Abstract
Objectives
To compare the sensitivity of MRI to detect colorectal cancer liver metastases (CRLM) after ingestion of manganese-based contrast agent (CMC-001) with that of a comprehensive intravenous gadobenate dimeglumine protocol, and to assess the safety and acceptability of oral manganese.
Methods
20 patients suspected of having 1–6 CRLM were included prospectively in this randomised cross-over study. Liver MRI was performed with a one-week interval at 1.5 T and included T1-w VIBE and T2-HASTE, before and after administration of 1.6 g CMC-001 or 0.1 mmol/kg gadobenate dimeglumine. The metastasis-to-liver signal intensity (SI) ratio was calculated. Standard of reference was histopathology after surgery, or combination of other imaging studies and/or follow up. Adverse events (AE) and clinicolaboratory tests were monitored.
Results
Of 44 metastases, 41 were detected after CMC-001 (93%) and 42 after gadobenate dimeglumine (95%). Fifteen false–positive lesions were found after CMC-001 and 2 after gadobenate dimeglumine. The metastasis-to-liver SI ratio was significantly higher after CMC-001 than after gadobenate dimeglumine (0.51 and 0.21 respectively, P < 0.0001). More AE occurred after manganese compared to gadobenate dimeglumine.
Conclusions
CMC-001 is as sensitive as an extensive intravenous gadobenate dimeglumine protocol in detecting CRLM. It was relatively well tolerated but had higher rates of gastrointestinal AE.
Key Points
• Liver MRI after ingestion of manganese is highly sensitive for detecting metastases
• High false–positive rate necessitates further evaluation, in some cases
• The MR examination time is short
• Oral ingestion of manganese seems safe and relatively well tolerated by patients
• Manganese compounds may be useful for liver metastasis surveillance after colorectal cancer
Keywords: Magnetic resonance imaging, Liver, Neoplasm metastasis, Colorectal neoplasm, Contrast media
Introduction
The liver is the most common site of metastases from the gastrointestinal tract, including pancreatic, gastric, small bowel, and colorectal cancers. In surveillance for liver metastases, the primary imaging technique is computed tomography (CT) [1, 2]. Contrast-enhanced ultrasound is also reported to perform well [3]. However, MRI due to its superior diagnostic sensitivity and specificity when evaluating liver lesions plays a major role [4, 5].
To improve the detection and characterisation of lesions, a number of intravenous MRI contrast agents have been developed. Among these, two of the T1-shortening contrast agents have liver-specific characteristics: gadoxetic acid (Primovist®) and gadobenate dimeglumine (MultiHance®). These contrast agents have to be administered intravenously and contain gadolinium, which increases the risk of nephrogenic systemic fibrosis (NSF) in patients with low glomerular filtration rate (GFR) [6].
A liver-specific contrast medium that is not administered intravenously and which does not contain gadolinium would be of great value for evaluating patients at high risk of liver metastases. A new contrast medium where the manganese is administered orally has been developed [7, 8]. The uptake of manganese from the bowel is normally very limited, but can be facilitated when it is mixed with alanine and vitamin D3. The manganese is then extracted from the blood circulation by the liver and is subsequently excreted with bile [9–11]. The high manganese uptake in the liver by the hepatocytes significantly decreases their T1 relaxation time, causing the liver parenchyma to appear bright on T1-weighted images. Non-functioning hepatocytes and metastases will not take up manganese and will therefore be of low signal intensity on T1-weighted images [7, 8, 12].
The primary aim of this phase II trial was to evaluate whether the sensitivity of the new oral contrast medium to detect colorectal liver cancer metastases (CRLM) was comparable to that of a comprehensive intravenous gadobenate dimeglumine protocol. The secondary aim was to assess the safety and acceptability of this new agent (CMC-001).
