Abstract
Cilia and flagella are highly conserved eukaryotic microtubule-based organelles that protrude from the surface of most mammalian cells. These structures require large protein complexes and motors for distal addition of tubulin and extension of the ciliary membrane. In order for ciliogenesis to occur, coordination of many processes must take place. An intricate concert of cell cycle regulation, vesicular trafficking, and ciliary extension must all play out with accurate timing to produce a cilium. Here, we review the stages of ciliogenesis as well as regulation of the length of the assembled cilium. Regulation of ciliogenesis during cell cycle progression centers on centrioles, from which cilia extend upon maturation into basal bodies. Centriole maturation involves a shift from roles in cell division to cilium nucleation via migration to the cell surface and docking at the plasma membrane. Docking is dependent on a variety of proteinaceous structures, termed distal appendages, acquired by the mother centriole. Ciliary elongation by the process of intraflagellar transport (IFT) ensues. Direct modification of ciliary structures, as well as modulation of signal transduction pathways, play a role in maintenance of the cilium. All of these stages are tightly regulated to produce a cilium of the right size at the right time. Finally, we discuss the implications of abnormal ciliogenesis and ciliary length control in human disease as well as some open questions.
Keywords: Length control, Intraflagellar transport, Ciliopathies, Ciliary signaling, Pharmacology
1. Cilium structure and function
Cilia are microtubule-based organelles that protrude from nearly all human cells. Notable exceptions are epithelia lining the gastrointestinal tract, non-ciliated Clara cells found in the bronchioles, and T lymphocytes. Cilia contain nine sets of microtubule doublets surrounded by a phospholipid membrane. This membrane is topologically continuous with the plasma membrane surrounding the remainder of the cell, but distinct in its lipid and protein composition. A variety of informative reviews are available that include detailed descriptions of ciliary structure (Carvalho-Santos et al., 2011; Ishikawa and Marshall, 2011; Rohatgi and Snell, 2010). The cylindrical doublets are extensions of the A and B tubules of the anchoring basal body triplets. Basal bodies are centriole-derived structures that migrate and dock to the cell surface when they are not being utilized during cell division in the spindle apparatus. Within the human body, most cells have a type of cilium known as a primary cilium. In cell types with many cilia per cell, such as in the airway, additional cilia form and are motile. Typically, primary cilia lack the additional central pair of microtubule singlets, dynein arms, and radial spokes required for motility. The cilia and flagella discussed here are essentially the same structure and the two terms will be used interchangeably.
Generation of cilia, termed ciliogenesis, occurs in several stages. First, cells must exit the mitotic cycle to free centrioles for axoneme nucleation. Centrioles are called basal bodies upon addition of distal appendages and docking to a ciliary vesicle that fuses with the plasma membrane. Finally, extension of the ciliary axoneme and membrane is mediated by a process termed intraflagellar transport (IFT). This process involves the bidirectional transport of microtubule motors and associated protein complexes, known as IFT, proteins and is discussed in several excellent reviews (Pedersen and Rosenbaum, 2008; Silverman and Leroux, 2009). Section 2 will highlight each stage of ciliogenesis separately.
As one might expect, the increased surface area of the cell produced by cilium extension is ideal for sensing the extracellular environment. The major function of primary cilia is to sense a variety of external stimuli including flow, ligands, and light. Motile cilia are capable of generating flow to clear mucus over tracheal epithelial cells in the airway, move cerebrospinal fluid over ependymal cells in brain ventricles, and transport eggs over the epithelial cells of the female reproductive tract. This is not to say that cilia without a central pair of microtubules cannot move, as is the case in nodal cilia, which are required for leftward flow of morphogens and left-right asymmetry determination (Nonaka et al., 1998). Motile cilia can also perform sensory functions, as is the case in airway epithelial cilia, which are also chemosensory (Shah et al., 2009). In addition to the sensory and motile functions of cilia, there is increasing evidence that the presence of cilia antagonizes cell cycle progression (reviewed in (Kim and Tsiokas, 2011; Pan and Snell, 2007; Plotnikova et al., 2008). This is due, in part, to the shared requirement for basal bodies/centrioles in ciliogenesis and cell division. Due to the essential functions performed by cilia on virtually all cell types, defects in ciliary structure and function result in a variety of diseases and syndromes termed ciliopathies. Cilia on each cell type have a normal length range and even slight deviations outside of this range are often sufficient to generate pathogenic phenotypes. Ciliopathies related to abnormal ciliogenesis and cilia length are discussed in greater detail in section 4.
2. Cilium formation
2.1. Deciding when to make a cilium: cell cycle regulation and ciliogenesis
Cilia are found on quiescent cells and on proliferating cells in the G1 phase of the cell cycle. In dividing cells, they are resorbed before S phase or during G2. There appears to be a bidirectional crosstalk between cilium formation and cell division as improper division can result in abnormal ciliogenesis and failure to form a cilium can regulate the cell cycle. For example, overproliferative cancer cell lines generally lack cilia and cells that cannot properly form a cilium undergo inappropriate cell division and cystogenesis, as is the case for IFT defects that cause cystic kidneys. Identification of a variety of intracellular signaling pathways in these processes has provided some clues about the molecular mechanisms coupling ciliogenesis and the cell cycle.
One class of cilium-related proteins regulating the cell cycle includes mediators of axoneme disassembly. Loss of a centrosome protein, Nde1, which interacts with a cytoplasmic dynein subunit LC8, causes increased cilia length and delays cell cycle re-entry (Kim et al., 2011), whereas phosphorylation of Tctex-1, another cytoplasmic dynein light chain, induces ciliary resorption and promotes S phase entry (Li et al., 2011). In addition to dynein subunits, aurora kinases are essential for disassembly of cilia and flagella. Loss of the aurora-like kinase CALK prevents flagellar disassembly in Chlamydomonas (Pan et al., 2004) and CALK phosphorylation state is a marker of the absence of flagella, as well as flagellar length (Luo et al., 2011; Pan et al., 2004). CALK is a distant relative of the human Aurora A kinase, which was found to be both necessary and sufficient for cilia disassembly in hTERT-RPE1 cells and regulates mitotic entry via spindle organization (Pugacheva et al., 2007). Other key regulators of cilium disassembly are ubiquitination and methylation. In Chlamydomonas flagella, there is an increase in ubiquitination of flagellar proteins including tubulin, dynein subunit IC2, and several signaling proteins during resorption (Huang et al., 2009). Similarly, several axonemal proteins were found to be differentially methylated during flagellar resorption (Schneider et al., 2008).
In addition to impaired ciliary disassembly, defects in ciliary axoneme elongation caused by loss of IFT proteins also result in a variety of cell cycle progression defects. For example, loss of IFT27 results in cell cycle elongation and impaired cytokinesis (Qin et al., 2007). Additionally, loss of IFT88 has recently been shown to be required for proper spindle orientation during mitosis in a variety of systems (Delaval et al., 2011) and was previously shown to result in overproliferative kidney cysts in mouse (Yoder et al., 2002). Tuning of timing of cilium formation, resorption and length appears to be essential for cell cycle regulation and blocking cancer phenotypes.
One contributor to ciliopathy-associated cystogenesis may be aneuploidy, a common pathogenic phenotype seen in cancer cells (reviewed by (Davoli and de Lange, 2011; Ganem et al., 2007)). Impaired ciliogenesis has indeed been shown to result in premature chromosome separation and aneuploidy in vertebrates (Chen et al., 2011a; Miyamoto et al., 2011). It is also conceivable that excessively long cilia or cilia with impaired resorption can delay chromosome separation and result in abnormal ploidy. For example, in mouse models for juvenile cystic kidney (jck) disease with mutations in Nek8, renal cilia are excessively long (Sohara et al., 2008) and expression of mutant Nek8 results in multinucleated cells (Bowers and Boylan, 2004; Liu et al., 2002; Trapp et al., 2008).
NIMA(never in mitosis)-related kinases (NRKs or NEKs), like Nek8, are involved in cell cycle control (reviewed in (O’Regan et al., 2007)). Several NRKs have been shown to have dual roles in cilium regulation and mitotic progression. For example, in Chlamydomonas, cells can release their flagella by microtubule severing in response to stress. This process, termed deflagellation or flagellar autotomy, is mediated by an NRK, Fa2p, which is important for timing of the G2/M transition (Mahjoub et al., 2002). Microtubule severing is also triggered by flagellar resorption prior to mitosis (Parker et al., 2010; Parker and Quarmby, 2003). Another NRK/NEK, Cnk2p, promotes flagellar resorption and progression through mitosis by decreasing the cell size required for mitotic commitment (Bradley and Quarmby, 2005). Finally, Nek1 can directly alter centrosome stability and regulate both ciliogenesis and cell cycle progression (White and Quarmby, 2008).
Several proteins, previously identified as cell cycle modulators, have now been shown to influence ciliogenesis as well. Among them is Cdc14b, an antagonist of Cdk1, which was found to be essential for proper ciliogenesis and ciliary length regulation in zebrafish (Clement et al., 2011). Additionally, the spindle checkpoint regulator BubR1 was found to be required for proper primary cilium formation in fish and humans (Miyamoto et al., 2011).
It appears that ciliogenesis can sequester the basal body to inhibit cell cycle progression, improper cell cycle progression can prevent timely cilium formation, and proteins like the NRKs can play a role in each of these processes by regulating centrosome and cilium structure.
