Abstract
Polymeric nanoparticles are emerging as an attractive treatment options for cancer due to their favorable size distribution, drug carrying capacity, and tunable properties. In particular, intelligent nanoparticles that respond to biological cues are of interest because of their ability to provide controlled release at a specific site. Tumor sites display abnormal pH profiles and pathophysiology that can be exploited to provide localized release. In this expert opinion, we discuss passive and active targeting of nanoparticles and several classes of pH-responsive nanoparticles.
Keywords: Drug Delivery, pH-responsive polymers, cancer therapy, nanoparticles, nanogels, polymer micelles
Introduction
Modern advances in science and medicine have brought about the advent of highly specific biological pharmaceutical agents, including proteins (monoclonal antibodies, growth factors, hormones, enzymes, synthetic oligopeptides) and nucleic acids (plasmid DNA, antisense oligonucleotides, siRNA, miRNA) that can be used to treat a variety of cancers [1, 2]. However, a significant obstacle in the emergence of highly specific cancer therapies remains the delivery of these macromolecules to their subcellular site of action. Extracellular and intracellular trafficking barriers represent a significant limitation in the delivery of fragile therapeutics as systemically administered macromolecules are rapidly sequestered by the reticuloendithelial system (RES) and internalized molecules are quickly trafficked to lysosomes for acidic and enzymatic degradation [3].
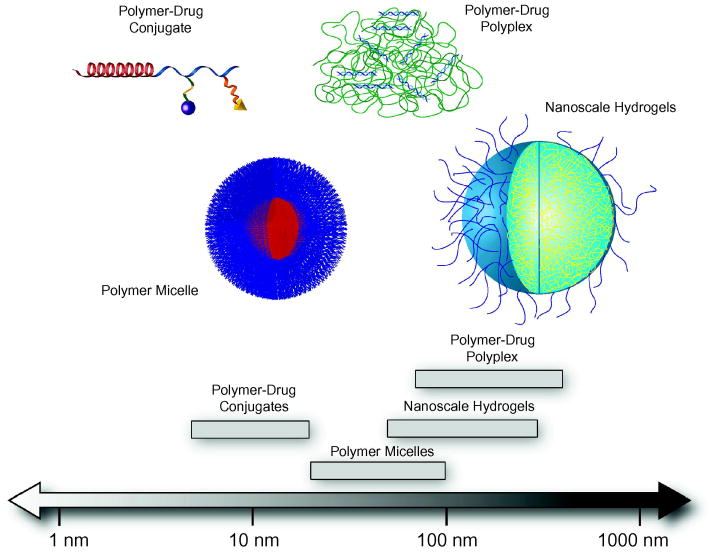
Conventional chemotherapy distributes anti-cancer drugs randomly throughout the body, indiscriminately killing tumor cells and healthy cells. Cancer patients worldwide stand to benefit from both efficient delivery systems of novel, specific therapeutics and improved, targeted delivery systems for existing therapeutics. Couvreur and colleagues, among others, have provided some of the earliest commentary on the use of nanoparticles for controlled release of therapeutic agents [4, 5]. Recently, the use of polymer-mediated delivery systems, such as polymer-drug conjugates, polymer micelles, polymer-drug polyplexes, and nanoscale hydrogels (Figure 1), have been investigated to improve efficacy of these drugs by providing protection from rapid clearance and enzymatic digestion, as well as offering the potential for controlled release [6, 7]. Of particular interest are intelligent systems able to respond to biological cues for tissue specific targeting or controlled drug release. Molecular design of intelligent delivery systems must consider stability, administration, absorption, metabolism, and bioavailability at target site. By controlling the level and location of biotherapeutics within the body, lower doses are needed and potentially harmful side effects can be minimized [3, 8, 9].
Figure 1. Typical length scales of responsive nanoscale drug delivery systems.