Material and methods
Population and standard of reference
Local ethics committee approval and informed patient consent were obtained for this open, randomised cross-over prospective study in which each patient constituted his/her own control. Twenty patients, 10 men and 10 women, aged 51–79 years (mean 64 years) were recruited prospectively and consecutively. All patients had been referred to the multidisciplinary team for evaluation of 1–6 suspected CRLMs before partial liver resection. The suspected metastases had been observed at contrast-enhanced CT in 17 patients and at MRI in 3 patients. In addition, nine of the patients had undergone a contrast-enhanced transabdominal ultrasound. At the final analysis (histopathology, additional imaging or follow-up) one or more metastases were found in 16 of the 20 patients; in 4 patients there were no metastases. In total, 44 metastases were identified (mean size: 19.5 mm, standard deviation: 15.6, size range: 3–73 mm). Metastases were verified at histopathology after surgery (n = 25), at combined evaluation of contrast-enhanced ultrasound and gadobenate dimeglumine-enhanced MRI by a consensus committee consisting of radiologists and surgeons at the multidisciplinary conference (n = 7), contrast-enhanced ultrasound performed at least 3 months later (n = 8), intra-operative contrast-enhanced ultrasound (n = 3) or contrast-enhanced CT 3 months later (n = 1). The absence of metastatic lesion in four patients was confirmed by histopathology in two, by combined evaluation of contrast-enhanced ultrasound and gadobenate dimeglumine-enhanced MRI in one patient and, finally, by MDCT examination performed three months later in one patient. In these four patients, the suspected metastasis had been observed at CT in three patients and at gadoxetic acid enhanced MRI in one.
Imaging
Two MRI examinations were performed on each patient at an interval of 1 week; orally administered manganese-based contrast medium CMC-001 (manganese chloride tetrahydrate MnCl2(H2O)4, CMC Contrast, Lyngby, Denmark) or intravenously administered gadobenate dimeglumine (MultiHance®, Bracco, Milan, Italy) was used in random order, based on sealed envelopes.
The patients arrived at the MR unit early in the morning after one night of fasting. Imaging was performed at 1.5 T (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany), combining the spine and the flexible body array coil before administration of contrast medium and either 3 h after oral ingestion of CMC-001 or after intravenous gadobenate dimeglumine using a 5-phase imaging protocol (unenhanced, arterioportal, portal venous, 5 min and 2 h), serving as our standard protocol in daily practice. The dose of CMC-001 was equivalent to 1.6 g MnCl2 and was dissolved in 200–400 mL of water. To facilitate its uptake, the manganese is combined with 1 g alanine and 1600 IU vitamin D3. The dose of gadobenate dimeglumine was 0.1 mmol/kg.
For T1-weighted imaging, axial breath-hold 3D T1-weighted images [volumetric interpolated breath hold examination (VIBE), echo time (TE) 2.03–2.09 ms, repetition time (TR) 4.05–4.29 ms, field of view 40 cm and 120 1.8 mm thick slices] were obtained. For T2 imaging, 60 axial half-Fourier acquisition single-shot turbo spin-echo (HASTE) images with an echo time (TE) 76 ms, repetition time (TR) 1000 ms, field of view 38 × 28.5 cm and 4 mm thick slices) were used. Total MR examination time for CMC-001 session was about 10 min each for pre- and post-contrast imaging. At the gadobenate dimeglumine session, the total MR examination time was 60 + 10 min (Table 1).
Table 1.