2.2. Centrosome maturation and membrane docking
In dividing cells, centrosomes can act to generate the mitotic spindle as well as perform other functions in cell cycle progression. Each centrosome contains a mother and a daughter centriole. For ciliogenesis to occur, the mother centriole acquires a variety of distal and subdistal appendages. The distal appendages, containing Cep164 protein (Graser et al., 2007), are thought to correspond to the transition fibers and alar sheets seen by electron microscopy (Anderson, 1972). This distal region of the mother centriole interacts with a post-golgi vesicle which flattens upon ciliary extension by the mother centriole and fuses with the plasma membrane (Sorokin, 1962). Vesicular transport to the centrosome occurs in a series of steps, including the recruitment to the pericentriolar endosomal recycling compartment (ERC) of the guanine nucleotide exchange factor (GEF) Rabin8, by Rab11 GTPase (Knodler et al., 2010). This involves members of the TRAPPII complex (Westlake et al., 2011). A seven protein complex associated with a pleiotropic ciliopathy, the BBsome, interacts with both Rabin8 at the centrosome and membrane structures. GTP loading on Rab8a by Rabin8 appear to promote docking and fusion of vesicles to the base of the ciliary membrane and promote ciliogenesis (Nachury et al., 2007). The localization of Rab8a to the centrosome depends on trafficking by the sorting nexin, SNX10, and the V-ATPase proton pump (Chen et al., 2011b). Another GTPase-GEF pair critical for ciliogenesis is Cdc42-Tuba. Cdc42 interacts with the exocyst complex and is required for its localization to the primary cilium (Zuo et al., 2011). Centriole docking itself requires many other centrosomal proteins including Talpid3 (Yin et al., 2009), Chibby (Love et al., 2010), Ofd1 (Singla et al., 2010), Odf2 (Ishikawa et al., 2005), and a variety of proteins associated with the Meckel-Gruber syndrome and Nephronophthisis ciliopathies that reside in the transition zone distal to the basal body (Williams et al., 2011).
In the last decade, there has been accumulating evidence implicating the role of actin dynamics in regulating ciliogenesis and ciliary length. In many cases it appears that cortical actin provides a scaffold hospitable to ciliogenesis, while cytoplasmic actin stress fibers inhibit the formation of cilia. Disruption of the F-actin network with cytochalasin D, which preferentially disrupts stress fibers, in cultured mammalian cells resulted in cilium elongation (Bershteyn et al., 2010; Kim et al., 2010; Sharma et al., 2011). There is some disagreement about whether Cytochalasin B (Friedland-Little et al.) preferentially disrupts stress fiber or cortical actin (Burgoyne and Cheek, 1987; Letourneau et al., 1987) and this may be cell type specific. CB treatment in primary dermal cells resulted in ciliary elongation and resistance to induced ciliary disassembly (Bershteyn et al., 2010). In zebrafish, loss of apical actin organization resulted in reduced cilia number and length in Kupffer’s vesicle and left-right asymmetry (Oishi et al., 2006). In endothelial cells, increased cAMP, PKA activation and PKC activation increase cilia length and cause a redistribution of actin from stress fibers to the cell cortex (Abdul-Majeed et al., 2011). In these cases, disruption of stress fibers or redistribution of actin to an apical network appear to stabilize cilia or inhibit disassembly.
While cortical actin appears to stabilize existing cilia, basal body docking and positioning in the cell cortex is also influenced by the distribution of cytoplasmic and apical actin networks. Cytoplasmic actin stress fibers appear to interfere with basal body migration while cortical actin can stabilize and organize basal body orientation and positioning. In human RPE1 cells, contractile actin bundles in spatially extended cells can alter the nucleus-centrosome axis and prevent ciliogenesis compared to spatially confined cells (Pitaval et al., 2010). Loss of the protein meckelin, which impairs cilium formation and interacts with an actin maintenance scaffolding protein, Nesprin-2, has also been shown to dramatically redistribute actin to cytoplasmic stress fibers (Dawe et al., 2009). Another protein, p160ROCK, is a rho-GTP binding protein which regulates actin organization, localizes to the pericentriolar matrix of the mother centriole in HeLa cells and is required for centriole positioning (Chevrier et al., 2002). The role of actin in also seen in multiciliated cells. In these cells, basal body positioning and orientation is important for coordinated beating of cilia. Interestingly, Werner et al. showed that in the multiciliated epithelium of Xenopus laevis embryos, two populations of cortical actin exist. This includes an actin meshwork in the same plane as the basal bodies and a slightly subapical network ~0.5 μM below the surface of the cell which links the basal body of one cilium to the tip of the rootlet of the adjacent cilium. Direct perturbations of the actin cytoskeleton using a concentration of cytochalasin D sufficient to disrupt only the subapical network impairs proper basal body spacing across the surface of individual cells without altering local ciliary orientation (Werner et al., 2011). A previous report of Xenopus morphants of PCP effectors inturned and fuzzy showed that a less dense apical actin network resulted in altered ciliary orientation (Park et al., 2006). In mouse primary culture airway epithelial cells, timely activation of RhoA (actin remodeling GTPase and a downstream component of the PCP pathway) is required for apical actin web formation, basal body docking and ciliogenesis (Pan et al., 2007).
Actin-binding proteins that regulate dynamics may provide important clues on how cilium formation and length may be influenced by actin network redistribution. A positive regulator of the Shh pathway localized to the basal body, MIM, promotes ciliogenesis by antagonizing cortactin phosphorylation (Bershteyn et al., 2010). Active cortactin can promote actin polymerization and branching. In a functional screen performed in human retinal pigment epithelium (htRPE) cells to identify regulators of ciliogenesis and ciliary length by RNAi, several other modifiers of actin dynamics were identified (Kim et al., 2010). Loss of ACTR3, a component of ARP2/3, which promotes polymerization at actin branches, caused an increase in ciliary length. Depletion of actin severing proteins GSN and AVIL decreased cilia numbers. Knockdown of a focal adhesion protein, α-PARVIN, which regulates actin dynamics, also lengthened cilia. Phosphorylation of α-PARVIN, also known as actopaxin, was previously shown to impair stress fiber formation (Clarke et al., 2004; Nikolopoulos and Turner, 2000) and may regulate ciliogenesis by regulation of stress fiber-plasma membrane association. These observations suggest that branched actin networks inhibit ciliogenesis and restrict cilium length. Actin severing can significantly remodel the actin network but it is unclear which resulting organization promotes ciliogenesis in this case. Gelsolin can sever filaments and cap the barbed ends inhibiting further polymerization, however, once uncapped, many more barbed ends are available for polymerization into a new network (Sun et al., 1999). A careful characterization of actin network organization when disrupting actin-binding proteins will be critical to understanding how changes in cilium regulation might take place.
It should come as no surprise that the apical actin network can affect docking of basal bodies and formation of cilia on the apical surface of cells. However, the nature of the interaction and specific molecular interfaces remain obscure. Some clues may be provided by the many ciliary signaling pathways that are now being uncovered (see section 3.3). Virtually every identified pathway regulating ciliogenesis and ciliary length can involve downstream interaction or modification of the actin network or actin binding proteins. An important advancement was the association of signaling- and actin-mediated regulation of cilium length with levels of soluble tubulin (Sharma et al., 2011). However, much more work is needed to begin to understand the actin-mediated regulation of a largely microtubule-based organelle.
2.3. Intraflagellar transport and cilium assembly/disassembly
The final step of ciliogenesis is the actual building of the axoneme by molecular motors and associated proteins by assembling tubulin at distal growing ends in a process termed intraflagellar transport (IFT). IFT was first described by Kozminski and others as bidirectional movement of “granule-like” particles along the axoneme of the flagellar axoneme in Chlamydomonas (Kozminski et al., 1993). Around the same time, a novel heterotrimeric kinesin was isolated from sea urchin embryos (Cole et al., 1993). Later, IFT was found to be dependent upon a kinesin motor, FLA10 (kinesin-II in mammals) (Kozminski et al., 1995) and FLA10 identified as a subunit of the heterotrimeric kinesin (Cole et al., 1998). Kinesin-II, a member of the kinesin-2 family, was also found to be involved in the assembly of motile cilia containing the central microtubule pair (Morris and Scholey, 1997). The IFT particles were originally found to consist of 15 polypeptides (now thought to be ≥20) in two complexes, A and B (Cole et al., 1998). Complex B is kinesin-II associated and is thought to contribute to anterograde transport for axoneme assembly (Cole et al., 1998), and complex A, dependent on dynein-2, is involved in retrograde transport to the ciliary base (Pazour et al., 1999; Pazour et al., 1998) for recycling of IFT components. The IFT proteins contain many protein-protein interaction motifs that are presumably used for cargo specification. In Chlamydomonas, IFT proteins bind flagellar proteins, suggesting a mode of cargo transport by direct binding (Qin et al., 2004), and in C. elegans, it has been directly demonstrated that tubulin is transported by IFT to the tips of cilia (Hao et al., 2011). Proteins destined for the cilium are produced in the cell body and may be recruited by an IFT protein, IFT52, localized to distal transition fibers (Deane et al., 2001). Other microtubule-binding motors play important roles in cilia regulation. In C. elegans, a second anterograde member of the kinesin-2 family, homodimeric Osm-3 (Kif17 in mammals) was identified. Osm-3 travels on distal microtubule singlets of cilia and moves at a faster rate (Snow et al., 2004). This motor works in concert with the slower kinesin-II for the intermediate transport rate seen in wild-type cilia (Pan et al., 2006). While distal microtubule singlets are known to exist in mammalian systems and C. elegans is an excellent model that has greatly informed the field on essential IFT mechanisms, its cilia are quite divergent. A kinesin in the kinesin-3 family, Klp-6, was found to regulate IFT in male-specific cilia (Morsci and Barr, 2011) in C. elegans. This motor did not require the hetrotrimeric or homodimeric kinesins for its movement and slowed the transport of the other two kinesins. While triple mutants of all three kinesins showed complete loss of cilia in most cases, occasionally some ciliary projections remained, leaving a possibility of yet another mechanism of ciliary elongation.
