Abstract
Cues that have been paired with food evoke dopamine in nucleus accumbens (NAc) and drive approach behavior. This cue-evoked dopamine signaling could contribute to overconsumption of food. One manner in which individuals try to restrict caloric intake is through the consumption of foods containing artificial (non-nutritive) sweeteners. We were interested in whether cues paired with a non-nutritive sweetener (saccharin) would evoke similar dopamine release as cues paired with a nutritive sweetener (sucrose). We trained food-restricted rats to associate distinct cues with sucrose or saccharin pellets. In the first group of rats, training sessions with each pellet took place on different days, maximizing the opportunity for rats to detect nutritional differences. After training, voltammetry recordings in NAc core revealed that sucrose cues evoked greater phasic dopamine release than saccharin cues. In a second group of rats, on each training day, sucrose and saccharin pellets were presented in pseudorandom order within the same session, to mask nutritional differences. In this condition, the difference in dopamine between sucrose and saccharin cues was attenuated, but not abolished. These results suggest that sucrose-paired cues will more powerfully motivate behavior than saccharin-paired cues. The differing responses to each cue seem to be driven by overall preference with both the nutritional value that the pellets predict as well as other factors, such as taste, contributing.
Keywords: phasic dopamine, nucleus accumbens core, artificial sweeteners, reward, rat
Introduction
The neurotransmitter dopamine is increasingly implicated in the pathology of obesity (Volkow et al., 2008a; Kenny, 2011) and recent studies have identified disruptions of the dopamine pathway in obese individuals (Stice et al., 2008; Volkow et al., 2008b). Dopamine is released during feeding and is essential for guiding behavior towards obtaining and consuming food (Zhou and Palmiter, 1995; Roitman et al., 2004; Kelley et al., 2005). Thus, dopamine can affect feeding at many levels. One important route through which dopamine might contribute to the etiology of obesity is via its involvement in attributing salience to initially neutral environmental stimuli. Specifically, if a cue reliably predicts a reward, such as food, the cue itself comes to evoke a phasic increase in the activity of dopamine neurons (Schultz, 1998). Such cue-evoked activity is observed as a transient increase in dopamine at terminal regions, particularly within the nucleus accumbens (NAc) core (Roitman et al., 2004; Day et al., 2007). Reward-predictive cues drive approach behavior and the magnitude of cue-evoked dopamine release positively correlates with approach behavior (Flagel et al., 2011). In addition, there is extensive work, in both humans and animals, showing that food-associated cues can increase motivation to obtain food, and in some cases consumption of food (Fedoroff et al., 1997; Ghitza et al., 2007; Ferriday and Brunstrom, 2010; Martín-García et al., 2011). Furthermore, dopamine seems to be critically involved in this process (Roitman et al., 2001; Nair et al., 2009). Thus, the factors that contribute to food- and cue-evoked phasic dopamine release could play a crucial role in feeding, over-consumption and obesity.
Although pairing cues with food has been demonstrated to result in the neurochemical and behavioral effects described above, there has been little work comparing qualitatively different food rewards, for example, those that differ in nutritional value. In particular, we were interested in how responses to artificial sweeteners might differ to sugar. Artificial (non-nutritive) sweeteners taste sweet but lack nutritional value. Although they tend to be perceived as far sweeter than natural sugars, they are often not preferred, which may be due to additional bitter or metallic components (Schiffman et al., 1979). They are widely used as dietary aids but their utility in treating obesity has been questioned (Bellisle and Drewnowski, 2007). We hypothesized that one complicating factor might be that they do not engage cue-evoked dopamine signaling as robustly as their nutritive counterparts, natural sugars. This is the first study in which phasic dopamine responses to cues predicting nutritive and non-nutritive sweeteners are compared.
