e. 75SirT1), in response to TNFα (10). Interestingly, several reports establish that SirT1 enzymatic activity is regulated post-translationally, implying that it may be affected by several intra- or extra-cellular signals that fine tune SirT1 function (13,14). That human chondrocytes remain viable following TNFα induction (10) may imply that the stable 75SirT1 fragment serves an alternate role in chondrocyte biology and OA pathology.
Human OA chondrocytes undergo extensive cell death in-vivo, due to increased exposure to proinflammatory cues (15,16). FLSirT1 has been shown to prevent chondrocyte apoptosis, which is consistent with its elevated levels in normal AC (8,17,18). In fact, Takayama et al., (2009), suggested that FLSirT1 possesses the capacity to modulate mitochondrial levels of Bax and Bcl2 in nitric oxide (NO)-induced apoptosis. Additional observations by Gagarina et al., (2010) established that the proapoptotic protein PTP1B is elevated in OA cartilage and that FLSirT1 is capable of downregulating its levels to achieve enhanced chondrocyte survival. Given that FLSirT1 is enzymatically active, it may attain an antiapoptotic effect in human chondrocytes (hCh) by facilitating gene expression or affecting the function of several soluble cellular proteins through deacetylation, as previously described for RelA/p65 and p53 (19,20).
Here we hypothesize that the stable, but enzymatically inactive 75SirT1 fragment promotes chondrocyte survival under proinflammatory stress, providing it with an alternative role as a longevity factor. This report monitors sequential changes in 75SirT1 subcellular localization, protein associations and chondrocyte apoptosis under proinflammatory stress.
MATERIALS & METHODS
Reagents, cell culture and transfections
Human AC and synovial fluid (SF) were obtained from the knee joints of OA patients undergoing total knee arthroplasty (average age 73, average BMI 31.4) and in accordance with the Hadassah Medical Center Institutional review board and based on international Helsinki ethical principles for medical research, involving human subjects. All participants provided a written informed consent, according to the Helsinki ethical regulations. Age-matched normal human AC samples were supplied by NDRI, Philadelphia, PA. Human chondrocyte were isolated and plated (passage 0 or 1) according to Derfoul et al., 2007 (21). TNFα (R&D, MN) was added where indicated at a final concentration of 50ng/mL and incubated for 24h, unless otherwise indicated.
OA hCh were treated with TNFα in the presence of enriched serum-free media BIO-MPM (Beit-Haemek, Israel). Transfection of hCh was carried out using FuGENE 6 (Roche, IN) in the presence of OPTI-MEM (Invitrogen, CA). SiRNA transfection was carried out using PepMute transfection reagent (SignaGen Laboratories, MD) according to manufacturers' instructions.
Plasmids, SiRNA and growth media supplements
PcDNA-Flag-His-hSirT1 was a kind gift of Prof. Danny Rienberg, University of New York. Caspase inhibitors, ALLN (10µg/mL), were purchased from Calbiochem (NJ). SB202190 (Sigma-Aldrich, Saint Louis, MO), was supplemented at final concentration of 1µg/mL as a MAPK p38 inhibitor. Leptomycin B (LepB) was purchased from Sigma-Aldrich (Saint Louis, MO) and supplemented at a final concentration of 10 nM for 3 hours prior to treatment with TNFα or control. SirT1 and control (CTL) SiRNA were purchased from Santa Cruz (CA) and used as instructed.
RT/PCR analysis
Real-time PCR were carried out using 10 ng of cDNA and Syber Green mix (ABI, CA), as previously described by Dvir-Ginzberg et al., (2011). Quantitative analyses were performed using StepOne Real-time PCR software. Primers for real-time were human Cathepsin B (F- CAA ACA GGA CAA GCA CTA CG; R- AGC AGG AA GTC CGA ATA CAC) and human GAPDH (F - CAA GGC TGA GAA CGG GAA GC; R- AGG GGG CAG AGA TGA TGA CC).
Protein analysis and immunoblotting
Crude protein extracts (i.e. whole cell extracts; WCE) and immunoblot procedures were carried out according to Perkines et al., 2005 (22). Mitochondrial extracts were obtained using the Mitochondria Isolation Kit (Pierce, Rockford, IL), according to manufacturers' guidelines. Nuclear and cytosolic extracts were obtained using the NE-PER kit (Thermo Scientific, Rockford, IL), according to manufacturers' guidelines.
Primary and secondary antibodies used for immunoblotting, immunoprecipitation, immunofluorescence and immunocytology are specified in Dvir-Ginzberg et al., 2011. Antibodies for Acetyl-CoA synthetases and CoA synthetases were kind gifts from Prof. John M. Denu from the University of Wisconsin.
Immunofluorescence (IF) and confocal microscopy
For IF analyses, hCh (p0) were cultured on a glass coverslip within 6-well plates, rinsed with PBS, fixed in 4% paraformaldehyde in PBS for 15 min, at room temperature (RT). Thereafter cells were treated with 0.2% Triton X-100 in PBS for an additional 15 min at RT. The coverslips were then incubated for 30 min at RT with primary antibody at a 1:100 dilution in blocking solution (1% BSA within PBS). Following three washes with PBS, coverslips were incubated for 30 min at RT with a secondary antibody at a 1:1000 dilution. Finally, samples were incubated with 4',6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, Saint Louis, MO) at a final concentration of 1 µg/mL in PBS. Slides were mounted using Vectashield HardSet Mounting Medium (Vector laboratories, Burlingame, CA) and visualized under a confocal microscope (Zeiss LSM 510 Meta, Peabody, MA) with adequate fluorescent emission/excitation parameters.
Immunohistochemistry (IHC)
Cartilage sections from explants were fixed in 4% paraformaldehyde, dehydrated using a graded series of ethanol washes, and embedded in paraffin. Sections of 5 µm in thickness were pre-digested with hyaluronidase (Sigma) in 10mM Tris-HCl, pH 7.5, for 30 min at 37°C, then incubated overnight at room temperature with specified antibodies in Tris buffered saline (TBS, pH 7.4) containing 0.1% BSA. Slides were visualized under a Leica DMR Microscope (Leica Microsystems, IL).
Enzyme-Linked Immunosorbent Assay (ELISA) for synovial TNFα
Synovial fluid from 9 different OA patients were obtained and centrifuged at 1500g, for 30min at 4°C. Supernatant were then aspirated and subject to TNFα detection via Human TNFα ELISA Ready-SET-Go (eBioscience, CA), according to the manufacturers instructions. Samples were normalized against a standard curve of pure human TNFα ranging from 4–250 pg/mL.
Fluorescence-activated cell sorter (FACS) analyses
FACS analyses were carried out using an MEBCYTO Apoptosis kit (MBL, MA), according to the manufacturer’s instruction. Briefly, following indicated human chondrocyte stimulation, supernatant and adherent cells were collected centrifuged (1000g, 5min) and washed twice with PBS. Thereafter, cells were adjusted to 1×106/mL and stained with 5µl fluorescein isothiocyanate (FITC)-labeled annexin V (Annexin-V) and 10 µl propidium iodide (PI) simultaneously. As positive control human chondrocytes were treated with 1 µM Doxorubicin (Sigma-Aldrich, Saint Louis, MO) for 72h, to induce apoptosis before FACS analyses. The data was collected and analyzed using FACScan and CellQuest software (both from Becton Dickinson and Co., Franklin Lakes, NJ).