The comprehensive protocol used when using gadobenate dimeglumine
| Sequence | Imaging plane | Slice thickness (mm) | TE (ms) | TR (ms) | Imaging time | Comment |
|---|---|---|---|---|---|---|
| T2-weighted HASTE | Axial | 4 | 76 | 1000 | 3 min | Respiratory triggering |
| T2-weighted HASTE | Coronal | 4 | 76 | 1080 | 3 min | Respiratory triggering |
| True-FISP | Axial | 4 | 1.46–1.54 | 3.43–3.61 | 18 s | Breath-hold |
| True-FISP | Sagittal | 4 | 1.38 | 3.23–3.30 | 18 s | Breath-hold |
| T1-weighted in/out of phase | Axial | 4 | 5.04/2.38 | 126 | 1 min 11 s | Respiratory triggering |
| MRCP | Axial | 3 | 678 | 3339–8086 | 3 min | Respiratory triggering |
| MRCP | Coronal | 1.5 | 678 | 3383–10546 | 3 min | Respiratory triggering |
| T1-weighted 3D VIBE | Axial | 1.8 | 2.03–2.09 | 4.05–4.29 | 22 s | Breath-hold |
| Before and after Gd-injection1 |
1 unenhanced, arterioportal, portal venous, 5 min and 2 h post-contrast
In all cases of contrast-enhanced ultrasound, the microbubble-containing contrast agent sulphur hexafluoride (SonoVue®, Bracco, Milan, Italy) was injected in a single dose of 2.4 mg/ml followed by a flush of 5–10 ml isotonic saline; when needed, the injection was repeated to evaluate the whole liver.
For patient safety, all examinations were evaluated at the time of the examination by one of the participating radiologists (N.A.) and the imaging findings were discussed at the multidisciplinary conference for decision-making. After a period of at least 6 weeks all examinations were re-evaluated in consensus by the two participating radiologists (N.A. and T.B.) for the purposes of the present study. The participating radiologists were aware that all patients had been referred for surgical procedure, but did not have access to previous studies or surgical outcome.
Image evaluation-lesion detection analysis
The unenhanced series were first evaluated, then the corresponding contrast-enhanced series. In 10 patients, the CMC-enhanced examination was evaluated first; in the other 10, the comprehensive gadobenate dimeglumine examination was evaluated first. Irrespectively of order, the CMC-001 and the dimeglumine session were evaluated with an interval of at least 1 week. When evaluating CMC-enhanced images, both T1-weighted and T2-HASTE images were used. Criteria for the diagnosis of metastasis were low signal intensity at T1-weighted images and slightly high-signal intensity at T2-HASTE images. Criteria for classifying a lesion as non-metastatic (i.e. haemangioma or/and cyst) were low-signal intensity at T1-weighted images and markedly high-signal intensity at T2-HASTE images. When evaluating gadobenate dimeglumine-enhanced images, the whole comprehensive protocol was used. Criteria for the diagnosis of liver metastasis were slightly hyperintense appearance in the T2-HASTE series, discrete peripheral rim contrast enhancement in the arterial and/or portal venous phases, and hypointense appearance in the liver-specific phase of the contrast enhanced series (2 h after administration of contrast medium). Criteria for the diagnosis of haemangioma were presence of discontinuous nodular peripheral enhancement following intravenous contrast agent administration and markedly high-signal intensity at T2-HASTE series. Criteria for the diagnosis of cyst were absence of enhancement following intravenous contrast agent administration and markedly high-signal intensity at T2-HASTE series. The number of metastases and their greatest diameter were recorded.
Image evaluation-quantitative analysis
The two participating radiologists (N.A. and T.B.) performed the quantitative analysis shortly after the lesion detection analysis was completed. There were two sets of unenhanced images of the liver: one before CMC-001 and one before gadobenate dimeglumine. For the calculations of lesion signal intensity before contrast-medium administration, the chronologically first of the two unenhanced series was used; for the calculations after contrast administration, only the hepatospecific phases were used (i.e. the T1-weighted image 3 h after ingestion of CMC-001 or the T1-weighted image 2 h after intravenous injection of gadobenate dimeglumine, respectively).
The signal intensity of the metastasis was obtained from a centrally placed circular ROI with half the diameter of the metastasis. The signal intensity of the surrounding liver was obtained from a representative region near the metastasis, in the same image, on the same anteroposterior level. The metastasis-to-liver ratio was calculated as:
Safety and acceptability assessment
Patients were carefully monitored for occurrence of AE at 1 and 2 h after contrast- medium administration and were additionally contacted by telephone at 24 and 48 h after each product administration for a follow-up of any ongoing or additional AE. AE were judged by the investigator as mild, moderate, or severe and those judged as having a reasonable causal relationship to the contrast agents qualified as adverse drug reactions (ADR). Other safety variables evaluated both before and at various time points after contrast-medium administration included clinical laboratory assessments (serum clinical chemistry, haematology, serology, urinalysis), vital signs (blood pressure and heart pulse rate), electrocardiogram (ECG) and physical examination (data not shown).