Targeted Delivery
Barriers to targeted delivery
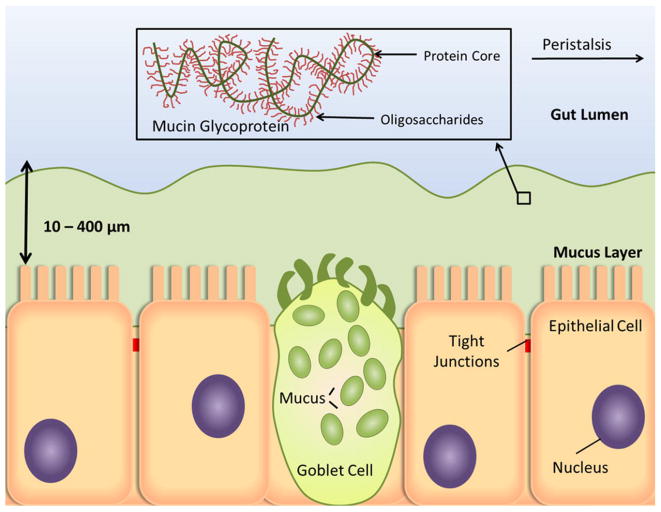
Though localized delivery represents an attractive method for achieving a high concentration of anti-tumor agent, most tissues are not accessible for localized treatment (e.g. intratumoral injection). Thus, systemic and oral administrations are commonly employed to introduce cancer therapeutics to the circulatory system. However, before reaching their site of action, anti-tumor formulations face a series of extracellular obstacles which threaten to reduce bioavailability and therapeutic benefit. The mononuclear phagocytic system (MPS), or reticuloendothelial system (RES), presents a major barrier to prolonged circulation of polymer nanoparticles [10]. Macrophages and monocytes of the MPS play an important physiological role by clearing foreign material, such as bacteria, viruses, and fungi, from the body. However, they are also highly efficient at removing unprotected polymer nanoparticles. Present in bloodstream and extracellular matrix of tissues, these phagocytotic bodies are not able to recognize circulating material directly, but must do so through the presence of opsonin proteins [10]. The association of opsonin proteins, termed opsonization, can occur within seconds after entering the bloodstream as complement proteins and immunoglobulins bind to the surface of the polymer particle [10]. Bound opsonins stimulate phagocytosis of the associated particle, ultimately resulting in its degradation or removal from the bloodstream. Particle aggregation with serum proteins such as serum albumin can also result in phagocytosis and clearance by the RES [11]. Typically, this process occurs through non-specific hydrophobic or electrostatic interactions between polymer particles and serum proteins [10, 12]. In addition to avoiding removal by the RES, polymer therapeutics must also escape filtration by the renal system. Current calculations, based on the sieving coefficient of the glomerular capillary wall, estimate that a polymer nanoparticle should be at least 10 nm in diameter to avoid first-pass renal filtration [13]. Extravasation, or escape from the blood vessels, poses a problem because molecules larger than 2 nm in diameter do not easily cross the capillary epithelia [13]. Once outside the capillaries, proteins and polysaccharides that comprise the extracellular matrix hinder diffusion of nanoparticles, increasing opportunity for phagocytosis by macrophages in the interstitial space [14]. Further obstacles face polymer therapeutics delivered through the oral route (Figure 2). The stomach, lumen of the small intestine, mucus layer, and the intestinal epithelial cell membrane must be successfully traversed [15]. Ingested formulations quickly encounter the harsh environment of the stomach, where digestive enzymes and low pH (pH ∼ 2) contribute to the rapid degradation of unprotected therapeutics, particularly proteins and nucleic acids. Though the pH is less extreme in the small intestine (pH 5 - 7.4), digestive enzymes in the intestinal lumen threaten to break down therapeutics before they cross the epithelium. The mucus lining, a dense network of glycoproteins, coats epithelial cells along the GI tract and provides resistance to macromolecular transport [16]. At the luminal surface of the epithelial cell layer, macromolecules are largely precluded from paracellular transport due to the presence of tight junctions. These junctions limit the paracellular space to approximately 10 - 50 Å [17], defending the body from entry of viruses and toxins through the GI tract. Additionally, intestinal motility reduces the residence time for release and absorption by the intestine.
Figure 2. Barriers to oral delivery of therapeutic molecules. Physical and diffusive barriers, such as the tight junctions and mucus layer, limit access of therapeutics to the underlying capillary structure in the lamina propria.
Intracellular trafficking barriers represent a significant obstacle in the delivery of biotherapeutics to their subcellular site of action. Most therapeutics must localize in the cytosol to exert functional activity. After passive or receptor-mediated endocytosis, macromolecules are rapidly delivered to early endosomes, where the vesicular pH is lowered by the action of ATP-dependent proton pumps [18]. Acidification is an important characteristic of endosomal trafficking, serving primarily to dissociate ligands from receptors. Although free receptors are recycled back to the cell surface, the early endosome contents are transferred to late endosomes and ultimately lysosomes for degradation [19]. The transit from early endosomes to late endosomes is fairly rapid, on the order of 2 – 3 minutes [18]. The luminal pH is progressively lowered throughout the pathway by ATP-dependent proton pumps. Typically the pH of early endosomes is 6.8 – 5.9, pH of late endosomes is 6.0 – 5.0, and pH of lysosomal compartments reaches 5.5 – 4.5 [18, 19]. Degradative enzymes populate the lysosome and readily break down foreign material. After successively protecting its cargo through numerous extracellular and intracellular barriers, a delivery vehicle must then release the therapeutic in functional form [12]. Therefore, enabling efficient translocation of cargo from endosome to cytoplasm is an essential feature of any polymer-mediated drug delivery system.