Statistical Analysis
Samples were obtained from 3–8 different donors for each experiment, as indicated in the figure legends. For immunoblot and IF analyses each donor sample was twice repeated per experiment. Statistical analysis carried out using one-way analysis of variance (ANOVA), assuming confidence levels of 95% (p<0.05) to be statistically significant. Students T-test was carried out to determine the differences between two equivalent treatments within a group, assuming confidence levels of 95% (p<0.05). Error bars indicate the standard deviation around the mean value of data point. Immunoblots were analyzed for intensity using Image J software densitometry.
RESULTS
Cathepsin B is induced by TNFα and elevated in OA cartilage
Given that TNFα plays a role in OA extracellular matrix (ECM) degradation via increasing the expression of cartilage catabolic enzymes (5,6), it may possess a similar effect on Cathepsin B levels in OA cartilage (7,11,12). Augmented levels of Cathepsin B could not only affect cartilage ECM degradation (11,12), but also contribute to SirT1 inactivation and thereby lead to impaired cartilage-ECM gene expression (8,10).
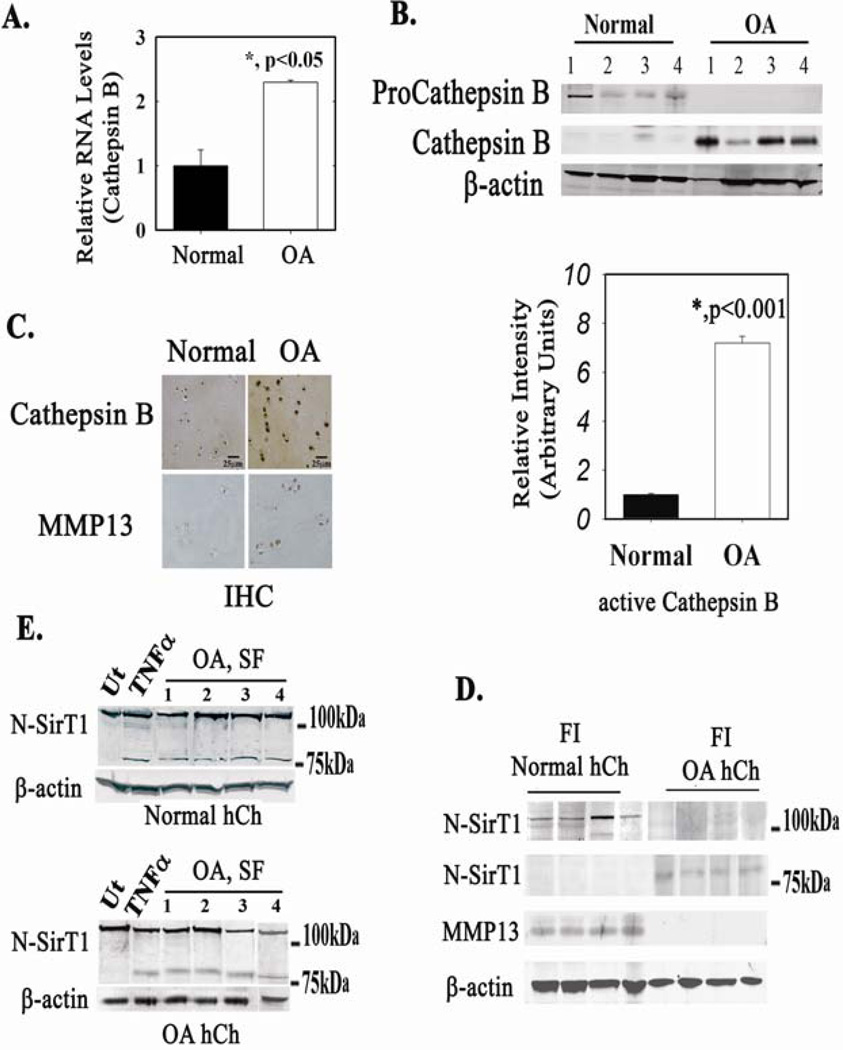
To determine if this is the case, RNA and protein levels of Cathepsin B were monitored in freshly isolated (FI) chondrocytes from human OA and normal AC knees (Figures 1A and 1B, respectively). As shown in Figures 1A and 1B, Cathepsin B RNA and active Cathepsin B (denoted as Cathepsin B) protein levels were significantly elevated in OA chondrocytes compared to normal chondrocytes. Consistently, immunohistochemical analyses exhibited elevated levels of active Cathepsin B in OA cartilage compared to normal cartilage (Figure 1C). Matrix metalloproteinase 13 (MMP13), a marker for accelerated joint destruction, also displayed increased levels in equivalent OA cartilage samples, consistent with previous reports (7,23). Next, protein extracts of normal and OA patients were immunoblotted with an N-terminally reactive SirT1 antibody (denoted N-SirT1, Figure 1D). While FLSirT1 is undetected in OA samples, 75SirT1 is notable (Figure 1D, right panel). On the other hand, normal FI chondrocytes display only FLSirT1 (Figure 1D, left panel). Previous reports support that the FLSirT1 is enhanced in normal vs. OA human chondrocytes (8,9). Incubating normal (Figure 1E, upper panel) and OA (Figure 1E, lower panel) hCh with synovial fluid derived from OA patients elicits the formation of 75SirT1 similar to TNFα-treated hCh of the same origin (Figure 1E). The fact that plated normal hCh are capable of generating 75SirT1, under the same conditions as OA hCh (Figure 1E), justifies that both hCh origins possess similar characteristics when in culture. Additionally, the data in Figure 1E, imply that synovial fluid from OA patients may consist of proinflammatory cytokines, in line with previous reports (5,24).
Figure 1. Osteoarthritic human chondrocytes display elevated levels of active Cathepsin B.
RNA (A) and protein (B) were obtained from freshly isolated (FI) human chondrocytes (hCh) derived from normal and osteoarthritic (OA) articular cartilage (AC). n=8 separate samples per normal and OA donors. C. IHC of OA and normal AC for Cathepsin B. Note elevated Cathepsin B in OA hCh and surrounding extracellular deposits as compared to normal AC. MMP13 IHC served as a marker for osteoarthritic AC in each equivalent sample. n=4 with 2 replicates per sample. D. Immunoblots of FI hCh from normal and OA cartilage were run with N-SirT1 antibody, n=8 separate samples per normal and OA donors. E. Normal (upper panel) and OA hCh (lower panel) were stimulated with TNFα and synovial fluid (SF, 10v% in BIO-MPM serum-free medium) derived from OA joints. Following 24h incubation, protein extracts were obtained and immunoblotted. Results in E, were repeated with three different origins of normal and OA hCh samples and four different SF samples from OA patients. "Ut" denotes untreated hCh and "TNFα" denotes TNFα treated (50 ng/mL, 24h) hCh. Immunoblots indicate 75 and 100 kDa protein ladder, on the right hand side.
To determine if TNFα also affects Cathepsin B expression in-vitro, OA hCh were analyzed for RNA and protein expression (SD-1A). As expected, RNA and protein levels of Cathepsin B were elevated following TNFα treatment, respectively. At the protein level, TNFα stimulation augmented both proCathepsin B and active Cathepsin B.
