Abstract
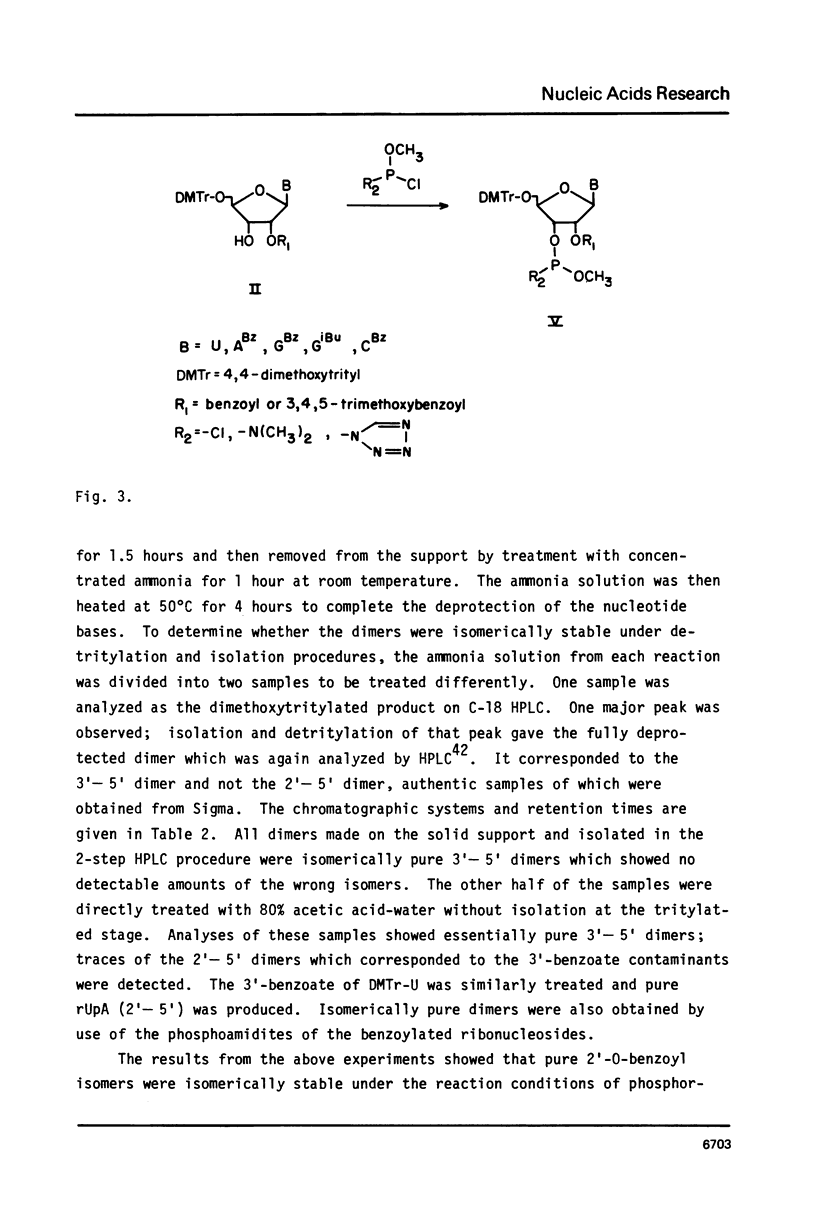
5'-0-(Dimethoxytrityl)-2'-0-(benzoyl or 3,4,5-trimethoxybenzoyl)-base protected ribonucleosides have been prepared by selective benzoylation of the 2'-hydroxyl group. The isomerization of the 2'-benzoates to the 3'-benzoates was studied. The protected ribonucleosides have been converted to either methylphosphochloridites or methylphosphoamidites and used to synthesize oligoribonucleotides on silica gel solid support. The synthetic RNA were deprotected and isolated using conditions that minimize internucleotide cleavage. The use of 2'-benzoates as protecting groups for ribonucleosides has made it possible to easily prepare and isolate mixtures of DNA and RNA.