Passive Targeting
Cellular barriers present formidable obstacles in the delivery of therapeutics for cancer treatment. Fortunately, certain aspects of cancer physiology can be exploited to achieve passive targeting to tumor sites. Rapid growth of tumors leads to aberrant angiogenic vasculature. The newly formed blood vessels are often disorganized and discontinuous, resulting in increased permeability to macromolecules. Moreover, lymphatic drainage systems are often poorly developed or non-existent in tumor sites, enabling accumulation of therapeutics [20]. This phenomenon, called the enhanced permeation and retention (EPR) effect [20], has increased the tumor concentration of anticancer agents up to 70-fold in some cases [21].
Since the pioneering work of Couvreur [22], nanoscale systems have been aggressively investigated for their utility in drug delivery applications. Nanoparticle size is known to play a critical role in achieving passive targeting. Nanoparticles above 10 nm in diameter are generally able to avoid filtration by the kidneys, while less well understood, the upper size limit for passively targeted nanoparticles is thought to be approximately 150 nm [13, 23]. Extravasation and diffusional barriers limit nanoparticle access to tumors when particle size is over 200 nm [13]. Additionally, previous studies have shown that nanoparticle clearance rate increases with size [24]. One such investigation demonstrated that the blood clearance of 80 nm nanoparticles was half as fast as the clearance of 170 and 240 nm particles [25]. Presumably, this effect is due to non-specific protein adsorption on the surface of larger nanoparticles, leading to opsonization and subsequent clearance by the RES [24].
Clearly, particles with longer circulation times have superior ability to reach the tumor site through passive targeting. As opsonization is an integral step in the removal of foreign macromolecules by the RES, many efforts for increasing serum stability and extending circulation time have focused on blocking absorption of opsonins onto the nanoparticle surface [10]. Hydrophobic and charged nanoparticles associate readily with opsonins and serum proteins. Attachment of polyethylene glycol (PEG), or PEGylation, has been a popular approach for conferring “stealth” properties to circulating nanoparticles. PEG is highly flexible, hydrophilic, non-degradable, and non-toxic [26]. PEGylation helps shield hydrophobic or charged nanoparticles and slows the process of opsonization through steric hindrance [10].
Active targeting
The primary goal of a cancer therapeutic is to eliminate cancerous cells while leaving normal cellular activities umimpaired. Therefore, it is highly advantageous for a polymer delivery system to preferentially localize in the tumor tissue rather than non-cancerous tissue in the body. Through careful engineering of polymer nanoparticles, various targeting ligands can be displayed to enhance selective delivery to a tumor site, thereby decreasing the preferential localization in the liver and spleen [2, 27]. Similar to tumor sites, these organs allow passage of macromolecules, generally up to 200 nm in size. Unchecked accumulation of non-degradable compounds may lead to undesired toxicity. Attachment of antibody fragments has been explored as a potential strategy to enhance tumor targeting due to their high binding specificity. By incorporating a fragment, specificity is retained and the antibody region responsible for eliciting an immune response is not present [28]. Despite some early success in antibody targeting [29], more recent attention has been focused on exploiting transport receptors upregulated in tumors, such as transferrin and folate. In normal cells, transferrin receptors exist to facilitate the endocytosis of transferrin, an iron-binding glycoprotein. Folate receptors function to allow transport of folic acid, a vitamin required for synthesis of purines and pyrimidines [28]. Transferrin and folate receptors are constitutively over expressed in many tumor cell types due to their increased metabolic demand.
Active targeting continues to command significant attention because many cancer-cell types display tumor-specific receptor upregulation. Strategies are being developed to specifically target breast, colorectal, lung, and prostate cancer by way of tumor-specific ligands [28]. As new genetic and physiological anomalies in cancer cells are discovered, incorporation of active targeting agents will play an important role in the local delivery of specialized therapeutic agents.