ELISA assay presented in Figure SD-1C displays an average level of 81.2±42 pg/mL human TNFα in synovial fluid of 9 different OA patients, which is consistent with previous reports (25). Additional in-vitro dose response data (SD-1D) reveal that 75SirT1 is generated at lower TNFα concentrations than 50 ng/mL (SD-1D). 75SirT1 is also enhanced in chondrocytes stimulated with physiological levels (i.e. approx. 100 pg/mL) of TNFα (SD-1E), indicating that in-vivo long-term cumulative effect of low TNFα concentration, may elicit OA pathology.
Previous reports indicate that Cathepsin B is released from the lysosomal compartment under TNFα stimuli (26–29). To determine if this is the case for OA hCh, Cathepsin B subcellular localization was monitored following TNFα stimulation (Figure 2A). Immunofluorescent confocal micrographs of active Cathepsin B show that following TNFα exposure, a fraction of active Cathepsin B is dispersed in the cytoplasm and nucleus (Figure 2A). In untreated hCh, active Cathepsin B is present solely within the lysosomes since it is completely colocalized with lysosomal-associated membrane marker I (LAMPI). Bioinformatic analyses of active Cathepsin B (AN# CAA77178) subcellular localization using PSORT II software (http://psort.hgc.jp/form2.html), revealed 13% of the enzyme may translocate to the nuclear compartment, while proCathepsin B (AN# AAH95408) is not predicted to undergo nuclear translocation.
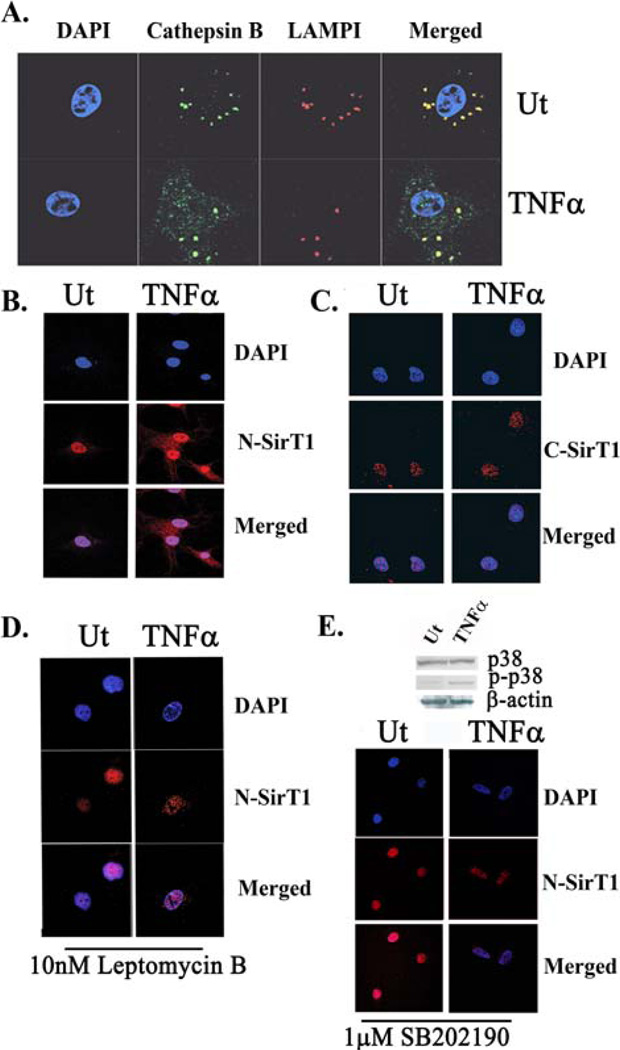
Figure 2. N-terminally intact SirT1 is exported to the cytoplasm in a MAPK p38 and CRM1-dependant fashion, following TNFα-treatment.
A. Confocal images of LAMPI (red fluorescence), active Cathepsin B (Cathepsin B; green fluorescence) and DAPI (blue fluorescence) TNFα treated (TNFα) and untreated (Ut) samples. B. Confocal images of hCh stained with an N-SirT1 antibody (red fluorescence). The nucleus was stained with DAPI, which appears in blue fluorescence. C. Confocal images of C- SirT1 (red fluorescence) and DAPI D. Human chondrocytes were treated with Leptomycin B (10nM LepB), a CRM1 (Exportin-1) inhibitor, for 3h prior TNFα treatment. The cells were then stained with an N-SirT1 antibody (red fluorescence) and DAPI (blue fluorescence). E. Immunoblots of MAPK p38 and phospho-p38 (upper panel) in untreated and TNFα-treated hCh. Lower panel exhibit hCh treated with SB202190 (1µg/mL), a MAPK p38 inhibitor, with and without TNFα stimulation. The samples were then stained with N-SirT1 antibody (red fluorescence) and DAPI. Results were repeated in duplicate samples of three separate OA hCh origins (n=3).
Supporting evidence shows that caspases are not involved in 75SirT1 generation and cellular targeting (SD-2), indicating that SirT1 cleavage is initiated in the nucleus through Cathepsin B site-specific cleavage (10).
75SirT1 is exported to the cytoplasm following TNFα stimulation
Several reports establish that SirT1 is capable of cytoplasmic translocation (30,31). Given that 75SirT1 is enzymatically inactive yet stable, we postulated that its presence in the nucleus may be impaired following TNFα exposure. To test this hypothesis, subcellular localization of the endogenous N-terminally intact SirT1 was monitored via confocal imaging. TNFα-treated and untreated OA hCh were stained with N-SirT1 antibody and visualized subcellularly (Figure 2B). The N-SirT1 antibody detects both FLSirT1 and 75SirT1, whereas the C-terminally reactive SirT1 antibody (C-SirT1) detects only FL-SirT1 (10). Results in Figure 2B reveal that approximately 50% of the N-terminally intact SirT1 (red fluorescence) is cytoplasmic following TNFα treatment (fluorescent intensity quantification throughout the cells axis is displayed in SD-3). On the other hand, confocal images using a C-SirT1 antibody revealed no changes in the location of the C-terminally intact FLSirT1 following TNFα stimulation (Figure 2C), implying that only the inactive cleaved SirT1 fragment (i.e. 75SirT1) is exported to the cytoplasm.
Tanno et al., (2007) established that CRM1 (Exportin 1) mediates SirT1 cytoplasmic export (31). To determine if CRM1 is also involved in TNFα-mediated export of 75SirT1, hCh were treated with Leptomycin B (Lep B), which blocks CRM1 transport activity. Confocal images established that using Lep B completely inhibited the export of cleaved SirT1, in OA hCh treated with TNFα (Figure 2D). Immunoblot analyses of OA hCh treated with TNFα and LepB, support that inhibiting CRM1-mediated export of 75SirT1 results in reduced 75SirT1 in the cytoplasmic extracts (CE), but did not affect the generation of 75SirT1 in nuclear extracts (NE) (SD-4A).