Statistics
The calculation was based on a one-sample t-test. Non-parametric tests were assessed by McNemar’s test (number of metastases after CMC-001 vs. unenhanced and after gadobenate dimeglumine vs. unenhanced). Parametric test (comparison of metastasis to liver ratio) was done by using Student’s paired t-test.
Results
At the unenhanced T1-weighted image series, 27 of 44 metastases were detected (sensitivity 61%, Table 2). After contrast-medium administration, a statistically significantly greater number of metastases were detected: after CMC-001, 41 of 44 metastases were detected (sensitivity 93%, P < 0.001) and after gadobenate dimeglumine 42 of 44 metastases were detected (sensitivity 95%, P < 0.001) (Fig. 1). There was no statistically significant difference in the number of detected metastases between CMC-001 and gadobenate dimeglumine. There were more false–positive lesions detected at CMC-001 than at gadobenate dimeglumine (Table 3). A false–positive result for both CMC-001 and gadobenate dimeglumine-enhanced liver MRI is presented in Fig. 2, while a false–negative result for CMC-001 and gadobenate dimeglumine is presented on Figs. 3 and 4, respectively. The mean metastasis-to-liver ratio increased from 0.22 (SD 0.16) before administration of contrast to 0.51 after CMC-001 (SD 0.17) P < 0.0001, but was unchanged after gadobenate dimeglumine [0.21 (SD 0.15) before and 0.21 (SD 0.09) after] (Table 4).
Table 2.
The number of liver metastases detected before and after contrast-medium administration
| Unenhanced | CMC-001 | Gadobenate dimeglumine | |
|---|---|---|---|
| True positive | 27a | 41b | 42c |
| False negative | 17 | 3 | 2 |
| False positive | 7 | 15 | 2 |
| Sensitivity (%) | 61 | 93 | 95 |
a–b P < 0.001, a–c P < 0.001, b–c not significant
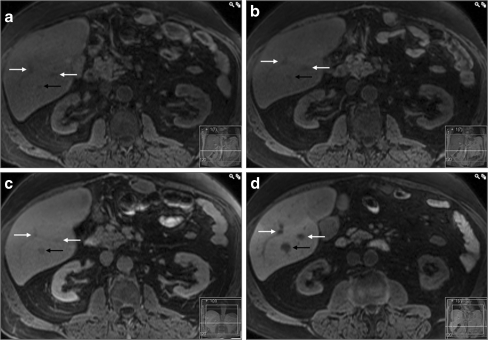
Fig. 1.
A typical case of colorectal liver metastasis. In the T1-weighted images the metastasis (black arrow) has lower signal intensity compared to the surrounding parenchyma at all phases: a, b before contrast administration, c two hours after intravenous administration of gadobenate dimeglumine and d three hours after oral ingestion of CMC-001. Some grade of enhancement of the lesion is observed after intravenous administration of gadobenate dimeglumine (c) but not after ingestion of CMC-001 (d). The white arrows indicate liver vessels
Table 3.
False–positive lesions
| CMC-001 | Gadobenate dimeglumine | |
|---|---|---|
| FNH | 4a | |
| Haemangioma | 4 | |
| Fibrotic haemangioma | 2 | 2 |
| Vessel | 2 | |
| Dysfunction/low uptake | 2b | |
| Clips | 1 | |
| Total | 15 | 2 |
aall focal nodular hyperplasias (FNH) were observed in the same patient
bboth false–positive lesions observed in the same patient
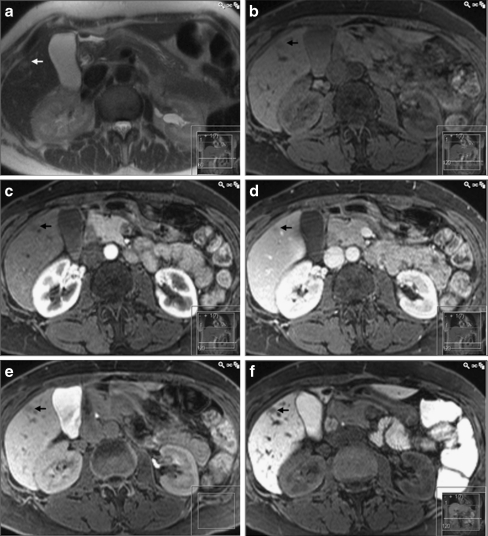
Fig. 2.