Responsive polymer delivery systems
Selective delivery of biotherapeutics hinges on a safe, efficient, and accurate delivery vector. In recent years, there has been much attention on the rational design of synthetic polymers for novel treatment modalities because of their facile manufacture, large carrying capacity [1], tunable physicochemical characteristics, and modulation of biological activity through attachment of targeting ligands and PEG [30]. Interdisciplinary research between polymer chemistry and biomedical science has yielded several promising systems for polymer based drug delivery, including polymer-drug conjugates, polymeric micelles, multi-component polyplexes, and nanoscale hydrogels [21, 31]. In particular, pH-responsive polymers have been investigated as delivery agents to overcome intracellular trafficking barriers. It is well known that diseased tissues, tumors, and sites of inflammation exhibit different pH profiles than normal tissue [32]. Also, intracellular pH varies substantially depending on organelle. Endosomes and lysosomes typically exhibit pH values of 6.8 - 4.5. This pH variation can be exploited to specifically deliver antitumor therapeutics to a specific intracellular or extracellular site of action. By judicious materials selection and careful engineering of molecular architecture, polymer nanoparticles can be developed to give well-controlled pH response and drug release [33, 34]. Many pH-responsive polymer delivery systems rely on acid catalyzed hydrolysis to control drug release or on weakly charged polymer chains to mediate endosomal disruption and enable cytoplasmic release.
Anionic polymers undergo a conformational change from charged open chains to compact, hydrophobically-stabilized structures capable of disrupting the membrane of a maturing endosome through pore formation and disruption of membrane integrity. The mechanism of membrane-destabilization by anionic polymers is thought to be related to their pH-dependent conformational transition [35] and the extent of polymer association with the lipid bilayer and cellular uptake can be enhanced by increased polymer hydrophobicity [36].
Cationic polymers, which are able to electrostatically bind negatively-charged nucleic acids such as DNA and RNA, are thought to promote endosomal rupture through the “proton sponge” mechanism. These polymers, which contain ionizable groups, absorb incoming protons during endosomal acidification. This action causes an accumulation of protons and counter ions, such as Cl-, within the endosome. The high osmotic strength within the endosomal compartment subsequently leads to osmotic swelling and endosomal rupture [12].
The general model for drug delivery through endosomal release has been well described [21]. After endocytosis, the decreasing endosomal pH induces a conformational change in the polymer. pH-responsive polymers can increase membrane permeability through hydrophobic associations or the proton sponge mechanism, releasing the anti-cancer agent into the cytosol. Lysosomal trafficking is undesirable, as harsh enzymatic conditions will readily degrade therapeutic molecules.
Polymer-drug conjugates
The use of synthetic polymer-drug conjugates for drug delivery was first proposed in 1975 [37]. Generally, these entities are composed of a reactive drug conjugated to a water soluble polymer backbone through a biodegradable linkage [38]. Common pH-responsive linkages include anhydrides, cis-aconityl, hydrazones, and acetals [21]. Targeting ligands, such as transferrin, can also be conjugated to the polymer backbone. The transferrin receptor is typically over-expressed in rapidly proliferating and cancer cells [39], providing an attractive opportunity for tumor-targeting.
Covalent conjugation of anticancer drugs to a polymer backbone via a pH-sensitive linkage has been explored as an option to liberate compounds such as doxorubicin in the local tumor environment. This type of delivery requires a conjugate that is stable in circulation and normal tissue but releases a fully active drug at the target site. Mayumi and colleagues have synthesized a copolymer of vinylpyrrolidone and dimethylmaleic anhydride, where the dimethylmaleic anhydride serves as the pH-responsive linker [40]. The dimethylmaleic anhydride is able to bind amine groups at pH > 8.0 and reverts back to anhydride form at pH < 7.0, releasing the bound drug. The copolymer showed negligible toxicity to cells at concentrations as high as 1 mg/mL. Typical concentrations of polymer drug delivery systems are near 200 μg/mL [41]. The polymer-drug conjugate was demonstrated to have increased circulatory half-life and enhanced anti-tumor activity compared to doxorubicin alone. In fact, intravenously injected polymer-doxorubicin conjugates were able to completely inhibit tumor growth in murine sarcoma models, confirming that the released drug was indeed fully active.
Doxorubicin has also been conjugated to a N-(hydroxypropyl)methacrylamide (HPMA) polymer via degradable hydrazone linkages [42]. Covalent attachment was stable at physiological pH, as less than 10% of doxorubicin was released from the polymer at pH 7.4. However, at pH 5.0, nearly 50% was released after 5 hours. Intravenous injection in mice significantly slowed tumor growth lasting 60 days and prolonged survival for 30 days over control treatments.