TNFα elicits two major intracellular pathways; one dependant on MAPK p38 and the other on JNK (32). To test if MAPK p38 or JNK facilitate TNFα-dependant cleavage and export of SirT1, we conducted immunoblot analyses for WCE of OA hCh treated and untreated with TNFα. Results revealed that MAPK p38 possesses increased phosphorylation (Figure 2E, upper panel), while JNK remains unaffected (SD-4B), following TNFα induction. Administering SB202190 (1µg/mL), an inhibitor of MAPK p38 activity, prevented SirT1 export (lower panel, Figure 2E), but had no effect on SirT1 cleavage (SD-4C).
Next, immunoblot analyses was carried out to verify whether the cytoplasmic SirT1 mainly consists of the cleaved 75SirT1 fragment (Figure 3A). Cytoplasmic and nuclear protein extracts (CE and NE; left and right panel, respectively) were obtained and immunoblotted with N-SirT1 and C-SirT1 antibodies. In line with confocal micrographs, immunoblots revealed that approximately 50% of the generated 75SirT1 is located in the cytoplasm following TNFα stimulation (Figure 3A, upper panel), whereas FLSirT1 is undetected in cytoplasmic extract under both conditions (Figure 3A, lower panel). Further time course experiments following 50 ng/mL TNFα exposure (0–48h), displayed increased levels of 75SirT1 in the cytoplasm, as a function of TNFα time exposure (Figure 3B). The CE blots show faint bands for FLSirT1 in short-term exposure (10min) to TNFα, possibly indicating that a minor portion of 75SirT1 may be generated via Cathepsin B-mediated cleavage of cytoplasmic FLSirT1. While CE display diminished FLSirT1 levels in longer exposure to TNFα, increased levels of 75SirT1 appear in CE (densitometry quantification in Figure 3B, lower panel). It appears from these data that the majority of 75SirT1 is generated via Cathepsin B-mediated cleavage of nuclear FLSirT1.
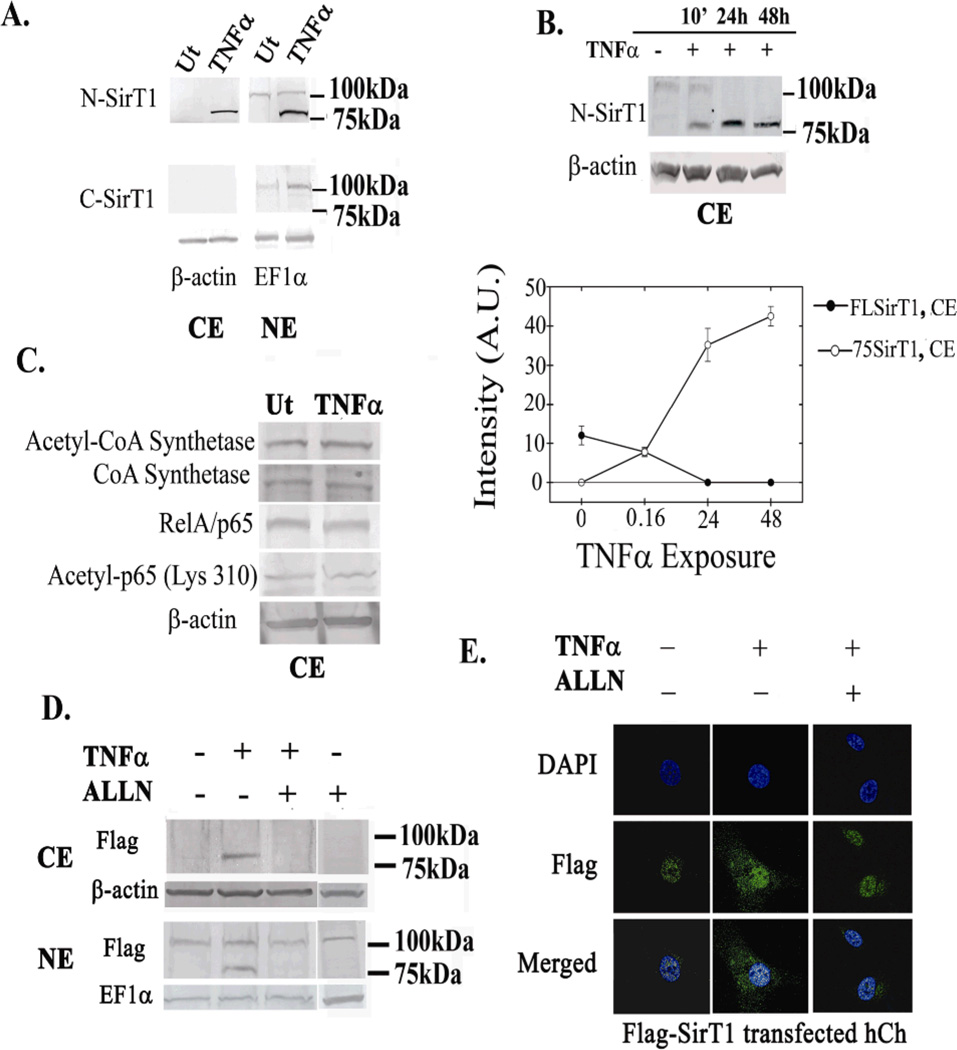
Figure 3. 75SirT1 fragment is exported to the cytoplasm upon TNFα stimulation.
A. Isolated CE and NE were immunoblotted for N-SirT1 (upper panel) and C-SirT1 antibody (lower panel). B. OA hCh are treated with 50 ng/mL TNFα for 10 min, 24h and 48h incubation, as indicated above the blot. CE were isolated and immunoblotted with N-SirT1 antibody. Lower panel indicated band intensity in arbitrary units (AU) of 75SirT1 (white circles) and FLSirT1 (black circles) as a function of TNFα time exposure. C. CE protein extracts were obtained and immunoblotted for cytoplasmic protein targets of SirT1 deacetylation. E. OA hCh were transfected with a SirT1 expression plasmid possessing a Flag tag on its N-terminal end (Flag-SirT1) and subsequently treated with TNFα and/or ALLN. Thereafter CE and NE were run for Flag antibody. F. Human OA chondrocytes were treated as indicated in E, stained for Flag antibody (green fluorescence) and DAPI (blue fluorescence) and inspected via confocal microscope. "Ut" denotes untreated hCh and "TNFα" denotes TNFα treated (50ng/mL, 24h) hCh. Immunoblots indicate 75 and 100 kDa protein ladder on the right hand side. Results were repeated in duplicate samples of three separate OA hCh origins (n=3).
To assess whether cytoplasmic 75SirT1 is enzymatically inactive, we monitored the acetylation of CoA synthetase and the p65 subunit of NFκB, which are both characterized as cytoplasmic protein target of SirT1 (33,19). Figure 3C presents undetectable acetylation changes in CoA synthetase and p65, in OA hCh treated or untreated with TNFα, supporting that the cytoplasmic 75SirT1 is indeed enzymatically inactive, as previously reported (10).