IFT regulates continuous tubulin turnover at the distal ends of the axoneme, which suggests a model for regulating ciliary length by balancing assembly and disassembly (Marshall et al., 2005; Marshall and Rosenbaum, 2001). The total amount IFT protein in flagella is independent of length (Marshall et al., 2005), but the number of IFT trains are not (Dentler, 2005). The size and number of IFT trains in flagella are inversely proportional to the length of flagella (Engel et al., 2009). A model for ciliary length control based on these observations is discussed in section 3.1.
The post-translational modification state of tubulin can also alter ciliary stability and motility. Stabilization of axonemal microtubules involves and acetylation (L’Hernault and Rosenbaum, 1985a, b) and detyrosination (Stephens, 1992). Polyglutamylation (Kubo et al., 2010; Suryavanshi et al., 2010) and polyglycylation (Redeker et al., 1994; Xia et al., 2000) also regulate assembly and motility of cilia. Recently, an axoneme tubulin acetyltransferase, αTAT1/MEC-17, was identified (Akella et al., 2010; Shida et al., 2010). Cilia disassembly has been shown to involve tubulin deacetylation (Pugacheva et al., 2007) ciliary protein methylation (Schneider et al., 2008) and ubiquitination (Huang et al., 2009).
As described in section 2.1, cilia are resorbed prior to cell division. The active axoneme disassembly rate is higher than the basal turnover rate (Pan and Snell, 2005) and is associated with higher IFT particle entry into the ciliary compartment. Disassembly of cilia is thought to be length independent, unlike assembly (Rosenbaum and Child, 1967), and can take place without active anterograde IFT (Marshall and Rosenbaum, 2001). Loss of anterograde IFT also induced microtubule severing in the presence of calcium and resorption was slowed in severing mutants indicating that microtubule severing pathways are involved in signaling or promoting disassembly (Parker and Quarmby, 2003). In Leishmania major and Giardia intestinalis, a microtubule depolymerizing kinesin, Kinesin-13, promotes flagellar shortening (Blaineau et al., 2007; Dawson et al., 2007). In Chlamydomonas, Kinesin-13 is transported into flagella during induced disassembly in an IFT-dependent manner (Piao et al., 2009). Another microtubule depolymerizing motor homologous to Kinesin-13, Kif24, acts at centrioles and restricts cilia extension as its loss promotes ciliogenesis (Kobayashi et al., 2011).
Several other ciliary and basal body components appear to regulate ciliogenesis. For example depletion of the microtubule tip tracking protein EB1, and a related protein EB3, which are both localized to the basal body, causes a significant reduction in the number of cilia in mammalian cell lines (Schroder et al., 2011). In these cells, cilia stumps are no longer anchored to the basal bodies. A significant loss of cilia was also seen when depleting the cells of an EB1 binding partner p150Glued.
Ciliogenesis requires enormous coordination of cell cycle regulatory signaling, cytoplasmic vesicular transport, and recruitment of all required IFT materials with proper stoichiometry. As disruption of genes involved in any step of this process can result in dramatic pathogenic phenotypes (see section 4), it is truly remarkable that this coordination is so robust. There has been very little evidence identifying redundant pathways that might contribute to this robustness. It has been shown that mutational load can contribute to the emergence of ciliopathic phenotypes (Williams et al., 2010). Functional overlap of the very large protein complexes, such as those associated with nephronophthisis and meckel syndrome (see section 4) can provide some redundancy (Williams et al., 2008).
3. Cilium maintenance
3.1. Ciliary length control models
In order to understand the factors that give rise to a steady state ciliary length and what perturbations may break that homeostasis, it is a useful exercise to consider various models of length control that are consistent with existing data and make predictions about untested hypotheses. The simplest model would be that a cell simply produces the exact quantity of flagellar precursor proteins to build a flagellum or a certain length (Fig. 1A). However we know that the availability of cytoplasmic precursors is not limiting because in experiments where protein synthesis is blocked with cyclohexamide and flagellar severing is subsequently induced, resulting in loss of all exisiting axonemal proteins, the flagella regenerate to up to half of normal length (Rosenbaum et al., 1969). Moreover, the weak dependence of flagellar length on the number of flagella per cell is not consistent with such a limiting precursor model (Kuchka and Jarvik, 1982; Marshall et al., 2005).
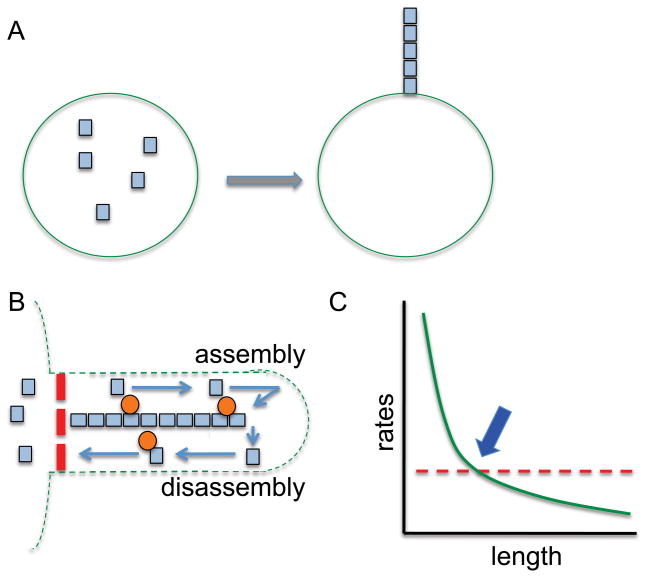
Figure 1. Limiting-precursor and balance-point models for length control.
(A) Limiting Precursor model, in which a fixed number of structural subunits (blue squares) contained with the cell (green circle) are incorporated into the final structure thus fixing its length by their initial quantity. (B) Axonemal tubulin dynamics underlying balance-point model. Tubulin subunits (blue squares) are synthesized in the cytoplasm and transported out to the tip of the cilium by intraflagellar transport (orange circles) where they assemble at the tip. Assembly at the tip is balanced by continuous disassembly of subunits from the tip, which are then returned to the cytoplasm. A putative pore or gate regulating entry of ciliary proteins and IFT proteins is indicated by a red dotted line. In this framework, steady-state length is achieved when the rates of assembly and disassembly are equal. (C) Balance point model for length control. Disassembly (red dotted line) is length-independent based on measurements of flagellar resorbtion in the absence of assembly. Assembly is a decreasing function of length because each IFT particle takes longer to move out to the tip as the length increases, and thus delivers cargo less efficiently. Because the number of IFT particles is fixed, independent of length, the overall efficiency of IFT is a decreasing function of length (green curve). The unique length where the two rates balance (blue arrow) is predicted to be the steady-state length of the cilium.
One alternative scheme is the balance point model proposed by Marshall (Marshall et al., 2005; Marshall and Rosenbaum, 2001), which is based on a few key observations from experiments in Chlamydomonas flagella (Fig. 1B). First, flagellar assembly during regeneration slows as the flagellum elongates (Marshall, 2002; Rosenbaum and Child, 1967). Second, when IFT is inhibited by a temperature sensitive mutation in the anterograde motor, flagella begin to shorten, suggesting that tubulin at flagellar tips is constantly being turned over (Marshall, 2002; Song and Dentler, 2001; Stephens, 1997). Finally, the disassembly that takes place during that shortening and induced resorption is constant (length independent) (Kozminski et al., 1995; Marshall et al., 2005; Marshall and Rosenbaum, 2001; Parker and Quarmby, 2003). The balance point model postulates that at the intersection of the decreasing assembly rate and the constant disassembly rate is the length set point (Fig. 1C). At axoneme lengths longer than this set point, the disassembly rate would exceed the assembly rate and the flagella would shorten. At lengths shorter than the set point, the assembly rate would predominate and the flagella would lengthen. The length-dependent assembly rate is now thought to arise from a fixed amount of IFT protein being packaged into smaller IFT trains (with a smaller tubulin carrying capacity) as the length of the flagellum increases (Engel et al., 2009). The length independence of the total IFT protein content can be explained if the same cycling IFT proteins must be retrieved from the flagellar tip in order to form new trains. This retrieval for individual particles would take longer as the flagellar length increases, decreasing the amount of IFT protein available for redeployment at any given time. However, there is currently no direct evidence for such a restriction of IFT protein exchange, and so we must consider how the quantity of IFT protein could be held constant if IFT proteins are constantly moving in an out of the flagellum. Moreover, a simple model in which an initial bolus of IFT protein is confined within the flagellum does not explain the remodeling of trains as a function of length. It therefore seems likely that the flagellum will employ some sort of length sensing or measuring mechanism to adjust the quantity of IFT particle proteins that are imported into the flagellum.