Polymer micelles
Many polymer micelles are formed by amphiphilic block copolymers. These supramolecular structures self-assemble to form particles with hydrophobic interior and a hydrophilic corona. Hydrophobic or water-insoluble drugs can be encapsulated in the hydrophobic core. The thermodynamic and kinetic stability of micelle structures allow them to retain integrity, even after extreme dilution (e.g. intravenous injection) or extended circulation time [43]. Typical micelle size (20 - 100 nm) is large enough to avoid renal clearance yet small enough to avoid rapid uptake by the RES [13]. Physical encapsulation of hydrophobic drugs within micelles may be a more favorable approach than conjugation as covalent attachment of drugs to a polymer carrier may negatively affect their bioavailability or require conjugation to the drug's active site [43].
Kataoka and colleagues [44] have developed pH-sensitive micelles demonstrating release of adriamycin. Adriamycin was conjugated to poly(aspartate) through an acid-labile hydrazone linker. Following acid-catalyzed hydrolysis, the micellar structure was disrupted, releasing free drug. A recent study by Geng, et al. describes the formation of cylindrical micelles, or filomicelles, from copolymers of poly(ethyleneglycol) and poly(caprolactone) (PCL) [45]. These particles, owing to their extended shape, were not readily taken up by the RES and achieved circulation times of over one week after systemic administration. Acid catalyzed degradation of PCL promotes micellar fragmentation and subsequent extravasation and cellular uptake. In vivo studies in tumor-bearing mice confirmed the benefit of prolonged circulation. Paclitaxel-loaded filomicelles caused significant increase in tumor apoptosis and decrease in tumor volume relative to free drug. Additionally, the magnitude of apoptotic response and tumor size reduction increased with filomicelle length (i.e. longer filomicelles delivered paclitaxel more efficiently than did short filomicelles). Long-circulating, pH degradable structures such as this could have significant utility in cancer therapy. Chemotherapeutic agents, such as paclitaxel or doxorubicin, could be administered less frequently, decreasing the intensity of conventional chemotherapy and improving quality of life for the patient.
pH-responsive micelles based on acetal polymers have shown promise in controlled release of doxorubicin [46]. The acetal block forms a hydrophobic core able to efficiently encapsulate doxorubicin (12 wt%). Upon exposure to mildly acidic conditions, the acetal core begins to hydrolyze, exposing polar groups in the micelle core. This alters solubility of the core block and disrupts the micelle, releasing free doxorubicin to the surrounding environment. This system releases 50% of doxorubicin content after 5 hours in pH 4.0 and 40 hours in pH 5.0, while the release rate at pH 7.4 is negligible. Thus, the time scale of doxorubicin release suggests this system would be applicable for sustained release to the acidic interstitial fluid surrounding tumors. As intracellular trafficking from endosomes to lysosomes occurs on the order of minutes, a carrier designed to enhance intracellular delivery through endosomal rupture should possess a comparable response time.
Polymer-Drug Polyplex
Polyplexes are formed by electrostatic or hydrophobic interactions between polymers and nucleic acids, proteins, or low molecular weight drugs. These structures are thought to have increased mechanical stability over micelles due to chain entanglements and hydrophobic interactions [47]. For example, polycations such as polyethyleneimine are routinely used to bind negatively-charged plasmid DNA for gene therapy in cancer treatment. These systems contain DNA-binding amines, and may contain targeting ligands to direct cell-specific delivery and receptor-mediated endocytosis [12]. In some cases, the addition of pH-responsive polyanions or hydrophobic comonomers may aid in the endosomal release of multi-component polyplexes [48-50]. In fact, DNA transfer has been increased up to three orders of magnitude by introduction of a membrane-lytic peptide [12].
Work by Shenoy and Amiji [51-53] has focused on using poly(β-amino esters) as pH-responsive polyplexes for delivery of chemotherapeutic agents. Copolymers of poly(ethylene oxide) and poly(propylene oxide) were blended with poly(β-amino ester) to form spherical nanoparticles of 150 - 200 nm. These polymers can be formulated to efficiently deliver small molecular weight drugs or polynucleotides in the form of oliogonucleotides or plasmid DNA. Cytotoxicity studies have demonstrated that poly(β-amino esters) are significantly less toxic than conventional polymers used for nucleic acid delivery, such as poly(ethyleneimine) or poly(L-lysine) [51]. Paclitaxel was efficiently loaded into these nanoparticles, achieving 20 wt% drug in polymer concentration. The particles exist in a stable, insoluble form at physiological pH but undergo a rapid dissolution at pH < 6.5, releasing drug to the surrounding environment in a sudden burst. When injected intravenously to tumor-bearing mice, paclitaxel-loaded nanoparticles significantly inhibited tumor growth relative to paclitaxel injection alone [53]. Moreover, body weight and blood count measurements indicated little to no adverse toxicity in mice injected with poly(β-amino ester).