To further confirm that cleavage of SirT1 is necessary for its export to the cytoplasm, CE and NE protein extracts were obtained from OA hCh ectopically transfected for an N-terminally Flag-tagged SirT1 expression plasmid (Flag-SirT1) and treated with TNFα and ALLN, which is a known Cathepsin B inhibitor (Figure 3D). In both CE (Figure 3D, upper panel) and NE (Figure 3D, lower panel) ALLN inhibited the formation of 75SirT1, which is positive for Flag due to its intact N-termini. FLSirT1 was apparent only in NE and not in the CE immunoblots, consistent with previous reports (10). Figure 3E displays immunofluorescent confocal images of OA hCh transfected with Flag-SirT1 and treated with TNFα and ALLN. The images show that similar to untreated cells, 75SirT1 (immunofluorescently labeled in green for Flag) is not located in the cytoplasm in the presence of ALLN and TNFα. These results were also supported by confocal imaging of endogenous SirT1 stained with N-SirT1 antibody (SD-4D).
So far, our results support that FLSirT1 cleavage via Cathepsin B, is necessary for its export to the cytoplasm as an inactive 75SirT1 fragment. Inhibiting the activity of CRM1 and p38 in TNFα-induced OA hCh, does not prevent the generation of 75SirT1, rather restricts its export.
75SirT1 colocalizes with mitochondrial proteins following TNFα stimulation
Attempts were next made to determine if the cytoplasmic 75SirT1 was targeted to a particular organelle. Antibodies targeting specific organellar proteins of lysosome and mitochondria were used in co-immunofluorescent studies with the N-SirT1 antibody. While no change in LAMPI-SirT1 colocalization was found in TNFα stimulated OA hCh (SD-5), SirT1 did exhibit increased colocalization with the mitochondrial marker Cytochrome Oxidase IV (CoxIV) (Figure 4A), following TNFα treatment. This is evident by the yellow fluorescent mark produced by overlap of the red (N-SirT1) and green (Cox IV) fluorescence. Additionally, the data in Figure 4A reveal that the mitochondria have increased in size following TNFα treatment. This observation is consistent with previous reports describing enlarged mitochondria in different cell types following treatment with a variety of agents to induce stress response (34–37). Mitochondrial swelling is frequently reported as a morphological characteristic of Mitochondrial Permeability Transition (MPT), which is associated with Cytochrome C release under stress-inducing conditions (38).
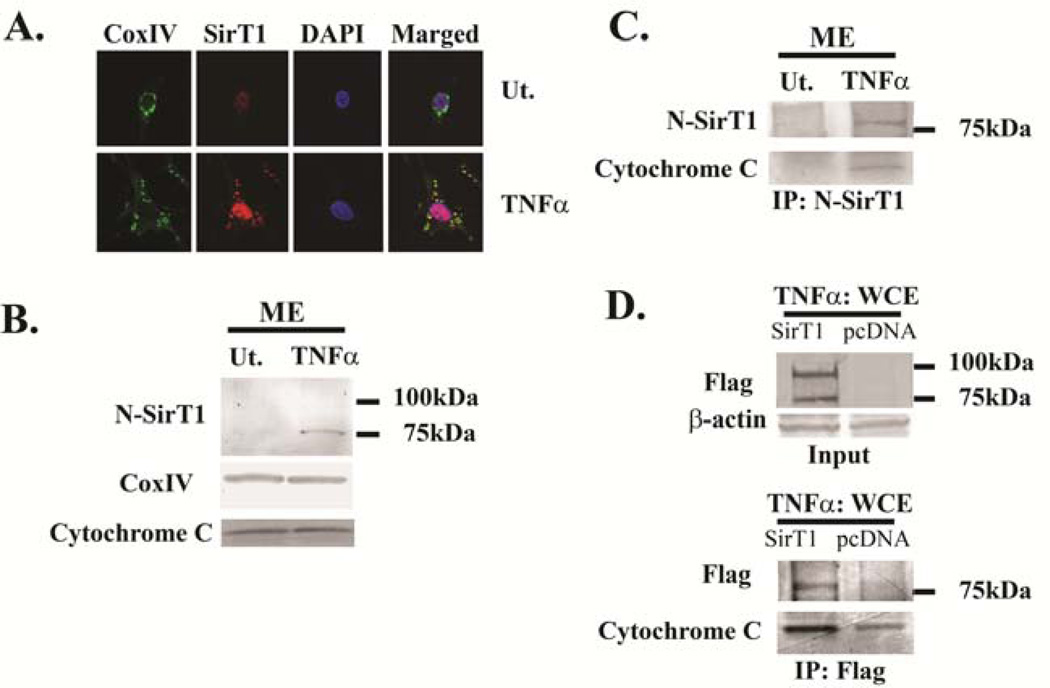
Figure 4. 75SirT1 colocalizes with the mitochondrial membrane and Cytochrome C following TNFα treatment.
OA hCh were treated with TNFα (TNFα) or untreated (Ut), as indicated. A. Confocal images stained with Cytochrome Oxidase Subunit IV (Cox IV; green fluorescence) and N-SirT1 (red fluorescence) antibodies display enhanced colocalization of cytoplamic 75SirT1 on the mitochondrial membrane, following TNFα stimuli. B. Mitochondrial protein extracts (ME) were immunoblotted for N-SirT1 (upper panel), CoxIV and Cytochrome C. C. MEs of TNFα-treated (TNFα) or untreated (Ut) OA hCh were obtained, immunoprecipitated with endogenous N-SirT1 and subsequently immunoblotted for both N-SirT1 and Cytochrome C antibodies. D. Human OA chondrocytes were transfected with Flag-SirT1 or pcDNA and treated with TNFα. Whole cell extract (WCE) inputs confirmed elevated SirT1 levels as compared to pcDNA derived extracts (upper panel). The lower panel of D displays Flag immunoprecipitants (IP:Flag) from TNFα-treated WCE, which were subsequently immunoblotted for Flag and Cytochrome C. Immunoblots indicate 75 and 100 kDa protein ladder on the right hand side. Results were repeated in duplicate samples of three separate OA hCh origins (n=3).
To confirm that the 75SirT1 was targeted to the mitochondria, mitochondria-enriched extracts (ME) were generated from the OA hCh. As shown in Figure 4B, 75SirT1, but not the FLSirT1, was present in ME following TNFα stimulation. Interestingly, overall levels of CoxIV and Cytochrome C in inputs from ME remain constant despite TNFα treatment.
Since TNFα-mediated apoptosis is often exerted by increased Cytochrome C release from the mitochondrial membrane, we sought out to evaluate a possible association between Cytochrome C and 75SirT1, which may block the formation of downstream apoptosome complex. MEs were immunoprecipitated with N-SirT1 antibody (Figure 4C) and immunoblotted for Cytochrome C. Pulling down the endogenous 75SirT1 in TNFα-treated ME, resulted in enriched protein levels of Cytochrome C (Figure 4C). In Figure 4D, OA hCh were ectopically transfected with Flag-SirT1 expression plasmid vs. pcDNA control and treated with TNFα (Figure 4D, upper panel). Following transfection whole cell extracts (WCE) were blotted for Flag and β-actin to validate input levels (Figure 4D, upper panel). Immunoprecipitating Flag from TNFα treated WCE confirmed that Cytochrome C possessed augmented association with 75SirT1 as compared to TNFα-treated pcDNA controls (Figure 4D, lower panel). The data support that 75SirT1 associates with Cytochrome C, possibly blocking its association with the apoptosome complex.