Some candidates have been implicated in regulating this type of sensing mechanism. Recent evidence has shown the critical function of the Cep290 protein. Cep290 is a Meckel syndrome associated protein that is located at the transition zone and appears to regulate levels of IFT complexes and ciliary entry of membrane associated proteins (Craige et al., 2010). Cep290 appears to be involved in membrane attachment to the transition zone. The gating activity of Cep290 may be regulated by its associated protein CP110. CP110 restricts cilia formation and requires the interaction with CEP290 for this function (Tsang et al., 2008). One model of ciliary entry based on import of the Kif17 ciliary motor involves the shuttling of cilium-targeted proteins by the nuclear import protein, importin β2 (Dishinger et al., 2010). In this model, cargo is released into the ciliary compartment due to displacement on importin by GTP-bound Ran GTPase. An enrichment of RanGTP in the cilium is thought to give rise to ciliary import. Other proteins thought to regulate ciliary gating are the septins, which were shown to form a membrane diffusion barrier (Hu et al., 2010). Recently, the idea of a septin diffusion barrier has been challenged by the proposal that proteins are excluded from the cilium by anchoring to the cortical actin cytoskeleton (Francis et al., 2011).
Several findings must be addressed in order to further evaluate the balance point model and alternative models involving feedback mechanisms. The following two sections highlight recent identification of modulators of flagellar length that involve direct modification of IFT or axoneme stability (Section 3.2) and signaling mechanisms that can induce ciliary length changes (Section 3.3).
3.2. Length regulation related to axoneme modification
Because of recent explosion interest in regulation of ciliogenesis and ciliary length, a great deal more is known about proteins essential for maintenance. Some of these proteins modify mechanics of IFT, thereby axonemal assembly and elongation. Others directly alter microtubule stability by altering post-translational modification state. In addition to the mechanisms that can directly alter axoneme formation or stability, a great many signaling pathways can also alter the percentage of ciliated cells in a population or alter ciliary length (see section 3.3) by unknown mechanisms. It is possible that the pathways regulating direct axoneme assembly involve yet unidentified downstream effectors of altered signaling. A summary of ciliary length altering proteins can be found in Table 1.
Table 1.
Cilium Length Altering Proteins
One class of proteins known to directly regulate axoneme structure includes both cilia specific and cilia non-specific microtubule motors. Anterograde motors such as the members of the Kinesin-2 and -3 family described in section 2.3 are required for axoneme formation and result in shortened or absent cilia in mice (Takeda et al., 1999), C. elegans (Cole et al., 1998; Morsci and Barr, 2011; Perkins et al., 1986; Snow et al., 2004) and Chlamydomonas (Cole et al., 1998; Kozminski et al., 1995) among others when disrupted. As discussed previously, in Leishmania and Giardia, microtubule depolymerizing Kinesin-13 promotes flagellar disassembly (Blaineau et al., 2007; Dawson et al., 2007), but this kinesin appears to be required for proper flagellar regeneration as well in Chlamydomonas (Piao et al., 2009). In addition to kinesins, dyneins also play a role in ciliary length regulation. A light chain of cytoplasmic dynein, Tctex-1, is responsible for restricting cilia length. Loss of this subunit results in increased cilia length (Palmer et al., 2011). Retrograde intraflagellar transport is mediated by cytoplasmic dynein-2. In Tetrahymena, loss of dynein-2 results in lengthened cilia (Asai et al., 2009; Rajagopalan et al., 2009). Decreased expression of a dynein intermediate chain, d2lic, by blocking its transcription results in abnormally short nodal cilia (Bonnafe et al., 2004).
Alteration of intraflagellar transport proteins has also been shown to prevent proper cilium formation. A murine hypomorph of IFT88 called Tg737orpk results in shortened kidney cilia and is a model for polycystic kidney disease (Pazour et al., 2000). The small GTPase Arl-13 appears responsible for coupling IFT complexes A and B. Its loss results in short cilia, an effect that may be rescued by another GTPase Arl-3 (Li et al., 2010). IFT70, a complex B component which binds directly to IFT46, is essential for flagellar assembly (Fan et al., 2010). Defects in IFT170, a complex A protein, also shows phenotypes of abnormal cilia and VACTERL-like features including a motile cilia phenotype, hydrocephalus (Friedland-Little et al., 2011).
As we have seen, loss of IFT proteins can prevent proper cilium assembly. It is also of great interest to determine how IFT is regulated in mutants with excessively long cilia. A previous report described an increase in IFT protein within flagella in a null mutant of the lf3 gene in Chlamydomonas, based on Western blot analysis of isolated flagella, implicating this gene in regulating IFT injection into the flagella (Tam et al., 2003). However, this result is potentially difficult to interpret, because the mutant analyzed has short stumpy flagella and therefore equal protein loading would result in a larger number of short flagella being loaded onto the gel compared to wild-type flagella. Because long and short flagella have equal amounts of IFT protein (Marshall et al., 2005), any increase in the number of loaded flagella per well would lead to the appearance of increased IFT protein in these mutants on a Western blot. Our own observations show that in studies of long flagella mutants in Chlamydomonas, IFT profiles are altered such that rather than switching from larger IFT trains to smaller trains as flagella elongate, larger trains persist (our unpublished observations). These cells inappropriately behave as if their flagella are shorter and must elongate further even after they reach wild-type length. Much more work needs to be done to determine the mechanism of injection of inappropriately sized trains, and more generally to understand how particular mutations that alter length may affect IFT or axonemal dynamics.
In addition to ciliary motors and IFT proteins, several direct modifiers of microtubule stability or microtubule binding proteins can control ciliary length. As one might expect, modulating levels of cytosolic tubulin by a variety of mechanisms can alter cilia length (Sharma et al., 2011). Tubulin acetylation is known to stabilize microtubules and is regulated by the tubulin deacetylase, HDAC6 (Hubbert et al., 2002). While tubascin, a small molecule inhibitor of HDAC6, does not influence changes in ciliary length in mammalian cells (Sharma et al., 2011), ciliary disassembly does require activation of HDAC6 by Aurora A kinase (Pugacheva et al., 2007). Additionally, loss of HDAC binding domain-containing proteins Cep70 and Cep131 results in shorter cilia in zebrafish (Wilkinson et al., 2009). As mentioned in section 2.1, ubiquitination and methylation programs are also activated in resorbing cilia (Huang et al., 2009; Schneider et al., 2008). Another method of direct axoneme regulation may be the binding of doublecortin (DC) domain proteins, which can directly bind microtubules and facilitate polymerization. A DC-domain containing protein, DCDC2, increases the length of cilia two-fold when overexpressed in hippocampal neurons and fibroblasts (Massinen et al., 2011). Overexpression of another DC domain protein, RP1, in mammalian photoreceptors increases cilium length (Omori et al., 2010) and it’s disruption causes autosomal dominant retinitis pigmentosa (a disorder of degenerating photoreceptor cilia). RP1 is phosphorylated by a male germ cell kinase (MAK). Defects in this kinase result in excessively long cilia that have an extended region of acetylated tubulin labeling (Omori et al., 2010). This hyperacetylation may inhibit the normal turnover required for ciliary maintenance at an appropriate length.
In addition to effects of integral cilia proteins, some basal body and transition zone proteins have also been shown to be essential regulators of ciliary length. In RPE1 cells, siRNA depletion of Nphp-8, a protein associated with renal and retinal function, resulted in elongated cilia (Patzke et al., 2010). In C. elegans, the same protein is required for proper ciliary elongation in a subset of ciliated neurons (Liu et al., 2011). A complex of disease-associated proteins localized to the transition zone was found to be required for ciliary formation or length maintenance in a tissue-specific manner. This complex contains Tctn1, Tctn2, Tmem67, and Cc2d2a (Garcia-Gonzalo et al., 2011). While it is easy to see how basal body docking can influence formation of cilia, it is less clear how proteins localized there are regulating cilium length. It is possible that they some of these proteins are responsible for recruitment of proteins necessary for cilium assembly or that they are regulating entrance of IFT trains into the cilium at the transition zone. Further work is needed to elucidate mechanisms of ciliary length control by basal body proteins.
3.3. Length regulation related to intracellular signaling
Clearly the days of viewing primary cilia as vestigial or static organelles are over. However, the role of cilia, not simply as sensory structures, but also as signaling hubs is under-appreciated. It is important to think of the signaling proteins and molecules that regulate ciliary length and function as large interconnected super-networks, but individual pathways can be identified to fill in this picture. The identified signaling proteins include Aurora, CDK, NIMA and MAP kinases. Additionally, ciliogenesis and ciliary length can be regulated by the Wnt, PCP, Shh and Notch developmentally regulated pathways. Finally, modulation of sensory pathways by altering G-protein coupled receptor signaling can alter ciliary length, as can regulation of second messengers.