A histopathologically proven fibrotic haemangioma (arrow) being falsely classified as metastasis on both gadobenate dimeglumine and CMC-001 enhanced MRI. In a T2-HASTE image, the lesion is faintly hyperintense. In the T1-weighted images, both b before and after injection of gadobenate dimeglumine (c arterial, d portal venous and e hepatobiliary phases) as well as f 3 h after ingestion of CMC-001, the lesion has low-signal intensity, being impossible to differentiate from metastasis
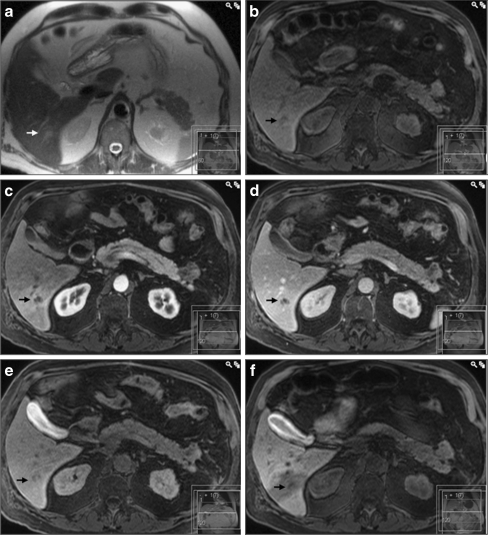
Fig. 3.
A false–negative result for CMC-001 enhanced MRI. In a T2-HASTE and b unenhanced T1-weighted images, the lesion (arrow) is hardly discernible from an adjacent liver vessel. In the T1 images after injection of gadobenate dimeglumine (c arterial, d portal venous and e hepatobiliary phases), the lesion has clearly lower signal intensity compared to the adjacent liver parenchyma. In f the T1-weighted image 3 h after ingestion of CMC-001, the lesion is difficult to detect as it is situated in a part of the liver with decreased uptake of CMC-001 due to decreased portal perfusion and/or hepatobiliary dysfunction
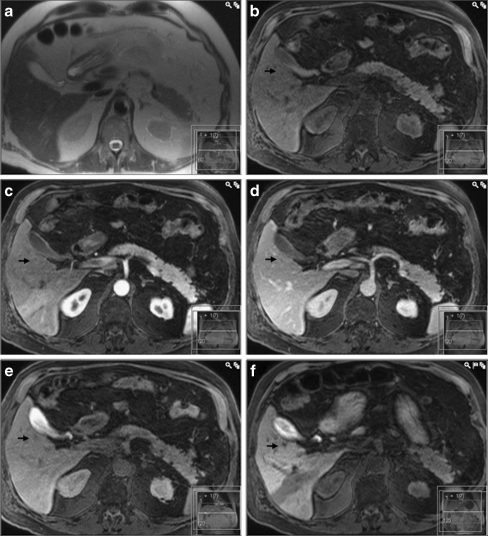
Fig. 4.
A false–negative result for gadobenate dimeglumine enhanced MRI (same patient as in Fig. 3). Both in the a T2-HASTE image as well as in the T1-weighted images after injection of gadobenate dimeglumine (c arterial, d portal venous and e hepatobiliary phases, the lesion is difficult to identify. However, in the f T1-weighted image 3 h after ingestion of CMC-001, the lesion (arrow) has clearly lower signal intensity compared to the adjacent parenchyma allowing for correct diagnosis of a metastasis
Table 4.