In an effort to explore synergistic advantage of both pH- and temperature-responsive behavior, Kang, et al. have prepared a series of graft copolymers based on temperature-responsive N-isopropylacrylamide and pH-responsive sulfamethoxypyridazine [47]. Doxorubicin was loaded into the polyplex at 10 wt % drug in polymer and released from the matrix by inducing a hydrophobic to hydrophilic phase transition. As expected, the release rate of doxorubicin was highest when the temperature-sensitive and pH-sensitive blocks were simultaneously converted to the hydrophilic state.
Nanoscale Hydrogels
Nanoscale hydrogels, or nanogels, are materials with diverse biomedical applications. Because of their inherent mechanical integrity and biocompatibility, hydrogels have utility in imaging, biosensing, molecular recognition, and therapeutic delivery [41, 54]. Nanoscale hydrogels are of particular interest in drug delivery because of their tunable nanoscale dimensions (i.e. large enough to avoid renal clearance and small enough to evade RES), drug loading capacity, colloidal stability, and large surface area for conjugation of active targeting moieties [54]. Furthermore, the nanoscale dimensions ensure a rapid response to pH changes, an attractive attribute in drug delivery applications. The pH response of hydrogels can be modulated by the pKa of polymer chains and choice of monomer.
Cationic monomers with pKa > 7, such as poly(dimethylaminoethyl methacrylate) (PDMAEMA), will exist in an ionic state at physiological pH. The positively charged amino groups will repel each other and permit the osmotically-driven flux of water into the polymer matrix. However, increasing the pH above the nanogel pKa will deprotonate the amino groups and hydrophobic interactions will collapse the gel, excluding water. Polymers based on anionic monomers (pKa < 7), such as methacrylic acid, will behave in the opposite manner. At pH below the pKa, pendant carboxyl groups are protonated and hydrophobic interactions maintain the gel in the collapsed state. Increasing the pH above the pKa will deprotonate the carboxyl groups, swelling the gel through electrostatic repulsions and influx of water.
Nanogels are being explored as drug delivery agents for cancer due to their ability to efficiently encapsulate therapeutics through simple equilibrium partitioning or electrostatic interactions. Using a cationic chitosan-based nanogel (180 nm diameter), researchers have delivered antitumor therapeutic metotrexate disodium to tumor cells [54]. Blanchette and Peppas have explored the use of methacrylic acid and ethylene glycol nanogels for oral delivery of chemotherapeutic agents [55]. Bleomycin was encapsulated in the hydrogel during polymerization and release studies demonstrated favorable release kinetics. The therapeutic agent was well protected in the hydrogel at low pH and nearly 100% of bleomycin was released after 1 hour at pH 7.4.
Recently, Peppas and coworkers described the synthesis of polycationic nanogels capable of well-defined hydrophilic-hydrophobic transitions near physiological pH [34, 41, 56] and successfully demonstrated loading of biological therapeutics (e.g. insulin) and imaging agents (e.g. gold nanoparticles). Akiyoshi and colleagues recently completed a Phase I clinical trial using a self-assembled, cholesterol-modified pullulan nanogel to deliver a HER2 protein fragment for vaccination against cancer [57]. HER2 protein has been implicated in increased aggressiveness of tumors, particularly in breast cancer [28]. Their results indicate that administration of the protein fragment successfully initiated an innate immune response against HER2.
Conclusions
The advantages of intelligent, pH-responsive delivery systems able to carry potent therapeutics are clear. Such systems would allow efficient delivery at a target site, thereby avoiding accumulation in non-target tissues, lowering therapeutic dosage, and minimizing harmful side effects. Much of the work ongoing in pH-responsive nanoparticles for cancer therapy remains focused on delivering conventional chemotherapeutic agents, such as doxorubicin and paclitaxel. Recent advances in our understanding of cancer pathology, growth, survival, and apoptotic pathways have unlocked a host of potential therapeutic targets which offer the opportunity for highly specific therapies. Customization and optimization of polymer delivery systems to efficiently deliver new therapeutics is an integral step in aiding their progression to clinical practice. Additionally, pH-responsive polymer delivery systems have not progressed well to clinical studies for cancer therapy. Inefficient translation of in vitro properties to in vivo efficacy, toxicity concerns, regulatory issues, and crowded drug pipelines may be factors slowing this process. Though the current investigations on pH-responsive nanoparticles are encouraging, there is an urgent need for meaningful data on pharmacokinetics, biodistribution, and physicochemical properties in vivo and particularly how these responsive nanoparticles behave in humans. The solution to this problem will require increased cooperation between polymer scientists, chemical and biomedical engineers, and medical scientists.