Section 2.1 highlighted the links between ciliogenesis and cell cycle progression. Modulation of several cell cycle related kinases can alter ciliary length. In zebrafish Cdc14b, the phosphatase that antagonizes Cdk1, was required for both primary and motile cilia to reach full length (Clement et al., 2011). In Chlamydomonas, a hypomorphic mutation of a CDK related kinase Lf2p results in excessively long flagella, while null mutants have stumpy flagella that are often of unequal flagellar length (Tam et al., 2007). The mechanism that regulates this variable phenotype is not yet understood. In C. elegans, defects in daf-19, an LF2 related protein localized to the ciliary base, result in truncated cilia (Phirke et al., 2011). As mentioned previously, the mitotic kinase Aurora A and aurora-like kinase CALK are required for ciliary disassembly (Luo et al., 2011; Pugacheva et al., 2007). In Chlamydomonas, the phosphorylation state of CALK also shifts when flagellar length reaches 6 μm (half of wild-type length), even in the case of long flagella mutants for which 6 μm is not half length (Luo et al., 2011). CALK is the first molecule to show absolute dependence on ciliary length for its modification state.
Another set of kinases involved in cell cycle control, the NIMA-related kinases (NRKs or NEKs) are responsible for cilia length regulation. In Tetrahymena, a variety of NRKs promote ciliary disassembly including Nrk2p, Nrk17p, and Nrk30p (Wloga et al., 2006). The same holds true in Chlamydomonas, in which knockdown of the NRK Cnk2p results in longer flagella (Bradley and Quarmby, 2005). In mice, loss of Nek8 produces excessively long renal cilia (Smith et al., 2006; Sohara et al., 2008). Other Neks appear to have the opposite effect on ciliary length. Loss of Nek1 results in reduced cilia with significantly shorter length and altered morphology (Thiel et al., 2011) and Nek4 reduces ciliary assembly in RPE1 cells, but this does not appear to be due to defects in mitotic progression (Coene et al., 2011).
It is well known that in many mammalian cell types cell proliferation can be slowed and ciliogenesis can be induced by serum starvation. Serum contains the growth factors that activate cell surface receptor tyrosine kinases for mitogenic signaling through MAP kinase activation. In quiescent fibroblasts, it has been shown that the PDGF receptor is localized to cilia and that proper cilia formation is required for mitogenic MAP kinase signaling (Schneider et al., 2005). In MDCK cells, knockdown of both the GTPase cdc42 and ciliogenic trafficking exocyst proteins results in MAP kinase activation (Zuo et al., 2011). Impairment in MAP kinase signaling has repeatedly been shown to result in increased ciliary length. In Chlamydomonas, null mutants of the LF4p MAP kinase have flagella several times longer than wild-type (Asleson and Lefebvre, 1998; Berman et al., 2003). MAK, the germ cell kinase that phosphorylates and antagonizes the microtubule RP1 (Omori et al., 2010), is a MAP kinase that appears to restrict cilium length as described in section 3.2. Finally, In C. elegans, mutants of the dyf-5 MAP kinase result in increased cilia length due to changes in ciliary motor docking (Burghoorn et al., 2010). Chlamydomonas LF4p, mammalian MAK, and C. elegans dyf-5 all have similar sequences due to conserved motifs found in all MAP kinases and appear to have similar loss of function phenotypes. However, each organism expresses several other MAP kinases with equally high sequence identity. Readers are cautioned not to make conclusions about functional orthologs as these related kinases are also uncharacterized with regard to ciliary phenotypes.
Although much of our knowledge of signaling molecules that affect ciliary assembly or length comes from direct measurements of cilia in experimental cells, another source of insights comes from ciliary disease genes and disease model mutations in genes that encode signaling molecules. For example, a major cause of developmental phenotypes seen in ciliopathy patients is loss of sonic hedgehog (Shh) signaling. Localization of the seven transmembrane protein smoothened (Smo) to the primary cilium was shown to be essential for Shh signaling (Corbit et al., 2005). Shh signal transducing Gli transcription factors are localized to cilia tips. Additionally loss of IFT88 results in altered Gli3 processing and alleviates repression of Gli1 transcription (Haycraft et al., 2005). Several proteins that affect Shh signaling turn out to play a role in ciliary formation or length regulation. Broad-minded (Bromi) is a zebrafish protein that interacts with cell cycle-related kinase (CCRK), a homolog of Chlamydomonas LF2p. Bromi is thought to coordinate membrane and axoneme assembly by regulating attachment of the ciliary membrane (Ko et al., 2010). MIM, which is the protein capable of regulating ciliogenesis by regulating actin polymerization via cortactin described in section 2.2, positively regulates the Shh pathway by binding suppressor of fused (Sufu) and Gli (Callahan et al., 2004). Additionally, over-expression of the forkhead transcription factor Foxj1 is sufficient to increase ciliary length and can decrease the activity of Gli proteins (Cruz et al., 2010). While cilia are required for proper hedgehog signaling in vertebrates, they are despensible for hedgehog signaling in Drosophila (Han et al., 2003; Ray et al., 1999).
In addition to Shh, modulation of proteins in the Wnt and planar cell polarity (PCP) pathways have ciliary consequences. For example, Gsk3β is a kinase that can phosphorylate and lead to the degradation of β-catenin in the Wnt pathway. Inhibition of Gsk3β with lithium chloride results in significantly elongated flagella in Chlamydomonas (Nakamura et al., 1987; Wilson and Lefebvre, 2004) and also lengthened cilia in mammalian cells and tissues (Miyoshi et al., 2009). In the non-canonical Wnt signaling PCP pathway Par3/Par6/aPKC, which reside at the base of the cilium, can regulate ciliogenesis (Sfakianos et al., 2007) and interact with ciliary kinesin-II (Fan et al., 2004).
Recently, Notch signaling has been implicated in ciliogenesis and cilium length regulation. In Xenopus laevis embryonic epidermis, microRNAs were identified that promote centriole duplication by inhibition of Notch signaling and allow cells to become multiciliated (Marcet et al., 2011a; Marcet et al., 2011b). Additionally, in zebrafish, inhibiting Notch signaling shortened cilia in the Kupffer’s vesicle and Notch signaling hyperactivation resulted in elongated cilia (Lopes et al., 2010). Finally, directly inhibiting ciliogenesis in murine epidermal keratinocytes caused hyperproliferation, prevented Notch signaling activation and caused a failure of these cells to differentiate (Ezratty et al., 2011). In these cells, Notch signaling seems to balance proliferation and differentiation consistent with a role in centriolar regulation however, the mechanism by which ciliary length control is achieved remains obscure.
Finally, many extracellular signaling pathways with variable effects on cilia length can utilize the same second messengers for transduction. Therefore it is difficult to infer the cilia length-altering effects of changes in second messengers alone. Primary cilia length can be increased by increases in cAMP (Abdul-Majeed et al., 2011; Besschetnova et al., 2010). Paradoxically, inhibition of adenylate cyclase III was found to significantly increase cilia length (Ou et al., 2009). A decrease in intracellular calcium was shown to increase cilia length in cultured mammalian cells (Besschetnova et al., 2010), but activators of calcium dependent protein kinase C result in increased cilia length as well (Abdul-Majeed et al., 2011).
Currently we are faced with a growing list of signaling molecules that affect ciliary length, without having much mechanistic information as to how they exert such an effect. In each case it is essential to ask which aspect of ciliogenesis and length control is being modulated by a given signaling pathways. A great deal of work is being done to understand how changes in the regulation of IFT that can result in abnormal ciliary length, as well as to identify ciliary phenotypes resulting from abnormal signaling pathways involved in other cellular processes. In the upcoming phase of ciliary exploration, it is important we begin to connect these distributed fields and identify the modulation in IFT rate and frequency in conditions of altered cellular signaling. We must continue to identify novel proteins that are essential players in ciliogenesis for which proteomics (Keller et al., 2009; Pazour et al., 2005), transcriptomics (Blacque et al., 2005), and genetic screening (Kim et al., 2010) have already played essential roles. However, modeling efforts remain essential for generating plausible hypotheses and testing predictions informed by existing functional data.
4. Diseases of abnormal ciliary formation and maintenance
Ciliopathies produce many phenotypes affecting a wide variety of organ systems. These include cystic kidneys (defects in renal cilia), retinal degeneration (photoreceptor outer segment malformation), left-right asymmetry defects (abnormal nodal cilia), infertility (defective sperm and oviduct flagella and cilia), polydactyly (disruption of Hedgehog signaling), obesity (neuronal cilia defects), and airway abnormalities (dysfunctional tracheal motile cilia). Loss of genes required for ciliogenesis and length control affect both motile and primary cilia. Table 2 summarizes the phenotypes and genes involved in selected ciliopathies.
Table 2.