Mean signal intensity (SI), of liver and metastases before and after contrast-medium administration. The SI is in arbitrary units (one standard deviation within parenthesis)
| CMC-001 | Gadobenate dimeglumine | |||
|---|---|---|---|---|
| Unenhanced | 3 h post contrast | Unenhanced | 2 h post contrast | |
| Liver SI | 160 (35) | 268 (91)a | 160 (37) | 267 (75)b |
| Metastasis SI | 122 (27) | 122 (31)c | 127 (32) | 207 (53)d |
| Metastasis to liver ratio | 0.22 (0.16) | 0.51 (0.17)e | 0.21 (0.15) | 0.21 (0.09)f |
a-bnot significant
c–d P < 0.0001
e-f P < 0.0001
A total of 38 unique AE were reported in 19 patients. After CMC-001 administration, there were 31 AE reported (24 judged to be ADR) and after gadobenate dimeglumine, there were 9 AE reported (3 judged to be ADR). The number of patients with at least 1 AE was 19 after CMC-001 and 8 after gadobenate dimeglumine (Table 5). The most common AE were diarrhoea (12 AE in total, 12 after CMC-001 and 0 after gadobenate dimeglumine) and nausea (4 after CMC-001 and 2 after gadobenate dimeglumine). The most common ADR was diarrhoea (12 after CMC-001 and 0 after gadobenate dimeglumine) followed by supraventricular extrasystoles (1 after CMC-001 and 0 after gadobenate dimeglumine), back pain (1 after CMC-001 and 0 after gadobenate dimeglumine), headache (1 after CMC-001 and 0 after gadobenate dimeglumine) and urticaria (0 after CMC-001 and 1 after gadobenate dimeglumine).
Table 5.
Total number of participants, number of participants with at least one adverse event (AE), adverse drug reactions (ADR)/number of AE, and intensity of AE for CMC-001 and gadobenate dimeglumine enhanced liver MRI
| CMC-001 | Gadobenate dimeglumine | |
|---|---|---|
| Total number of participants | 20 | 20 |
| Participants with at least one AE | 19 (95%) | 8 (40%) |
| Number of ADR/AE | 24/31 | 3/9 |
| Intensity of AE (mild/moderate/severe) | 20/10/1 | 7/2/0 |
The majority of adverse events were mild (20 AE after CMC-001 and 7 after gadobenate dimeglumine) or moderate (10 AE after CMC-001 and 2 after gadobenate dimeglumine). One AE (back pain judged to be possibly related to CMC-001) was recorded as severe in intensity. No serious AE were reported. There were no clinically significant changes in clinical laboratory changes, vital signs, ECG or at physical examination.
Discussion
The sensitivity to detect CRLM after orally administered manganese (93%) was comparable to that of intravenously administered gadobenate dimeglumine (95%). The high level of sensitivity concur with previously published studies on detection of liver metastases at MRI using intravenously administered mangafodipir trisodium [13] and gadobenate dimeglumine [14, 15]. CRLM as small as 3 mm could be detected, regardless of which of the two liver-specific contrast agents was used. The novel contrast agent CMC-001 has, therefore, the potential to be used for surveillance of patients at high risk of liver metastases, distinguishing those cancer patients who need more extensive liver evaluation from those who do not. Such a group of patients are those with colorectal cancer. In this patient group liver metastases are present in up to 25% of the patients at the time of diagnosis [16] and in up to 50% five years later [17]. Patients who have undergone “curative” liver surgery for colorectal cancer metastases have a high recurrence rate, more than 60% [18]. Hence, patients with CRLM require follow up imaging for many years, preferentially with a fast and sensitive method. However, further studies assessing the effectiveness of CMC-001 enhanced liver MRI as a surveillance tool are required. An advantage of CMC-001 is that it allows patients to self-administer the contrast agent orally 2–3 h before the imaging acquisition session and obviating the need for intravenous injection.