Acknowledgments
Portions of our laboratory work discussed here were supported in part by grants National Institutes of Health (No. EB-00246-18), and National Science Foundation (No. 1033746). W.B.L is a National Science Foundation Fellow
References
- 1.Dincer S, Turk M, Piskin E. Intelligent polymers as nonviral vectors. Gene Ther. 2005;12:S139–S145. doi: 10.1038/sj.gt.3302628. [DOI] [PubMed] [Google Scholar]
- 2.Couvreur P, Brigger I, Dubernet C. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliver Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006. [Google Scholar]
- 4.Couvreur P, Puisieux F. Nanoparticles and Microparticles for the Delivery of Polypeptides and Proteins. Adv Drug Deliver Rev. 1993;10:141–162. [Google Scholar]
- 5.Couvreur P, Dubernet C, Puisieux F. Controlled Drug-Delivery with Nanoparticles -Current Possibilities and Future-Trends. Eur J Pharm Biopharm. 1995;41:2–13. [Google Scholar]
- 6.Langer R. Drug Delivery and Targeting. Nature. 1998:5–10. [PubMed] [Google Scholar]
- 7.Duncan R. Polymer conjugates as anticancer nanomedicines. Nature Reviews Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 8.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 9.Peppas NA. Intelligent therapeutics: biomimetic systems and nanotechnology in drug delivery. Adv Drug Deliver Rev. 2004;56:1529–1531. doi: 10.1016/j.addr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Vauthier C, Couvreur P. Nanotechnology: Intelligent design to treat complex disease. Pharm Res-Dordr. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 12.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discovery. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 13.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discovery. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka YJ, Leong KW. Engineering strategies to enhance nanoparticle-mediated oral delivery. Journal of Biomaterials Science-Polymer Edition. 2008;19:1549–1570. doi: 10.1163/156856208786440479. [DOI] [PubMed] [Google Scholar]
- 16.Bhavsar MD, Shenoy Dinesh B, Amiji Mansoor M. Polymeric Nanoparticles for Delivery in the Gastro-Intestinal Tract. In: Torchilin VP, editor. Nanoparticulates as Drug Carriers. Imperial College Press; London: 2006. [Google Scholar]
- 17.Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 2006;11:905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 21.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 22.Couvreur P, Kante B, Roland M, Guiot P, Bauduin P, Speiser P. Polycyanoacrylate Nanocapsules as Potential Lysosomotropic Carriers - Preparation, Morphological and Sorptive Properties. J Pharm Pharmacol. 1979;31:331–332. doi: 10.1111/j.2042-7158.1979.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 23.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Delivery Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. In vivo tumor targeting of tumor necrosis factor-a-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Tong R, Cheng JJ. Anticancer polymeric nanomedicines. Polym Rev (Philadelphia, PA, U S) 2007;47:345–381. [Google Scholar]
- 27.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Delivery Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama K, Takahashi N, Tagawa T, Nagaike K, Iwatsuru M. Immunoliposomes bearing polyethyleneglycol-coupled Fab' fragment show prolonged circulation time and high extravasation into targeted solid tumors in vivo. FEBS Lett. 1997;413:177–180. doi: 10.1016/s0014-5793(97)00905-8. [DOI] [PubMed] [Google Scholar]
- 30.des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for Drug Delivery Systems. Annual Review of Chemical and Biomolecular Engineering. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Leobandung W, Ichikawa H, Fukumori Y, Peppas NA. Monodisperse nanoparticles of poly(ethylene glycol) macromers and N-isopropyl acrylamide for biomedical applications. J Appl Polym Sci. 2003;87:1678–1684. [Google Scholar]
- 34.Liechty WB, Caldorera-Moore M, Phillips MA, Schoener C, Peppas NA. Advanced molecular design of biopolymers for transmucosal and intracellular delivery of chemotherapeutic agents and biological therapeutics. J Control Release. doi: 10.1016/j.jconrel.2011.06.009. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yessine MA, Leroux JC. Membrane-destabilizing polyanions: interaction with lipid bilayers and endosomal escape of biomacromolecules. Adv Drug Delivery Rev. 2004;56:999–1021. doi: 10.1016/j.addr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd JB, Pratten MK, Duncan R, Kooistra T, Cartlidge SA. Substrate Processing and Selection in Endocytosis. Biochem Soc Trans. 1984;12:977–978. doi: 10.1042/bst0120977. [DOI] [PubMed] [Google Scholar]