Ciliopathies, Phenotypes and Associated Genes
| Disorder | Overall Phenotype | Ciliary Phenotype | Genes |
|---|---|---|---|
| Bardet-Biedl Syndrome |
|
Truncated | BBS1-12 |
| Nephrononphthisis |
|
Fewer, Truncated | NPHP1-9, Nek8 |
| Senor-Loken Syndrome |
|
Fewer, Truncated | NPHP1, NPHP4, NPHP5/IQCB1, NPHP6/CEP290, SDCCAG8 |
| Joubert Syndrome |
|
Fewer, Truncated | INPP5E, TMEM216, AHI1, NPHP1, NPHP6/CEP290, TMEM67, RPGRIP1L, ARL13B, CC2D2A, OFD1 |
| Jeune Asphyxiating Thoracic Dystrophy |
|
None (defects from abnormal hedgehog signaling) | IFT80 |
| Polycystic Kidney Disease |
|
Fewer, Truncated | IFT88, PKD1, PKD2 |
| Juvenile Cystic Kidney Disease |
|
Elongated | Nek8 |
| Sensenbrenner Syndrome |
|
Fewer, Truncated | IFT22, IFT43 |
| Situs Inversus |
|
Absent, Truncated, Immotile | Kif3A/B, NPHP2 |
| Meckel-Gruber Syndrome |
|
Elongated | MKS1,3,5,6; CEP290, BD91,BD92 |
| Tuberous Sclerosis |
|
Elongated | Tsc1, Tsc2 |
| Alstrom Syndrome |
|
Positioning defect, Truncated (in some models) | ALMS1 |
| Orofaciodigital Syndrome 1 |
|
Fewer | OFD1 |
| Primary Ciliary Dyskinesia |
|
Immotile | DNAI1, DNAH5, TXNDC3, DNAH11, DNAI2, KTU, RSPH4A, RSPH9, LRRC50 |
| Kartegener Syndrome |
|
Immotile |
Disorders resulting from immotile cilia include Kartagener’s Syndrome characterized by situs inversus, sinusitis from failed mucociliary transport, and bronchiectasis (Afzelius, 1976). Loss of a dynein intermediate chain (Ostrowski et al., 2010), or dynein heavy chains DNAH7 (Zhang et al., 2002) and DNAH5 (Olbrich et al., 2002) result in primary ciliary dyskinesia (PCD). Interestingly, mutations in several thioredoxins, which regulate cellular processes via redox reactions, are also found to result in PCD (Duriez et al., 2007).
IFT abnormalities result in a variety of disorders including polycystic kidney disease from aberrant IFT88 (Yoder et al., 2002). Defective IFT80 produces a zebrafish model of jeune thoracic asphyxiating dystrophy (Hudak et al., 2010). Mutations in IFT22 and IFT43 result in Sensenbrenner syndrome, a disorder featuring craniofacial abnormalities due to defects in Shh signaling (Arts et al., 2011; Walczak-Sztulpa et al., 2010).
A consistent pathogenic phenotype in many ciliopathies is disruption of left-right asymmetry. This occurs because cilia on the embryonic node (similar to Kupffer’s vesicle in zebrafish) rotate to generate leftward flow for left-right determination (Nonaka et al., 1998). Functional loss of these nodal cilia, through defects in the ciliary kinesin-II motor for example, result in the assymetry randomization condition known as situs inversus.
Abnormalities in many of the discussed ciliogenesis proteins result in pleiotriopic disorders affecting multiple tissues containing primary cilia. These include bardet-biedl syndrome (BBS), nephronophthisis (NPHP), meckel syndrome (MKS), joubert syndrome (JBTS). Many ciliary transition zone proteins are involved in the pathogenesis of MKS. These include MKS-1, MKS-3/TMEM67, MKS-5/RPGRIP1L, MKS-6/CC2D2A, CEP290, and BD91 (Dowdle et al., 2011). Loss of centrosomal docking occured in TMEM216 mutants and resulted in Joubert and Meckel Syndromes. BBS, containing many ciliopathy hallmarks results from mutations in BBS proteins, which are thought to form a coat that traffics membrane proteins to the primary cilium (Jin et al., 2010).
While many ciliopathies result from abnormally short cilia or impaired ciliogenesis some are characterized by excessively long cilia. These include juvenile cystic kidney disease resulting from abnormal Nek8 (Sohara et al., 2008) and meckel syndrome resulting from mutations in Mks1 and Mks3 (Tammachote et al., 2009). A zebrafish model of tuberous sclerosis knockdown of Tsc1 results in elongated cilia (DiBella et al., 2009). In addition to elongated cilia, loss of Nek8 also results in cystogenesis (Trapp et al., 2008) and NPHP (Otto et al., 2008). Understanding the pathologies of the long cilia phenotypes is critical to understanding ciliary maintenance. While abnormally short and absent cilia can certainly tell us about critical genes and functions required for ciliogenesis, it is difficult to identify which effects are upstream and downstream of primary defects when cilia are so dramatically disrupted.
Until cilia were implicated in the phenotypes described above, it was difficult to understand the basis of the varied pathologies affecting distributed organ systems. It would be a mistake to assume that only the known ciliopathies can be ameliorated by modulation of ciliogenic and length regulatory pathways. Due to the increasing evidence for the role of cilia in cell cycle progression, the requirement for ciliary proteins for vesicular and membrane transport, and the feedback of ciliary alterations on multiple signaling pathways, it is likely that an increasing number of disorders may be targeted using ciliary intervention. It is tempting to speculate that various forms of cancer, signaling disorders and channelopathies may eventually be primarily considered ciliopathies. An advantage to the promiscuity of many signaling pathways in ciliary regulation is that pharmaceuticals that are already approved for treatments of other disorders may be useful for treating ciliopathies. One possibility might be the use of lithium, a drug currently being administered for the treatment of depression, but with known cilia lengthening effects. We hypothesize that the many ciliopathies with abnormally short cilia may respond favorably to lithium. Also, a small molecule screen of flagellar length regulators identified the dopamine family of G-protein coupled receptors (GPCRs) as the most frequent targets of length-altering compounds (our unpublished observations). Currently, the largest percentage of FDA approved drugs target GPCRs. Cross-application of some of these treatments that have already been shown to be safe for patients may be a rapid road to approval of new ciliopathy treatments.
5. Conclusions
Cilia should be considered critical organelles, like any other, necessary for cellular homeostasis. As we have seen, many cellular processes are dependent on proper timing of ciliogenesis or on proper ciliary maintenance (Fig. 2). We have just begun to scratch the surface on how cilia are regulated and many questions remain. For example, the ciliary transition zone is a hotbed of activity and a hub for accumulation of ciliary proteins. It is unclear how activity is coordinated at this region. Determining the nature of the ciliary gate, as well as identifying factors regulating injection of IFT trains is essential for understanding cilium maintenance. It is also becoming increasingly evident that ciilary proteins serve dual functions outside the cilium. In fact, one of the few cell types that does not form cilia still expresses IFT proteins (Baldari and Rosenbaum, 2010). Regulation of the availability of ciliary proteins for IFT and determination of factors influencing differential targeting of ciliary proteins are also largely unanswered questions. We now have many tools for the visualization of intracellular trafficking in real time. The combination of these technologies and our new understanding of potential ciliary regulators will lead to an explosion of new data in this exciting field in the near future.
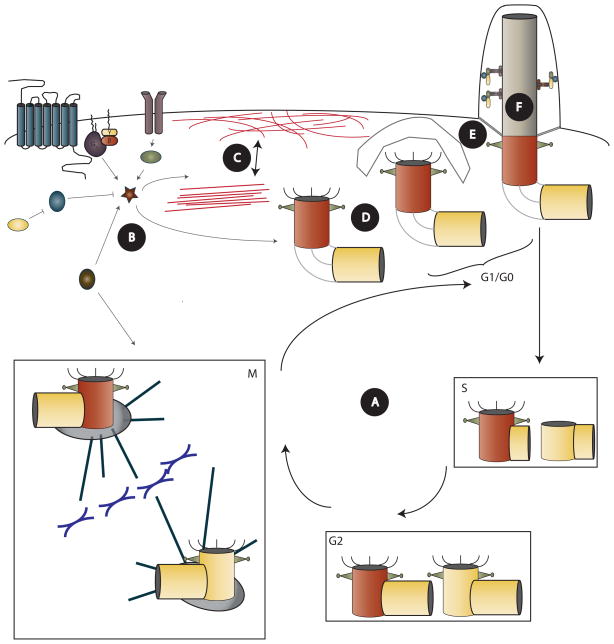
Figure 2. Mechanisms regulating ciliogenesis and ciliary length.
(A) Cycle cycle regulators modulate the centrosome cycle to regulate timing of ciliary resorption, centriole maturation, and ciliogenesis. Ciliation inhibits cell cycle progression. (B) Extracellular signals and intracellular kinase cascades alter their own pathways or converge on common second messengers for centriole or ciliary regulation. (C) Remodeling of actin between contractile stress fibers and a cortical network alters conditions hospitable for basal body docking and maintenance at the cell surface. (D) Proteins regulating centriole migration and formation of appendages regulate basal body docking to ciliary vesicles for fusion with the plasma membrane. (E) Large complexes of disease-related transition zone proteins regulate recruitment of ciliary proteins or gate ciliary entry. (F) Direct modification of axoneme by altering stability through acetylation state or assembly/disassembly through IFT tunes ciliary length.