Materials and Methods
Adult male Sprague-Dawley rats (325–375 g at the time of testing) were singly housed in standard conditions (temperature, 22°C; humidity, 30%; 12/12 h light/dark cycle with lights on at 7:00 a.m.). Prior to training and during recovery from surgery rats had ad libitum access to standard lab chow but during training/testing, rats were food-restricted to ~90–95% of their free-feeding weight (approx. 18 g chow/day). Water was available ad libitum. Sessions took place in custom-made behavioral chambers that allowed multiple pellets to be delivered to the same receptacle. Rats were trained with differently-flavored sucrose and 1.1% saccharin pellets (45 mg; grape and banana, counterbalanced; #s F06645/F06646/F06778/F06779; Bio-Serv, Frenchtown, NJ). A Pavlovian procedure was used in which delivery of each pellet (randomly selected inter-trial interval range: 30–90 s) was preceded by a distinct compound stimulus that occurred 3 s earlier (60 dB white noise or 50 dB tone), which turned off after 1 s, and left or right cue light, which remained lit until pellet delivery; see Figure 1b and 1e). Cues were counterbalanced across rats. Sessions lasted approximately 30 min. Pellets were either delivered on alternate days (30 pellets/session for 5 d with each pellet; Figure 1a) or on the same day (15 of each pellet/day for 10 d; Figure 1d). Following training, rats were surgically prepared for voltammetry recordings as described previously (Ebner et al., 2010). Briefly, under ketamine/xylazine anesthesia, rats were implanted with a guide cannula directed towards NAc core, a reference electrode in contralateral cortex, and a stimulating electrode in the midbrain. After 5–7 d recovery, rats were food-restricted again and subsequently two post-surgery training sessions (sucrose/saccharin) were run on separate days. These were identical to earlier sessions except rats were tethered to a commutator for habituation. 1–2 d following the final training session, dopamine, detected as current due to its oxidation and reduction, was recorded using fast-scan cyclic voltammetry while rats were presented with both types of cue/pellet in a pseudorandom order (10–30 trials with each cue/pellet; randomly selected inter-trial interval range: 30–90 s). During this session, a pre-calibrated carbon-fiber electrode was lowered into NAc core using a micromanipulator. Electrodes were pre-calibrated in a custom-made flow cell using 1 µM dopamine, diluted in aCSF, and yielded an average value of 23.9 ± 1.6 nA/µM. Dopamine concentration was extracted from current traces using principal component analysis (Heien et al., 2004; Keithley et al., 2010). 1–7 d after recording, a preference test was conducted in which rats were given 5 min to consume up to 20 sucrose and 20 saccharin pellets. Subsequently, rats were perfused and brains were sectioned for histology to confirm electrode placements. Pellets consumed during training were analyzed using repeated measures ANOVA. To examine event-related dopamine, mean concentration during several epochs (baseline: the 5 s before cue onset; cue: the 1 s following cue onset; pellet: the 1 s following pellet delivery; consumption: the 1 s following pellet epoch; see Figure 2A) was analyzed using a general linear model with epoch and trial type as within-subject factors. Post hoc Tukey tests were used where appropriate. The ratio of cue-evoked saccharin responses to sucrose responses was compared across training schedules using an unpaired t-test.
Figure 1.
Greater dopamine is evoked to sucrose-predictive cues than to saccharin-predictive cues. This difference is larger when rats are trained with pellets on alternate days (a–c) than on the same day (d–f). (a) Experimental timeline for rats trained with pellets on alternate days. (b) Top and center panel, averaged color plots showing current change (in color) across the applied voltages (Eapp; ordinate) over time (abscissa). Dopamine is evoked at the onset of the compound cue stimulus and is identified by its oxidation (green feature, ~0.6 V) and reduction (dark blue/yellow feature, ~−0.2 V) peaks. Bottom panel, dopamine concentration extracted using principal component analysis. Dashed lines show SEM. (c) Number of pellets eaten out of 30 during each training session. * P < 0.05 vs. sucrose on same day, post hoc Tukey test. (d) Experimental timeline for rats trained with pellets on the same day. (e) Averaged color plots and concentration traces showing dopamine evoked to each type of cue/pellet. (f) Number of pellets eaten out of 15 during each training session.
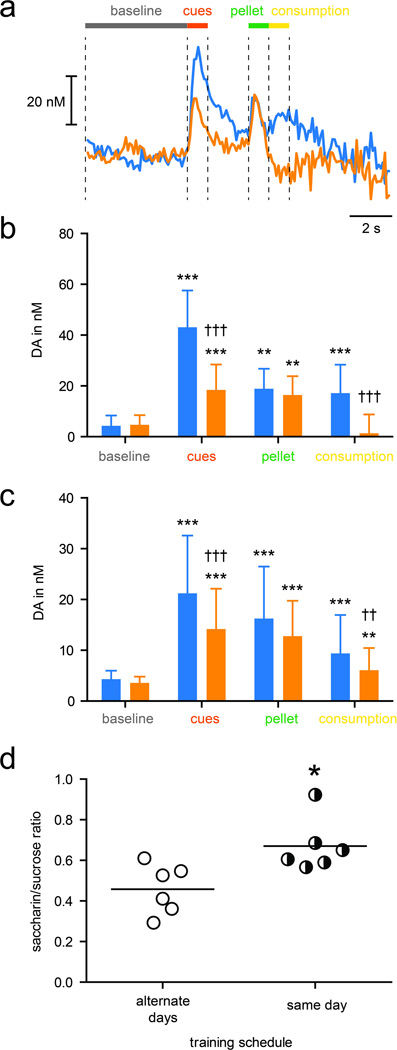
Figure 2.