75SirT1 blocks apoptosis in TNFα treated OA hCh
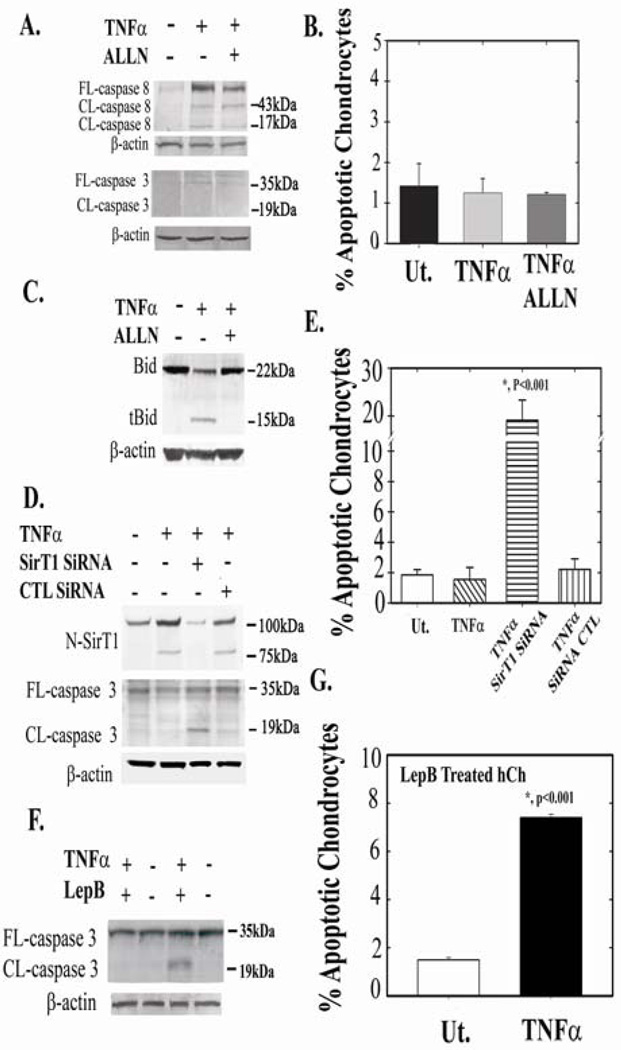
Based on the data accumulated so far, it appears that 75SirT1 associates with Cytochrome C on the mitochondrial membrane, thereby blocking downstream apoptosis via preventing apoptosome assembly and subsequent caspase 3 activation. Next, we investigated how downstream apoptotic events are affected by inhibiting the formation of 75SirT1, via ALLN, which inhibits Cathepsin B cleavage of FLSirT1 (see Figure 3D, ref#10). Although active caspase 8 is induced in TNFα treated cells (with and without ALLN), no detectable differences are observed for active caspase 3 (Figure 5A), in ALLN/TNFα treated OA hCh. Consistently, FACS results confirm that adding ALLN to TNFα-treated OA hCh does not induce apoptosis (Figure 5B). This is possibly the case since Cathepsin B, which is released from the lysosome following TNFα treatment (26), is capable of inducing Cytochrome C release from the mitochondria via cleavage and activation of Bid (39). Figure 5C shows truncated Bid (tBid) in TNFα treated chondrocytes, which is undetected when ALLN is added. Although, additional lysosomal proteases are targeted by the inhibitor ALLN, it is assumed that inhibiting Cathepsin B by ALLN, may deny tBid formation and thereby prevent downstream apoptosis. As further support, immunoblot analyses of freshly isolated OA vs. normal samples show augmented tBid in OA chondrocyte extracts, while normal samples display undetected tBid levels (SD-6).
Figure 5. 75SirT1 promotes human chondrocyte survival.
OA Human chondrocytes were treated as indicated above the immunoblots. A. Following treatment, whole cell extract (WCE) were immunoblotted for cleaved (CL) and full-length (FL) caspase 8 and 3. B. FACS analyses for apoptotic cell population was carried out using double staining for Annexin V and propidium iodide, for OA hCh treated as indicated in A. C. hCh were treated as indicated above the blot and run for Bid and tBid. D. Human chondrocytes were treated with SirT1 SiRNA, control (CTL) SiRNA, with or without TNFα (as indicated above the immunoblot). WCE were obtained and immunoblotted with N-SirT1 antibody, CL and FL caspase 3. E. FACS analyses for OA hCh in C. F. Human chondrocytes were treated with the CRM1 inhibitor, Leptomycin B (LepB; 10nM) and TNFα, as indicated above the immunoblot. WCE were immunoblotted for FL and CL caspase 3. G. FACS analyses for hCh treated as in E. FACS analyses were repeated twice in three different samples. Results determined significance according to t-test at p<0.001 confidence Immunoblots indicate 75 and 100 kDa protein ladder on the right hand side. Results were repeated in duplicate samples of three separate OA hCh origins (n=3).
To determine if 75SirT1 is capable of prohibiting Cytochrome C-mediated apoptosis, OA hCh were treated with SirT1 SiRNA in the presence of TNFα. Attenuating SirT1 levels (75SirT1 and FLSirT1) in the presence of TNFα induced apoptosis as judged by elevated cleaved caspase 3 and FACS analyses of Annexin V-positive cell population (Figure 5C and 5D, respectively). Control SiRNA treatments did not exhibit elevated apoptotic events as compared to TNFα and untreated cells.
Given that previous reports attributed antiapoptotic characteristics to FLSirT1 in hCh (17,18), we carried out further experiments to elucidate the possible attribute of 75SirT1 in preventing apoptosis in hCh. OA hCh were treated with LepB to inhibit nuclear export of 75SirT1 and subsequent 75SirT1-Cytochrome C association following TNFα treatment (as seen in Figure 2D). LepB/TNFα treatment increased levels of active caspase 3 (Figure 5E) and apoptosis (Figure 5F) 7-fold as compared to controls.
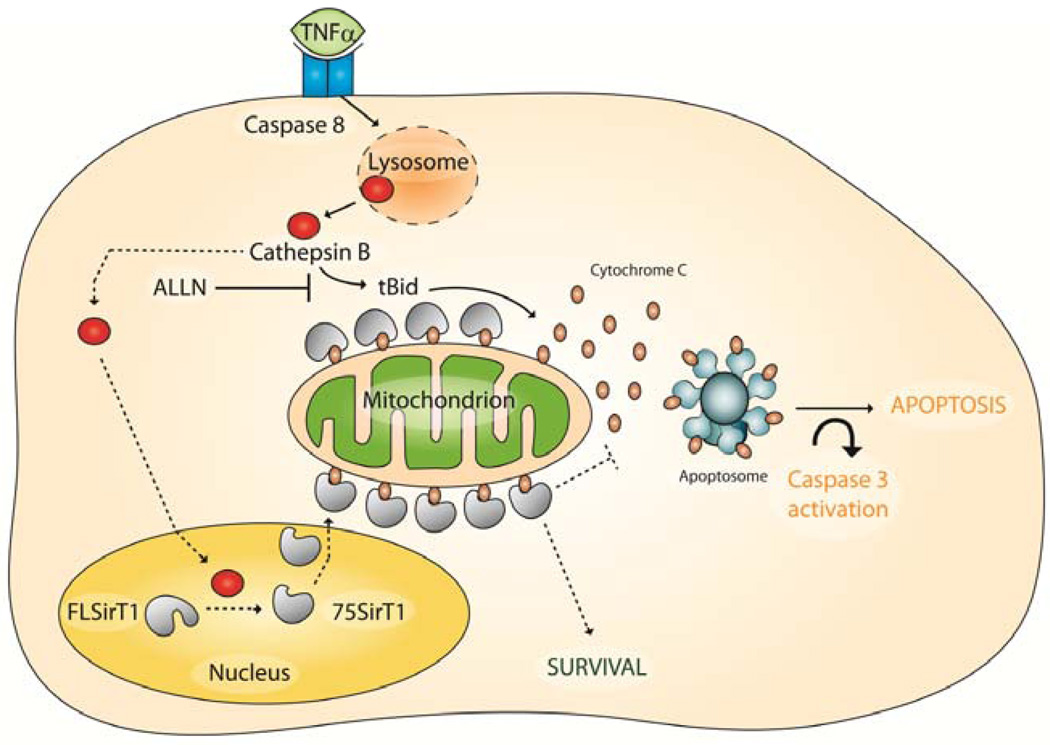
Overall, the data indicate that 75SirT1 prevents cell death through its enhanced association with Cytochrome C upon the mitochondrial membrane following hCh exposure to TNFα (illustrated in Figure 6). Our results support that 75SirT1 is capable of promoting cell survival through an enzymatically independent mechanism.