Full text
PDF

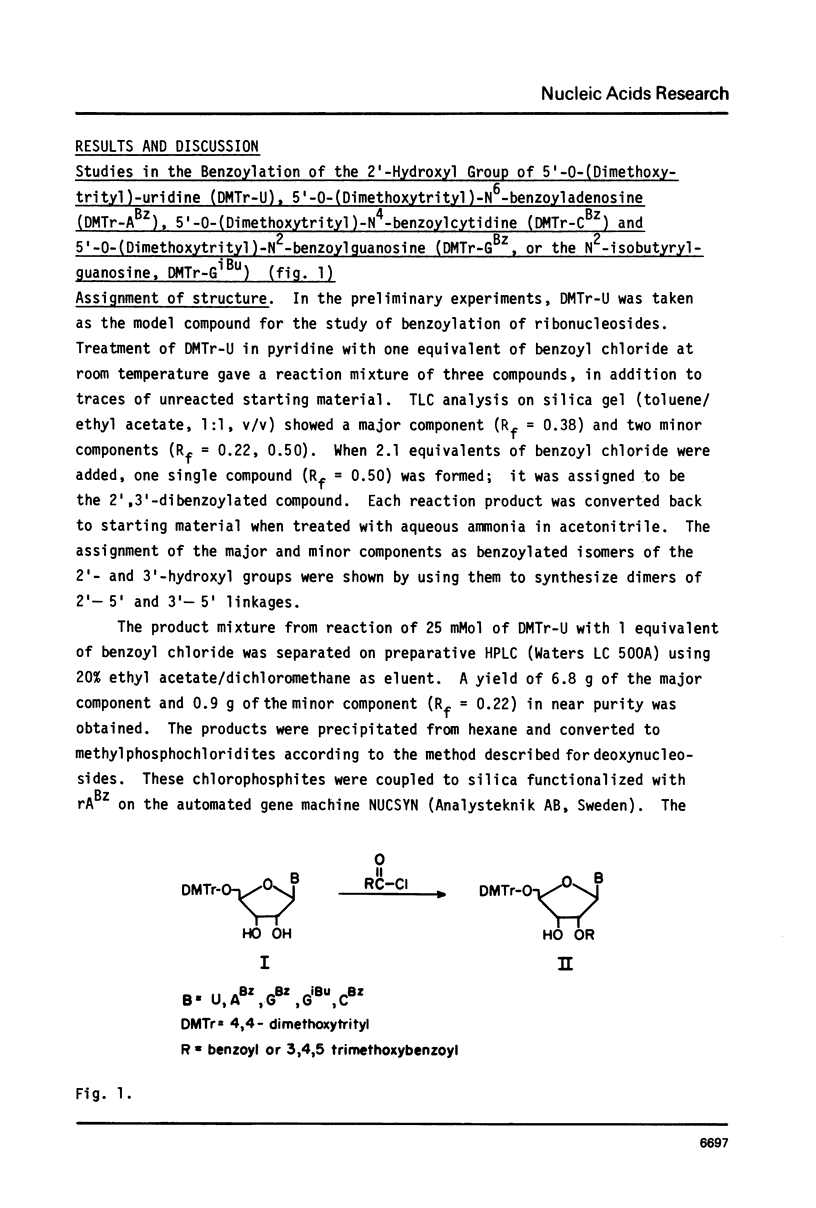