Comparison of dopamine responses evoked in different behavioral epochs. Masking the nutritional disparity between sucrose and saccharin pellets reduces the difference between cue-evoked dopamine responses to each pellet. (a) Representative trace taken from one rat (alternate day training group) to show epochs analyzed in (b) and (c). Baseline (gray bar), 5 s before cue; Cue (red bar), 1 s after cue; Pellet (green bar), 1 s after pellet delivery; Consumption (yellow bar), 1 s after Pellet epoch. (b) Mean ± SEM dopamine concentration for each epoch described in (a) for rats that received alternate day training. ** P < 0.01, *** P < 0.001 vs. baseline; †† P < 0.01, ††† P < 0.001 vs. sucrose. (c) Mean ± SEM dopamine concentration for rats that received same day training. (d) Mean dopamine response to saccharin cues plotted as a ratio of the mean response to sucrose cues for each rat. Circles show values for individual rats and lines show mean for each training schedule. * P < 0.05.
Results
Food-restricted rats were presented with distinct cues that preceded delivery of either sucrose or saccharin pellets, on alternate days. As training progressed, rats ate an increasing number of the available pellets (Figure 1c; time effect: F(4,40) = 5.98, P < 0.001). For the first two sessions with each pellet, sucrose pellets were more readily eaten than saccharin, but this difference disappeared with experience (pellet effect: F(1,10) = 7.76, P = 0.019; post hoc results shown in Figure 1c).
After training, rats were surgically prepared for voltammetry recordings. During post-surgery training sessions, and throughout voltammetry recordings, all pellets of both types were consumed.
In voltammetry recording sessions, cues/pellets were presented in a pseudorandom order, while phasic dopamine was recorded in the NAc core. Both sucrose and saccharin cues evoked a sharp rise in dopamine concentration. However, the dopamine response to sucrose cues was greater than that to saccharin cues (Figure 1b and 2b; P < 0.001, n = 6). A second peak was also evident at a time corresponding to delivery of the pellet but this did not differ between sucrose and saccharin trials (P = 0.976, n = 6). Lastly, a third elevation in dopamine concentration was apparent during the time corresponding to pellet consumption, but this feature was only present in sucrose, not saccharin trials (P < 0.001, n = 6).
To determine whether the nutritional disparity between the two pellets could account for this result, we conducted a second experiment in which we masked nutritional differences between the pellets. To accomplish this, rather than receiving pellets on alternate days during training, rats were given both types of pellet within the same training session, in a pseudorandom order as during testing described above (Figure 1d). In this way, the maximal interval between a sucrose and saccharin pellet is <5 min (compared to 24 h in the previous study). Under this training schedule, rats still ate an increasing number of available pellets as training progressed (Figure 1f; time effect: F(9,90) = 5.67, P < 0.001), but the preference for sucrose during early sessions appeared blunted and was not significant (pellet effect: F(1,10) = 2.62, P = 0.137). During testing, sucrose cues still evoked greater dopamine release than saccharin cues (Figure 1e and 2c; P < 0.001, n = 6), but this difference too appeared attenuated compared with when rats were trained with pellets on alternate days. We assessed this difference by comparing the ratio of dopamine released to saccharin vs. sucrose cues and found a significant increase in this ratio attributable to training schedule (Figure 2d; P = 0.016, n = 6). Thus, training rats in a way that obscured rather than maximized the nutritional disparity between sucrose and saccharin pellets, altered both behavioral and neurochemical consequences.
2–7 d later, after ad libitum feeding, all rats showed a strong preference for sucrose over saccharin pellets, however, no difference was attributable to training schedule (alternate day: sucrose, 20.0 ± 0.0 vs. saccharin, 7.5 ± 3.3; same day: sucrose, 19.7 ± 0.3 vs. saccharin, 3.3 ± 1.0; pellet effect: F(1,20) = 68.87, P < 0.0001).
Discussion
Here, we show that dopamine responses in NAc core to food-predictive cues are strongly modulated by the food’s characteristics. We compared the non-nutritive sweetener, saccharin, with its nutritive counterpart, sucrose, and find that sucrose cues evoke greater phasic dopamine than saccharin cues. When the nutritional value of each pellet was masked during training, the differential cue response was reduced.
Cues that reliably predict rewards come to drive phasic dopamine neuronal activity (Schultz, 1998; Matsumoto and Hikosaka, 2009) and phasic dopamine release in the NAc core (Roitman et al., 2004; Day et al., 2007). These signals are known to vary as a function of reward size, probability, delay, and ambiguity (Tobler et al., 2005; Roesch et al., 2007; Bromberg-Martin and Hikosaka, 2009; Day et al., 2010; Gan et al., 2010). However, how dopamine responses to qualitatively different rewards vary has not been studied.