Figure 6. Predicted role of 75SirT1 in chondrocyte survival.
Following TNFα stimulation caspase 8 dependant lysosomal permeability occurs. Lysosomal permeability exerts augmented levels of Cathepsin B in the cell cytoplasm and nucleus. Active Cathepsin B cleaves nuclear FLSirT1 to generate an inactive stable 75SirT1 fragment, which is exported via CRM1 to the cytoplasm (broken arrows). While in the Cytoplasm 75SirT1 interacts with Cytochrome C on the mitochondrial membrane to block downstream apoptososme assembly (broken arrows). Solid black arrows indicate the TNFα-Cathepsin B pathways through Bid activation. ALLN will block both Bid cleavage and 75SirT1 generation. The model may be relevant in-vivo for articular chondrocytes prone to develop OA (see SD-6).
DISCUSSION
Our previous findings display SirT1 as a positive regulator of genes encoding AC structural components as collagen 2α1 and aggrecan (8). It is therefore likely that SirT1s activity is altered by external cues as proinflammatory cytokines (10), which are involved in OA pathology (5,6).
Numerous reports indicate that SirT1 exerts a broad anti-inflammatory effect in various tissues and pathologies (40–43). This may be partly due to SirT1s capacity to deacetylate the p65 subunit of NFκB, denying its transcriptional ability (19) and leading to decreased transcription of proinflammatory-responsive genes. Despite these reports, very little is known about the effect inflammation holds on SirT1, especially regarding OA pathology. A previous report determines that SirT1 is cleaved and inactivated in TNFα stimulated OA hCh by Cathepsin B (10). Based on these data, we further examine the role of 75SirT1 in OA pathology and chondrocyte biology.
Our results confirm that 75SirT1 is exported to the cytoplasm dependent on MAPK p38 and CRM1 (exportin-1). MAPK p38 dependant CRM1-export has also been established for E2F1, during keratinocyte differentiation (44). Interestingly, inhibiting CRM1 activity doesn't prevent SirT1 cleavage, suggesting that this cleavage event is independent of SirT1s export and involves Cathepsin B-responsive TNFα stimulation, as previously suggested (10). Cleaving SirT1 on its C-terminal domain (i.e. residue 533), possibly exposes several nuclear export signal (NES) spanning between a.a 293–517 (30), facilitating more efficient CRM1- dependant export.
Data presented in this study establishes that approximately half of 75SirT1 generated under TNFα stimulation is exported to the cytoplasm. More importantly, inhibiting 75SirT1 formation via ALLN prevented this export, indicating that 75SirT1 export is dependant on FLSirT1 cleavage by Cathepsin B. Cytoplasmic 75SirT1 colocalized with the mitochondrial membrane, implying it may play a role in cell survival. Consistent with these data, Cytochrome C was found to associate with endogenous and exogenous 75SirT1, possibly blocking Cytochrome C assembly with the apoptosome complex (45) and downstream caspase-mediated apoptosis (see suggested mechanism illustrated in Figure 6). In line with this proposed mechanism, Hou et al., (2010), show that under increased glucose conditions endothelial cells (EC) display enhanced levels of cytoplasmic SirT1 (46). Cytoplasmic SirT1 was shown to prevent apoptosis through the regulation of Cytochrome C release and modulation of mitochondrial permeability in EC (46).
Surprisingly, administering ALLN, did not induce apoptosis despite preventing the generation of 75SirT1. ALLN possibly extends cell survival via inhibiting Cathepsin B-mediated Bid cleavage, which has been suggested to be necessary for induction of TNFα-responsive apoptosis and Cytochrome C release (26,39). Our data with freshly isolated normal and OA samples support that tBid may be involved in early stages of OA development and chondrocyte death (SD-6). Treating chondrocytes with SirT1 SiRNA or LepB, reduced levels of cytoplasmic 75SirT1 in the presence of TNFα, and presented a significant induction of chondrocyte apoptosis. Despite these results, the enzymatically active FLSirT1 has been demonstrated to promote chondrocyte survival (17,18), which could also be the case in TNFα stimulation. From our results, it appears that 75SirT1 may also achieve enhanced chondrocyte survival through its association with mitochondrial Cytochrome C. Therefore, we conclude that both FLSirT1 and 75SirT1 may complement each other in promoting chondrocyte survival under proinflammatory stress.
That lysosomal Cathepsins regulate gene expression is a relatively novel notion (10,47,48), since such proteases have been considered enzymatically active at low pH (48). Yet, Cathepsin B is amongst the lysosomal proteases that maintains much of its enzymatic activity in neutral pH (39,49–51), which enables its activity outside the lysosomal compartment.
In consistence with our preliminary clinical evidence (Figure 1) Baici et al., (1995) found that OA possess elevated levels of active Cathepsin B as compared to normal cartilage (11). Our in-vitro results also establish Cathepsin B is elevated upon TNFα treatment. However, despite its devastating cumulative effect on cartilage ECM following Cathepsin B secretion, it also appears to indirectly regulate ECM gene expression through cleavage and inactivation of SirT1 (8,10). Although 75SirT1 enables short term cell survival under proinflammatory stress, it comes at the expense of FLSirT1-mediated cartilage ECM gene expression and may perpetuate OA pathology in long term proinflammatory exposure.
Finally, we conclude that SirT1 may serve a dual role in its capacity to protect chondrocytes from apoptotic death. In its full length form, it maintains deacetylase activity and regulates expression of various inflammatory-responsive genes (19,20), while its inactive cleaved form (i.e. 75SirT1), mediates survival through its capacity to associate with Cytochrome C and possibly block downstream apoptosome assembly.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Kristien Zaal and Evelyn Ralston (NIAMS Light Imaging section) for help with microscopy. The authors thank Prof. Danny Reinberg for the generous gift of pHisFlag-hSirT1 and Prof. John Denu for kindly providing the antibodies for acetylated-CoA Synthetase and CoA Synthetase. Dr David J. Hall (NIAMS, NIH) and Prof. Avner Yayon (Prochon Ltd) provided helpful professional input. Ms Anat Daskal coordinated the Institutional Helsinki application that enabled the use of human OA samples. Authors thank Ms. Olga Mizrahi for her assistance with FACS preparation and analyses.