Acknowledgments
We apologize to those whose excellent contributions we were unable to include here. This work was funded by the National Institutes of Health F32 GM090562 and R01 GM097017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Majeed S, Moloney BC, Nauli SM. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an alpha-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J Cell Biol. 1972;54:246–265. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Bongers EM, Mans DA, van Beersum SE, Oud MM, Bolat E, Spruijt L, Cornelissen EA, Schuurs-Hoeijmakers JH, de Leeuw N, et al. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet. 2011;48:390–395. doi: 10.1136/jmg.2011.088864. [DOI] [PubMed] [Google Scholar]
- Asai DJ, Rajagopalan V, Wilkes DE. Dynein-2 and ciliogenesis in Tetrahymena. Cell Motil Cytoskeleton. 2009;66:673–677. doi: 10.1002/cm.20397. [DOI] [PubMed] [Google Scholar]
- Asleson CM, Lefebvre PA. Genetic analysis of flagellar length control in Chlamydomonas reinhardtii: a new long-flagella locus and extragenic suppressor mutations. Genetics. 1998;148:693–702. doi: 10.1093/genetics/148.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas. Current Biology: CB. 2003;13:1145–1149. doi: 10.1016/s0960-9822(03)00415-9. [DOI] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell. 2010;19:270–283. doi: 10.1016/j.devcel.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20:182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, et al. Functional genomics of the cilium, a sensory organelle. Curr Biol. 2005;15:935–941. doi: 10.1016/j.cub.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol. 2007;17:778–782. doi: 10.1016/j.cub.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, et al. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Molecular and Cellular Biology. 2004;24:4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AJ, Boylan JF. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene. 2004;328:135–142. doi: 10.1016/j.gene.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Bradley BA, Quarmby LM. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. Journal of Cell Science. 2005;118:3317–3326. doi: 10.1242/jcs.02455. [DOI] [PubMed] [Google Scholar]
- Burghoorn J, Dekkers MP, Rademakers S, de Jong T, Willemsen R, Swoboda P, Jansen G. Dauer pheromone and G-protein signaling modulate the coordination of intraflagellar transport kinesin motor proteins in C. elegans. Journal of Cell Science. 2010;123:2077–2084. doi: 10.1242/jcs.062885. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Cheek TR. Reorganisation of peripheral actin filaments as a prelude to exocytosis. Biosci Rep. 1987;7:281–288. doi: 10.1007/BF01121449. [DOI] [PubMed] [Google Scholar]
- Callahan CA, Ofstad T, Horng L, Wang JK, Zhen HH, Coulombe PA, Oro AE. MIM/BEG4, a Sonic hedgehog-responsive gene that potentiates Gli-dependent transcription. Genes Dev. 2004;18:2724–2729. doi: 10.1101/gad.1221804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen CF, Chiang HC, Pena M, Polci R, Wei RL, Edwards RA, Hansel DE, Chen PL, Riley DJ. Mutation of NIMA-related kinase 1 (NEK1) leads to chromosome instability. Mol Cancer. 2011a;10:5. doi: 10.1186/1476-4598-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu B, Xu L, Li H, Xia J, Yin W, Li Z, Li S, Lin S, Shu X, et al. A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res. 2011b doi: 10.1038/cr.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M, Narumiya S, Bornens M, Job D. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002;157:807–817. doi: 10.1083/jcb.200203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DM, Brown MC, LaLonde DP, Turner CE. Phosphorylation of actopaxin regulates cell spreading and migration. J Cell Biol. 2004;166:901–912. doi: 10.1083/jcb.200404024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement A, Solnica-Krezel L, Gould KL. The Cdc14B phosphatase contributes to ciliogenesis in zebrafish. Development. 2011;138:291–302. doi: 10.1242/dev.055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coene KL, Mans DA, Boldt K, Gloeckner CJ, van Reeuwijk J, Bolat E, Roosing S, Letteboer SJ, Peters TA, Cremers FP, et al. The ciliopathy-associated protein homologs RPGRIP1 and RPGRIP1L are linked to cilium integrity through interaction with Nek4 serine/threonine kinase. Hum Mol Genet. 2011;20:3592–3605. doi: 10.1093/hmg/ddr280. [DOI] [PubMed] [Google Scholar]
- Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C, Ribes V, Kutejova E, Cayuso J, Lawson V, Norris D, Stevens J, Davey M, Blight K, Bangs F, et al. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development. 2010;137:4271–4282. doi: 10.1242/dev.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. Journal of Cell Science. 2009;122:2716–2726. doi: 10.1242/jcs.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryotic Cell. 2007;6:2354–2364. doi: 10.1128/EC.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nature Cell Biology. 2011;13:461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J Cell Biol. 2005;170:649–659. doi: 10.1083/jcb.200412021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBella LM, Park A, Sun Z. Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum Mol Genet. 2009;18:595–606. doi: 10.1093/hmg/ddn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nature Cell Biology. 2010;12:703–710. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle WE, Robinson JF, Kneist A, Sirerol-Piquer MS, Frints SG, Corbit KC, Zaghloul NA, van Lijnschoten G, Mulders L, Verver DE, et al. Disruption of a ciliary B9 protein complex causes Meckel syndrome. American Journal of Human Genetics. 2011;89:94–110. doi: 10.1016/j.ajhg.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez B, Duquesnoy P, Escudier E, Bridoux AM, Escalier D, Rayet I, Marcos E, Vojtek AM, Bercher JF, Amselem S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2007;104:3336–3341. doi: 10.1073/pnas.0611405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Ludington WB, Marshall WF. Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J Cell Biol. 2009;187:81–89. doi: 10.1083/jcb.200812084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Fan ZC, Behal RH, Geimer S, Wang Z, Williamson SM, Zhang H, Cole DG, Qin H. Chlamydomonas IFT70/CrDYF-1 is a core component of IFT particle complex B and is required for flagellar assembly. Mol Biol Cell. 2010;21:2696–2706. doi: 10.1091/mbc.E10-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193:219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland-Little JM, Hoffmann AD, Ocbina PJ, Peterson MA, Bosman JD, Chen Y, Cheng SY, Anderson KV, Moskowitz IP. A novel murine allele of Intraflagellar Transport Protein 172 causes a syndrome including VACTERL-like features with hydrocephalus. Hum Mol Genet. 2011;20:3725–3737. doi: 10.1093/hmg/ddr241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Current opinion in genetics & development. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature Genetics. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nature Cell Biology. 2011;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genetics. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, et al. A Septin Diffusion Barrier at the Base of the Primary Cilium Maintains Ciliary Membrane Protein Distribution. Science. 2010 doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Diener DR, Rosenbaum JL. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol. 2009;186:601–613. doi: 10.1083/jcb.200903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Hudak LM, Lunt S, Chang CH, Winkler E, Flammer H, Lindsey M, Perkins BD. The intraflagellar transport protein ift80 is essential for photoreceptor survival in a zebrafish model of jeune asphyxiating thoracic dystrophy. Invest Ophthalmol Vis Sci. 2010;51:3792–3799. doi: 10.1167/iovs.09-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Kubo A, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nature Cell Biology. 2005;7:517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller LC, Geimer S, Romijn E, Yates J, 3rd, Zamora I, Marshall WF. Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol Biol Cell. 2009;20:1150–1166. doi: 10.1091/mbc.E08-06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle. 2011;10:2683–2690. doi: 10.4161/cc.10.16.17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nature Cell Biology. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev Cell. 2010;18:237–247. doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD. Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell. 2011;145:914–925. doi: 10.1016/j.cell.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Kuchka MR, Jarvik JW. Analysis of flagellar size control using a mutant of Chlamydomonas reinhardtii with a variable number of flagella. J Cell Biol. 1982;92:170–175. doi: 10.1083/jcb.92.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985a;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- L’Hernault SW, Rosenbaum JL. Reversal of the posttranslational modification on Chlamydomonas flagellar alpha-tubulin occurs during flagellar resorption. J Cell Biol. 1985b;100:457–462. doi: 10.1083/jcb.100.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Ressler AH. Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cytoskeleton. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nature Cell Biology. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189:1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang M, Xia Z, Xu P, Chen L, Xu T. Caenorhabditis elegans ciliary protein NPHP-8, the homologue of human RPGRIP1L, is required for ciliogenesis and chemosensation. Biochem Biophys Res Commun. 2011;410:626–631. doi: 10.1016/j.bbrc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- Lopes SS, Lourenco R, Pacheco L, Moreno N, Kreiling J, Saude L. Notch signalling regulates left-right asymmetry through ciliary length control. Development. 2010;137:3625–3632. doi: 10.1242/dev.054452. [DOI] [PubMed] [Google Scholar]
- Love D, Li FQ, Burke MC, Cyge B, Ohmitsu M, Cabello J, Larson JE, Brody SL, Cohen JC, Takemaru K. Altered lung morphogenesis, epithelial cell differentiation and mechanics in mice deficient in the Wnt/beta-catenin antagonist Chibby. PLoS One. 2010;5:e13600. doi: 10.1371/journal.pone.0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Cao M, Kan Y, Li G, Snell W, Pan J. The phosphorylation state of an aurora-like kinase marks the length of growing flagella in Chlamydomonas. Curr Biol. 2011;21:586–591. doi: 10.1016/j.cub.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub MR, Montpetit B, Zhao L, Finst RJ, Goh B, Kim AC, Quarmby LM. The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. Journal of Cell Science. 2002;115:1759–1768. doi: 10.1242/jcs.115.8.1759. [DOI] [PubMed] [Google Scholar]
- Marcet B, Chevalier B, Coraux C, Kodjabachian L, Barbry P. MicroRNA-based silencing of Delta/Notch signaling promotes multiple cilia formation. Cell Cycle. 2011a;10:2858–2864. doi: 10.4161/cc.10.17.17011. [DOI] [PubMed] [Google Scholar]
- Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nature Cell Biology. 2011b;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- Marshall WF. Size control in dynamic organelles. Trends in cell biology. 2002 doi: 10.1016/s0962-8924(02)02341-3. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massinen S, Hokkanen ME, Matsson H, Tammimies K, Tapia-Paez I, Dahlstrom-Heuser V, Kuja-Panula J, Burghoorn J, Jeppsson KE, Swoboda P, et al. Increased expression of the dyslexia candidate gene DCDC2 affects length and signaling of primary cilia in neurons. PLoS One. 2011;6:e20580. doi: 10.1371/journal.pone.0020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Porazinski S, Wang H, Borovina A, Ciruna B, Shimizu A, Kajii T, Kikuchi A, Furutani-Seiki M, Matsuura S. Insufficiency of BUBR1, a mitotic spindle checkpoint regulator, causes impaired ciliogenesis in vertebrates. Hum Mol Genet. 2011;20:2058–2070. doi: 10.1093/hmg/ddr090. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009;388:757–762. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- Morris RL, Scholey JM. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J Cell Biol. 1997;138:1009–1022. doi: 10.1083/jcb.138.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol. 2011;21:1239–1244. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takino H, Kojima MK. Effect of Lithium on Flagellar Length in Chlamydomonas-Reinhardtii. Cell Struct Funct. 1987;12:369–374. [Google Scholar]
- Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol. 2000;151:1435–1448. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- O’Regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div. 2007;2:25. doi: 10.1186/1747-1028-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nature Genetics. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Olbrich H, Haffner K, Kispert A, Volkel A, Volz A, Sasmaz G, Reinhardt R, Hennig S, Lehrach H, Konietzko N, et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nature Genetics. 2002;30:143–144. doi: 10.1038/ng817. [DOI] [PubMed] [Google Scholar]
- Omori Y, Chaya T, Katoh K, Kajimura N, Sato S, Muraoka K, Ueno S, Koyasu T, Kondo M, Furukawa T. Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc Natl Acad Sci U S A. 2010;107:22671–22676. doi: 10.1073/pnas.1009437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O’Neal WK, Grubb BR. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010;43:55–63. doi: 10.1165/rcmb.2009-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Ruan YB, Chen M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KJ, Maccarthy-Morrogh L, Smyllie N, Stephens DJ. A role for Tctex-1 (DYNLT1) in controlling primary cilium length. Eur J Cell Biol. 2011;90:865–871. doi: 10.1016/j.ejcb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell. 2005;9:431–438. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. Journal of Cell Science. 2007;120:1868–1876. doi: 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nature Genetics. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Parker JD, Hilton LK, Diener DR, Rasi MQ, Mahjoub MR, Rosenbaum JL, Quarmby LM. Centrioles are freed from cilia by severing prior to mitosis. Cytoskeleton (Hoboken) 2010;67:425–430. doi: 10.1002/cm.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Quarmby LM. Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport. BMC Cell Biology. 2003;4:11. doi: 10.1186/1471-2121-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzke S, Redick S, Warsame A, Murga-Zamalloa CA, Khanna H, Doxsey S, Stokke T. CSPP is a ciliary protein interacting with Nephrocystin 8 and required for cilia formation. Mol Biol Cell. 2010;21:2555–2567. doi: 10.1091/mbc.E09-06-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Developmental Biology. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Phirke P, Efimenko E, Mohan S, Burghoorn J, Crona F, Bakhoum MW, Trieb M, Schuske K, Jorgensen EM, Piasecki BP, et al. Transcriptional profiling of C. elegans DAF-19 uncovers a ciliary base-associated protein and a CDK/CCRK/LF2p-related kinase required for intraflagellar transport. Developmental Biology. 2011;357:235–247. doi: 10.1016/j.ydbio.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci U S A. 2009;106:4713–4718. doi: 10.1073/pnas.0808671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaval A, Tseng Q, Bornens M, Thery M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J Cell Biol. 2010;191:303–312. doi: 10.1083/jcb.201004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan V, Subramanian A, Wilkes DE, Pennock DG, Asai DJ. Dynein-2 affects the regulation of ciliary length but is not required for ciliogenesis in Tetrahymena thermophila. Mol Biol Cell. 2009;20:708–720. doi: 10.1091/mbc.E08-07-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K, Perez SE, Yang Z, Xu J, Ritchings BW, Steller H, Goldstein LS. Kinesin-II is required for axonal transport of choline acetyltransferase in Drosophila. J Cell Biol. 1999;147:507–518. doi: 10.1083/jcb.147.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bre MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science. 1994;266:1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Child FM. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967;34:345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969;41:600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Ulland M, Sloboda RD. A protein methylation pathway in Chlamydomonas flagella is active during flagellar resorption. Mol Biol Cell. 2008;19:4319–4327. doi: 10.1091/mbc.E08-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. Journal of Cell Science. 2011;124:2539–2551. doi: 10.1242/jcs.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianos J, Togawa A, Maday S, Hull M, Pypaert M, Cantley L, Toomre D, Mellman I. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol. 2007;179:1133–1140. doi: 10.1083/jcb.200709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell. 2011;22:806–816. doi: 10.1091/mbc.E10-03-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends in cell biology. 2009;19:306–316. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18:410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. Journal of the American Society of Nephrology: JASN. 2006;17:2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MRS, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nature Cell Biology. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Dentler WL. Flagellar protein dynamics in Chlamydomonas. J Biol Chem. 2001;276:29754–29763. doi: 10.1074/jbc.M103184200. [DOI] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RE. Tubulin in sea urchin embryonic cilia: post-translational modifications during regeneration. Journal of Cell Science. 1992;101(Pt 4):837–845. doi: 10.1242/jcs.101.4.837. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Synthesis and turnover of embryonic sea urchin ciliary proteins during selective inhibition of tubulin synthesis and assembly. Mol Biol Cell. 1997;8:2187–2198. doi: 10.1091/mbc.8.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- Suryavanshi S, Edde B, Fox LA, Guerrero S, Hard R, Hennessey T, Kabi A, Malison D, Pennock D, Sale WS, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol. 2010;20:435–440. doi: 10.1016/j.cub.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LW, Dentler WL, Lefebvre PA. Defective flagellar assembly and length regulation in LF3 null mutants in Chlamydomonas. J Cell Biol. 2003;163:597–607. doi: 10.1083/jcb.200307143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LW, Wilson NF, Lefebvre PA. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol. 2007;176:819–829. doi: 10.1083/jcb.200610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammachote R, Hommerding CJ, Sinders RM, Miller CA, Czarnecki PG, Leightner AC, Salisbury JL, Ward CJ, Torres VE, Gattone VH, 2nd, et al. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum Mol Genet. 2009;18:3311–3323. doi: 10.1093/hmg/ddp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stoss H, Beinder E, et al. NEK1 mutations cause short-rib polydactyly syndrome type majewski. American Journal of Human Genetics. 2011;88:106–114. doi: 10.1016/j.ajhg.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp ML, Galtseva A, Manning DK, Beier DR, Rosenblum ND, Quarmby LM. Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr Nephrol. 2008;23:377–387. doi: 10.1007/s00467-007-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, et al. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. American Journal of Human Genetics. 2010;86:949–956. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, Mitchell BJ. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J Cell Biol. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MC, Quarmby LM. The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biology. 2008;9:29. doi: 10.1186/1471-2121-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CJ, Carl M, Harris WA. Cep70 and Cep131 contribute to ciliogenesis in zebrafish embryos. BMC Cell Biology. 2009;10:17. doi: 10.1186/1471-2121-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Masyukova SV, Yoder BK. Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4. J Am Soc Nephrol. 2010;21:782–793. doi: 10.1681/ASN.2009060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Winkelbauer ME, Schafer JC, Michaud EJ, Yoder BK. Functional redundancy of the B9 proteins and nephrocystins in Caenorhabditis elegans ciliogenesis. Mol Biol Cell. 2008;19:2154–2168. doi: 10.1091/mbc.E07-10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryotic Cell. 2004;3:1307–1319. doi: 10.1128/EC.3.5.1307-1319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Camba A, Rogowski K, Manning G, Jerka-Dziadosz M, Gaertig J. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell. 2006;17:2799–2810. doi: 10.1091/mbc.E05-05-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Hai B, Gao Y, Burnette D, Thazhath R, Duan J, Bre MH, Levilliers N, Gorovsky MA, Gaertig J. Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J Cell Biol. 2000;149:1097–1106. doi: 10.1083/jcb.149.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, Whitley P, Genikhovich G, Technau U, Burt DW, et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–664. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, O’Neal WK, Randell SH, Blackburn K, Moyer MB, Boucher RC, Ostrowski LE. Identification of dynein heavy chain 7 as an inner arm component of human cilia that is synthesized but not assembled in a case of primary ciliary dyskinesia. J Biol Chem. 2002;277:17906–17915. doi: 10.1074/jbc.M200348200. [DOI] [PubMed] [Google Scholar]
- Zuo X, Fogelgren B, Lipschutz JH. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem. 2011;286:22469–22477. doi: 10.1074/jbc.M111.238469. [DOI] [PMC free article] [PubMed] [Google Scholar]