Here, the clearest result to emerge is that cues paired with the preferred reward – sucrose in all rats – evoked greater dopamine than cues that predicted non-preferred saccharin. Under both training schedules, all rats showed a clear preference for sucrose over saccharin, and correspondingly all rats exhibited greater dopamine responses to sucrose cues than to saccharin cues. Although this difference was blunted in rats in which the nutritional disparity between pellets was masked, it was not abolished. This suggests that the residual difference reflects dopamine encoding of cue value as it relates to preference. This nutrition-independent subjective preference could result from a difference in the orosensory qualities of the pellets or an aversion to other taste qualities in saccharin, such as its bitterness. Interestingly, such encoding of preference is observed in a population of orbitofrontal cortex neurons (Padoa-Schioppa and Assad, 2006) suggesting there might be a systems-level representation of subjective preference.
What is less clear is the extent to which the dopamine signal is sensitive to the nutritive status of the two sweeteners - sucrose and saccharin. In the second experiment, in which the nutritional disparity between pellets was masked, the difference between cue-evoked dopamine was lessened indicating that nutritional value may play a significant role in driving dopamine responses to food-paired cues, as suggested previously (de Araujo et al., 2008; Touzani et al., 2010). Since in these studies, as well as ours, conditioning is produced with relatively small caloric loads, the mechanism by which calories interact with reward circuitry is likely to be highly sensitive. Still, the post-ingestive signal responsible has yet to be elucidated.
An interesting feature of the data is that the main difference between the training schedules is the decrease of the dopamine response to sucrose cues between alternate day and same day training; the saccharin cue appears to be of a similar magnitude in both cases. One possibility is that an unpleasant aspect of the saccharin pellets, whether linked to nutritional value or not, is being generalized to the sucrose trials and leading to a reduction in the response to sucrose cues.
Finally, it is worth reiterating that all recordings here were made in NAc core; it is not known if dopamine responses in NAc shell distinguish between sucrose and saccharin cues under similar conditions. In NAc shell of naïve rats, intraoral infusions of both sucrose and saccharin increase dopamine by a similar magnitude (Roitman et al., 2008; Wheeler et al., 2011). Whether the response to saccharin would decrease with repeated experience, as rats in this paper received, or if differential dopamine would be evoked by cues paired with intraoral infusions of each substance remains to be tested. In general, dopamine responses in NAc shell are more readily evoked by primary stimuli than by cues suggesting that there would be a negligible NAc shell dopamine response for sucrose and saccharin cues in the paradigm we use here (Roitman et al., 2008; Aragona et al., 2009; Brown et al., in press; Wheeler et al., 2011) However, it is worth noting that some authors have observed strong cue responses in NAc shell (Owesson-White et al., 2009; Wanat et al., 2010). The apparent contradictions in these results could reflect a finer distinction between different areas within the shell or be a result of the different behavioral tasks used in each case.
In conclusion, cues predicting sucrose and saccharin are differentially encoded by dopamine, suggesting that sucrose cues will more powerfully motivate behavior. Understanding how food rewards are transduced by neural systems is an essential component for treating eating disorders, including obesity.
Acknowledgements
We wish to thank Eric Schmidt and Matt Schuck in the UIC Research Resources Center for their services and Jackson Cone and Heather Robbins for help with training rats and histology. Support for this work was provided by NIH R01 DA025634 to MFR.
References
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–126. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. doi: 10.1111/j.1460-9568.2011.07914.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry. 2010;68:306–309. doi: 10.1016/j.biopsych.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28:33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. ’i just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PEM. Dissociable cost and benefit encoding of future 11 rewards by mesolimbic dopamine. Nat Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide yy3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J Neurosci. 2007;27:11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fastscan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Keithley RB, Carelli RM, Wightman RM. Rank estimation and the multivariate analysis of in vivo fast-scan cyclic voltammetric data. Anal Chem. 2010;82:5541–5551. doi: 10.1021/ac100413t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-García E, Burokas A, Kostrzewa E, Gieryk A, Korostynski M, Ziolkowska B, Przewlocka B, Przewlocki R, Maldonado R. New operant model of reinstatement of food-seeking behavior in mice. Psychopharmacology (Berl) 2011;215:49–70. doi: 10.1007/s00213-010-2110-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, van Dijk G, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res. 2001;122:193–199. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Reilly DA, Clark T., 3rd Qualitative differences among sweeteners. Physiol Behav. 1979;23:1–9. doi: 10.1016/0031-9384(79)90113-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacol Biochem Behav. 2010;97:55–62. doi: 10.1016/j.pbb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Kuhnen CM, Phillips PEM. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]