There were 15 false–positive results at the CMC-001 session compared with 2 false–positive results at the gadobenate dimeglumine session. This higher false–positive rate is a downside of CMC-001 compared to gadobenate dimeglumine-enhanced liver MRI, where additional information about the contrast behavior of lesions is obtained at the dynamic phase after contrast injection, resulting in higher specificity. In clinical practice, this means that when lesions are detected at CMC-001 enhanced liver MRI, further evaluation by more specific techniques is necessary; once these lesions have been characterised, CMC-001 enhanced liver MRI should be highly accurate for detecting new lesions. CMC-001 should therefore be of special value in patients where repeated MRI exams are needed, i.e. in the surveillance situation. In the study setting, the radiologists were not allowed to evaluate previous examinations or patient history. This is a limitation of our study, because the number of false–positive lesions has probably been overestimated.
When comparing the signal intensity of the liver parenchyma before and after contrast-medium administration, there was no difference in the signal increase after CMC-001 compared with that after gadobenate dimeglumine (Table 4). However, the metastases did also show some grade of enhancement after gadobenate dimeglumine, but not after CMC-001. Thus, there was a significant increase in the metastasis-to-liver ratio after CMC-001, but not after gadobenate dimeglumine. In the present study, the sensitivity to detect CRLM on T1-weighted images without contrast media was low (61%), which is much lower compared with previously published data by Choi et al. [15] with sensitivity of 86% before and 96% after gadobenate dimeglumine. One possible explanation for their greater sensitivity before contrast media were administered can be the fact that the readers in Choi’s study also had access to T2-weighted images, while in our study readers evaluated only T1-weighted images at the unenhanced session. Interestingly, their sensitivity increased only to 87% when adding the dynamic phases after gadobenate dimeglumine injection (excluding the hepatobiliary phase), and the number of false–positive lesions increased from 0 to 1. Furthermore, published data from Kim et al. [14] show that the hepatobiliary (one-hour delayed) phase had a better diagnostic performance than the dynamic phase imaging after gadobenate dimeglumine injection for the detection of liver metastases (sensitivity of 96% and 77%, respectively). These findings indicate that the hepatobiliary phase can play an important role in the evaluation of CRLM.
The dominant excretion of manganese via the hepatobiliary pathway makes it theoretically attractive for use in patients with impaired renal function. When measuring manganese levels in blood levels and urine in healthy volunteers after ingestion of CMC-001, no significant increase in manganese concentration was detected [19]. Further safety studies are, however, needed before such recommendations can be issued.
There were no serious AE. All but one of the AE were considered by the investigator to be of mild or moderate intensity and no safety concerns were raised for any of the two products. The observed adverse events were related to the high ion-content resulting in gastrointestinal discomfort, diarrhoea and nausea. We are therefore currently examining if lowering the dose of MnCl2 tetrahydrate can result in fewer AE but with a sufficient diagnostic imaging quality. Apart from the gastrointestinal disorders, a limitation of using CMC-001 can be the inhomogeneous uptake in cases of low portal venous perfusion and/or of decreased liver function increasing the risk for suboptimal enhancement and diminishing lesion conspicuity (as is the case in Fig. 3).
In our study, all patients were evaluated for liver surgery. Therefore, a majority of the metastases (25/44) could be verified at histopathology. However, the remaining 19 lesions were not considered resectable and therefore they lack histopathological verification. The final diagnosis of these had to rely on other imaging studies and on follow up. This is a limitation of our study and a factor for potential bias and overestimation of sensitivity. Additionally, our patient cohort, i.e. those with colorectal cancer and high risk of having liver metastases, comprise a highly selected study population, potentially also contributing to an overestimation of sensitivity. However, these limitations are considered to be of low impact in the present comparison with the comprehensive gadobenate dimeglumine protocol.
In conclusion, the sensitivity to detect colorectal cancer liver metastases at CMC-001 enhanced MRI is comparably high to that of a comprehensive intravenous gadobenate dimeglumine protocol; and it is safe, without serious adverse events.