This work was supported by the Marie Curie European IRG reintegration grant and Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Iannone F, Lapadula G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15:364–372. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- 2.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 3.Petersson IF, Jacobsson LT. Osteoarthritis of the peripheral joints. Best Pract Res Clin Rheumatol. 2002;16:741–760. doi: 10.1053/berh.2002.0266. [DOI] [PubMed] [Google Scholar]
- 4.Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26(3):371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 6.Furuzawa-Carballeda J, Macip-Rodríguez PM, Cabral AR. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 2008;26:554–560. [PubMed] [Google Scholar]
- 7.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Frontiers in Bioscience. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 8.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008;283:36300–36310. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita N, Matsushita T, Ishida K, Kubo S, Matsumoto T, Takayama K, Kurosaka M, Kuroda R. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res. 2011;29(4):511–515. doi: 10.1002/jor.21284. [DOI] [PubMed] [Google Scholar]
- 10.Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. TNFα-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis and Rheumatism. 2011;63(8):2363–2373. doi: 10.1002/art.30279. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baici A, Horler D, Lang A, Merlin C, Kissling R. Cathepsin B in osteoarthritis: zonal variation of enzyme activity in human femoral head cartilage. Annal. Rheum. Dis. 1995;54:281–288. doi: 10.1136/ard.54.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baici A, Lang A, Hörler D, Kissling R, Merlin C. Cathepsin B in osteoarthritis: cytochemical and histochemical analysis of human femoral head cartilage. Annal. Rheum. Dis. 1995;54:289–297. doi: 10.1136/ard.54.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3(12):e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondroyctes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Yatsugi N, Tsukazaki T, Osaki M, Koji T, Tamashita S, Shindo H. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. J Orthop Sci. 2000;5:150–156. doi: 10.1007/s007760050142. [DOI] [PubMed] [Google Scholar]
- 17.Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, Kurosaka M, Kuroda R. SirT1 regulation of apoptosis of human chondrocytes. Arth. & Rheum. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 18.Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010;62(5):1383–1392. doi: 10.1002/art.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 21.Derfoul A, Miyoshi AD, Freeman DE, Tuan RS. Glucosamine promotes chondrogenicphenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarth. & Cart. 2007;15:646–655. doi: 10.1016/j.joca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Perkins GL, Derfoul A, Ast A, Hall DJ. An inhibitor of the stretch-activated cation receptor exerts a potent effect on chondrocyte phenotype. Differentiation. 2005;73:199–211. doi: 10.1111/j.1432-0436.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, Clark IM. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res. Ther. 2006;8:R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2(6):459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 25.Manicourt DH, Poilvache P, Van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis and rheumatism. 2000;43(2):281–288. doi: 10.1002/1529-0131(200002)43:2<281::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Guicciardi EM, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-α–mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leist M, Jäättelä M. Triggering of apoptosis by Cathepsins. Cell Death Differ. 2001;8(4):324–326. doi: 10.1038/sj.cdd.4400859. [DOI] [PubMed] [Google Scholar]
- 28.Li JH, Pober JS. The cathepsin B death pathway contributes to TNF plus IFN-gamma-mediated human endothelial injury. J. Immunol. 2005;175:1858–1866. doi: 10.4049/jimmunol.175.3.1858. [DOI] [PubMed] [Google Scholar]
- 29.Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am J Physiol Gastrointest Liver Physiol. 2002;283(4):G947–G956. doi: 10.1152/ajpgi.00151.2002. [DOI] [PubMed] [Google Scholar]
- 30.Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. Journal of Cellular Physiology. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- 31.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 32.Russo M, Mupo A, Spagnuolo C, Russo GL. Exploring death receptor pathways as selective targets in cancer therapy. Biochem Pharmacol. 2010;80(5):674–682. doi: 10.1016/j.bcp.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103(27):10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiche J, Rouleau M, Gounon P, Brahimi-Horn MC, Pouysségur J, Mazure NM. Hypoxic enlarged mitochondria protect cancer cells from apoptotic stimuli. J Cell Physiol. 2010;222(3):648–657. doi: 10.1002/jcp.21984. [DOI] [PubMed] [Google Scholar]
- 35.Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209(2):468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- 36.Berliner JA. Formation of enlarged mitochondria in a liver cell line in response to a synthetic glucocorticoid. J Cell Biol. 1975;64(3):711–716. doi: 10.1083/jcb.64.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers AE, Bentley P, Fisher RL. Consequences of mitochondrial injury induced by pharmaceutical fatty acid oxidation inhibitors is characterized in human and rat liver slices. Toxicol In Vitro. 2006;20(7):1173–1182. doi: 10.1016/j.tiv.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Büki A, Okonkwo DO, Wang KKW, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. Journal of Neuroscience. 2000;20(8):2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cirman T, Oresić K, Mazovec GD, Turk V, Reed JC, Myers RM, Salvesen GS, Turk B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279(5):3578–3587. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58(2):344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HN, Li L, Gao P, Chen HZ, Zhang R, Wei YS, Liu DP, Liang CC. Involvement of the p65/RelA subunit of NF-kappaB in TNF-alpha-induced SIRT1 expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2010;397(3):569–575. doi: 10.1016/j.bbrc.2010.05.160. [DOI] [PubMed] [Google Scholar]
- 42.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Hagarkatti M, Nagarkatti PS. Resveratrol(trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharm. Exptl. Therap. 2010;332:829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10(4):640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- 44.Ivanova IA, Dagnino L. Activation of p38- and CRM1-dependent nuclear export promotes E2F1 degradation during keratinocyte differentiation. Oncogene. 2007;26:1147–1154. doi: 10.1038/sj.onc.1209894. [DOI] [PubMed] [Google Scholar]
- 45.Beer HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115(10):2633–2639. doi: 10.1172/JCI26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan EM, Allis CD. Errors in erasure: links between histone lysine methylation removal and disease. Prog Drug Res. 2011;67:69–90. doi: 10.1007/978-3-7643-8989-5_4. [DOI] [PubMed] [Google Scholar]
- 48.Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, Allis CD. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–294. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turk B, Turk V, Turk D. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem. 1997;378:141–150. [PubMed] [Google Scholar]
- 50.Hazen LG, Bleeker FE, Lauritzen B, Bahns S, Song J, Jonker A, Van Driel BE, Lyon H, Hansen U, Köhler A, Van Noorden CJ. Comparative localization of cathepsin B protein and activity in colorectal cancer. J Histochem Cytochem. 2000;48:1421–1430. doi: 10.1177/002215540004801012. [DOI] [PubMed] [Google Scholar]
- 51.Spiess E, Brüning A, Gack S, Ulbricht B, Spring H, Trefz G, Ebert W. Cathepsin B activity in human lung tumor cell lines: ultrastructural localization, pH sensitivity, and inhibitor status at the cellular level. J Histochem Cytochem. 1994;42:917–929. doi: 10.1177/42.7.8014475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.