- 37.Ringsdorf H. Structure and properties of pharmacologically active polymers. Journal of Polymer Science Part C Polymer Symposium. 1975;51:135–153. [Google Scholar]
- 38.Duncan R, Vicent MJ, Greco F, Nicholson RI. Polymer-drug conjugates: towards a novel approach for the treatment of endrocine-related cancer. Endocr Relat Cancer. 2005;12:S189–S199. doi: 10.1677/erc.1.01045. [DOI] [PubMed] [Google Scholar]
- 39.Cassidy J, Schätzlein AG. Tumour-targeted drug and gene delivery: principles and concepts. Expert Reviews in Molecular Medicine. 2004;6:1–17. doi: 10.1017/S1462399404008269. [DOI] [PubMed] [Google Scholar]
- 40.Kamada H, Tsutsumi Y, Yoshioka Y, Yamamoto Y, Kodaira H, Tsunoda Si, Okamoto T, Mukai Y, Shibata H, Nakagawa S, Mayumi T. Design of a pH-Sensitive Polymeric Carrier for Drug Release and Its Application in Cancer Therapy. Clin Cancer Res. 2004;10:2545–2550. doi: 10.1158/1078-0432.ccr-03-0544. [DOI] [PubMed] [Google Scholar]
- 41.Fisher O, Kim T, Dietz S, Peppas N. Enhanced Core Hydrophobicity, Functionalization and Cell Penetration of Polybasic Nanomatrices. Pharm Res. 2009;26:51–60. doi: 10.1007/s11095-008-9704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etrych T, Jelínková M, Ríhová B, Ulbrich K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release. 2001;73:89–102. doi: 10.1016/s0168-3659(01)00281-4. [DOI] [PubMed] [Google Scholar]
- 43.Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(-amino acid) micelles for drug delivery. Adv Drug Delivery Rev. 2002;54:169–190. doi: 10.1016/s0169-409x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 44.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angewandte Chemie-International Edition. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 45.Geng Y, Dalhaimer P, Cai SS, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillies ER, Frechet JMJ. pH-Responsive Copolymer Assemblies for Controlled Release of Doxorubicin. Bioconjug Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 47.Kang SI, Na K, Bae YH. Physicochemical characteristics and doxorubicin-release behaviors of pH/temperature-sensitive polymeric nanoparticles. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2003;231:103–112. [Google Scholar]
- 48.Chen R, Khormaee S, Eccleston ME, Slater NKH. The role of hydrophobic amino acid grafts in the enhancement of membrane-disruptive activity of pH-responsive pseudo-peptides. Biomaterials. 2009;30:1954–1961. doi: 10.1016/j.biomaterials.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen RJ, Eccleston ME, Yue ZL, Slater NKH. Synthesis and pH-responsive properties of pseudo-peptides containing hydrophobic amino acid grafts. J Mater Chem. 2009;19:4217–4224. [Google Scholar]
- 50.Liechty WB, Chen R, Farzaneh F, Tavassoli M, Slater NKH. Synthetic pH-Responsive Polymers for Protein Transduction. Adv Mater. 2009;21:3910–3914. doi: 10.1002/adma.200901733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol Pharm. 2005;2:357–366. doi: 10.1021/mp0500420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shenoy D, Little S, Langer R, Amiji M. Poly(Ethylene Oxide)-Modified Poly(β-Amino Ester) Nanoparticles as a pH-Sensitive System for Tumor-Targeted Delivery of Hydrophobic Drugs: Part 2. In Vivo Distribution and Tumor Localization Studies. Pharm Res. 2005;22:2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devalapally H, Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model, Cancer Chemother. Pharmacol. 2007;59:477–484. doi: 10.1007/s00280-006-0287-5. [DOI] [PubMed] [Google Scholar]
- 54.Raemdonck K, Demeester J, De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–715. [Google Scholar]
- 55.Blanchette J, Peppas NA. Oral chemotherapeutic delivery: Design and cellular response. Ann Biomed Eng. 2005;33:142–149. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 56.Fisher OZ, Peppas NA. Polybasic Nanomatrices Prepared by UV-Initiated Photopolymerization. Macromolecules. 2009;42:3391–3398. doi: 10.1021/ma801966r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.K S, Kageyama Shinichi, Hirayama Michiko, Nagata Yasuhiro, Imai Hiroshi, Shiraishi Taizo, Akiyoshi Kazunari, Scott Andrew M, Murphy Roger, Hoffman Eric W, Old Lloyd J, Katayama Naoyuki, Shiku Hiroshi. Humoral immune responses in patients vaccinated with 1-146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008;99:601–607. doi: 10.1111/j.1349-7006.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]