Acknowledgements
N.A. is a consultant for CMC Medical, the company who financed the clinical trial.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.The Royal College of Radiologists (2007) RCR Referral Guidelines: Making the best use of clinical radiology services (MBUR), 6th Edition. London, pp 96–97
- 2.Schima W, Kulinna C, Langenberger H, Ba-Ssalamah A. Liver metastases of colorectal cancer: US, CT or MR. Cancer Imaging. 2005;5:149–156. doi: 10.1102/1470-7330.2005.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515–1526. doi: 10.1016/j.ultrasmedbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 5.Martin DR, Danrad R, Hussain SM. MR imaging of the liver. Radiol Clin North Am. 2005;43:861–886. doi: 10.1016/j.rcl.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen HS. Recent hot topics in contrast media. Eur Radiol. 2011;21:492–495. doi: 10.1007/s00330-010-2026-x. [DOI] [PubMed] [Google Scholar]
- 7.Chabanova E, Logager V, Moller JM, Dekker H, Barentsz J, Thomsen HS. Imaging liver metastases with a new oral manganese-based contrast agent. Acad Radiol. 2006;13:827–832. doi: 10.1016/j.acra.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Rief M, Huppertz A, Asbach P, Franiel T, Schwenke C, Hamm B, Taupitz M, Wagner M. Manganese-based oral contrast agent for liver magnetic resonance imaging: evaluation of the time course and dose response of liver signal intensity enhancement. Invest Radiol. 2010;45:565–571. doi: 10.1097/RLI.0b013e3181e9e120. [DOI] [PubMed] [Google Scholar]
- 9.Davis CD, Zech L, Greger JL. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc Soc Exp Biol Med. 1993;202:103–108. doi: 10.3181/00379727-202-43518. [DOI] [PubMed] [Google Scholar]
- 10.Malecki EA, Radzanowski GM, Radzanowski TJ, Gallaher DD, Greger JL. Biliary manganese excretion in conscious rats is affected by acute and chronic manganese intake but not by dietary fat. J Nutr. 1996;126:489–498. doi: 10.1093/jn/126.2.489. [DOI] [PubMed] [Google Scholar]
- 11.Finley JW, Johnson PE, Johnson LK. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am J Clin Nutr. 1994;60:949–955. doi: 10.1093/ajcn/60.6.949. [DOI] [PubMed] [Google Scholar]
- 12.Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725–1748. doi: 10.1148/rg.296095515. [DOI] [PubMed] [Google Scholar]
- 13.Bartolozzi C, Donati F, Cioni D, Procacci C, Morana G, Chiesa A, Grazioli L, Cittadini G, Cittadini G, Giovagnoni A, Gandini G, Maass J, Lencioni R. Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. Eur Radiol. 2004;14:14–20. doi: 10.1007/s00330-003-1966-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim YK, Lee JM, Kim CS, Chung GH, Kim CY, Kim IH. Detection of liver metastases: gadobenate dimeglumine-enhanced three-dimensional dynamic phases and one-hour delayed phase MR imaging versus superparamagnetic iron oxide-enhanced MR imaging. Eur Radiol. 2005;15:220–228. doi: 10.1007/s00330-004-2570-3. [DOI] [PubMed] [Google Scholar]
- 15.Choi JY, Choi JS, Kim MJ, Lim JS, Park MS, Kim JH, Chung YE. Detection of hepatic hypovascular metastases: 3D gradient echo MRI using a hepatobiliary contrast agent. J Magn Reson Imaging. 2010;31:571–578. doi: 10.1002/jmri.22076. [DOI] [PubMed] [Google Scholar]
- 16.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengmark S, Hafström L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::AID-CNCR2820230126>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen HS, Loegager V, Noergaard H, Chabanova E, Moller JM, Sonne J. Oral manganese for liver imaging at three different field strengths. Acad Radiol. 2004;11:630–636. doi: 10.1016/j.acra.2004.01.004. [DOI] [PubMed] [Google Scholar]