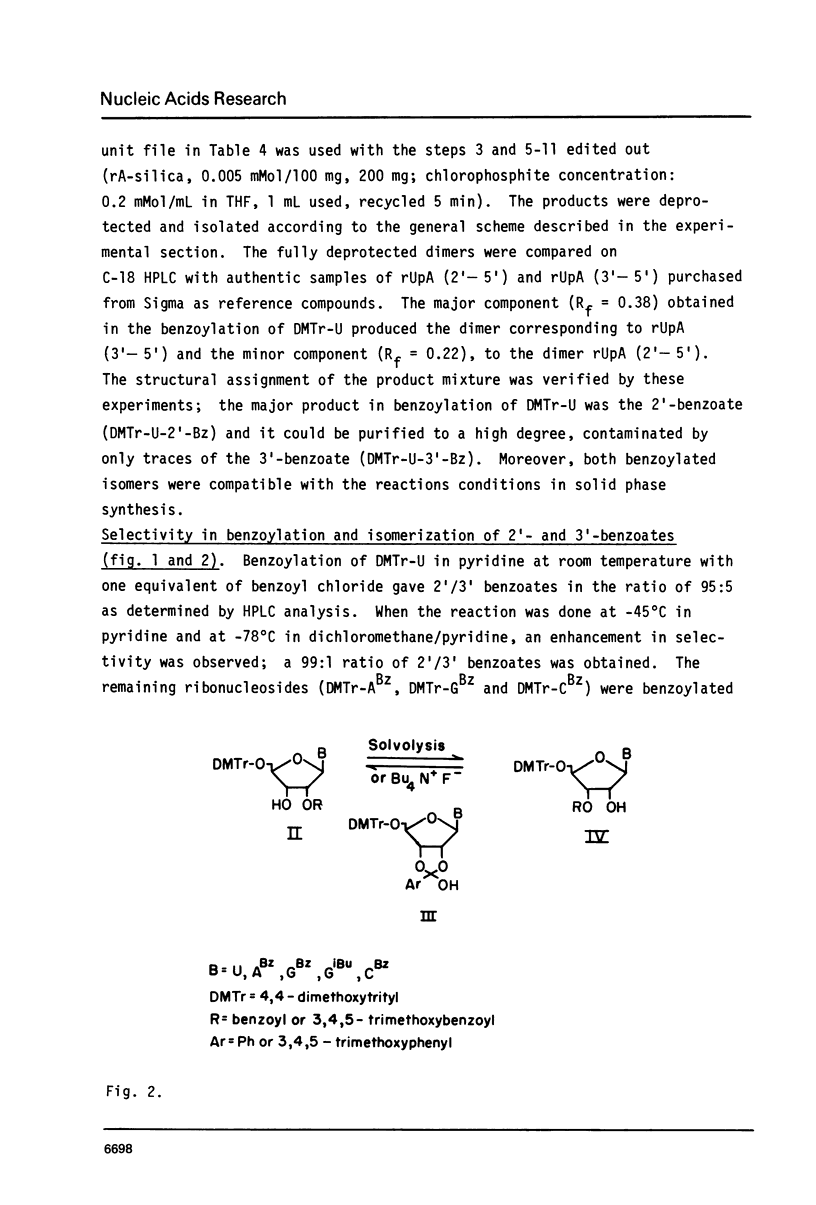

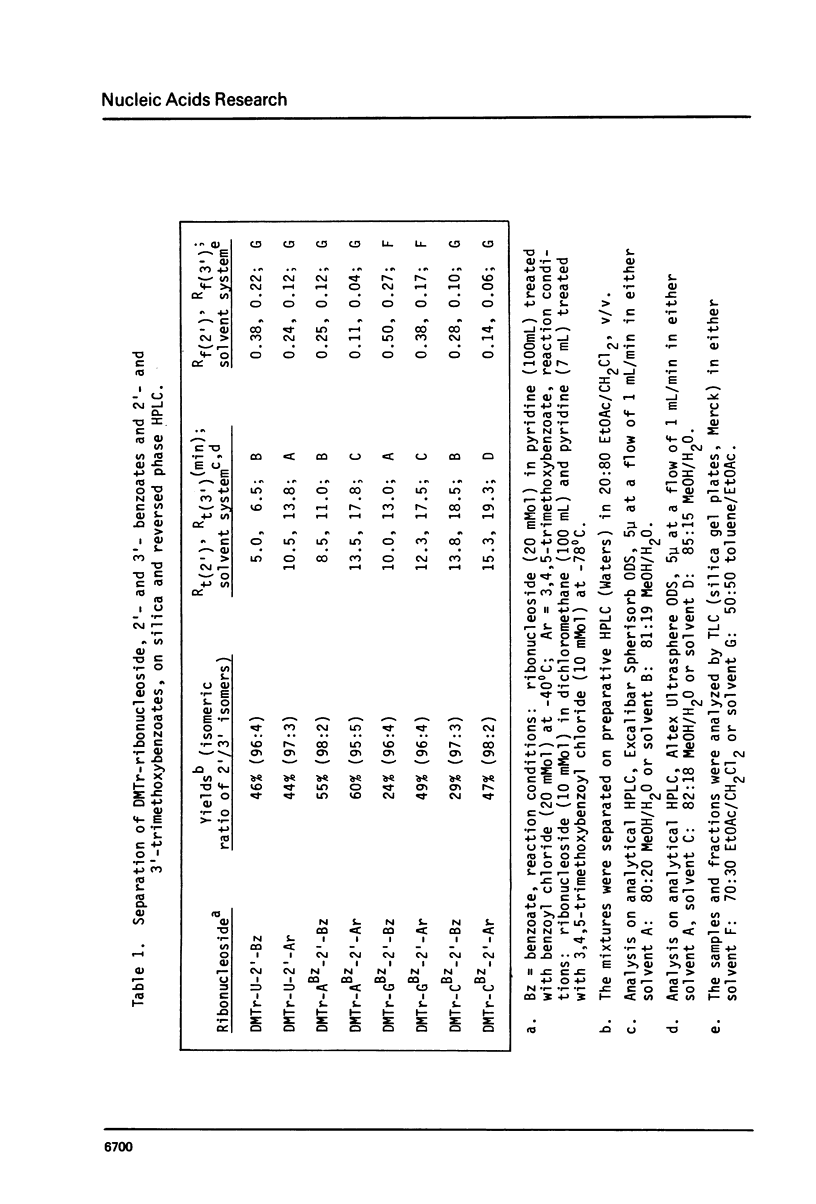
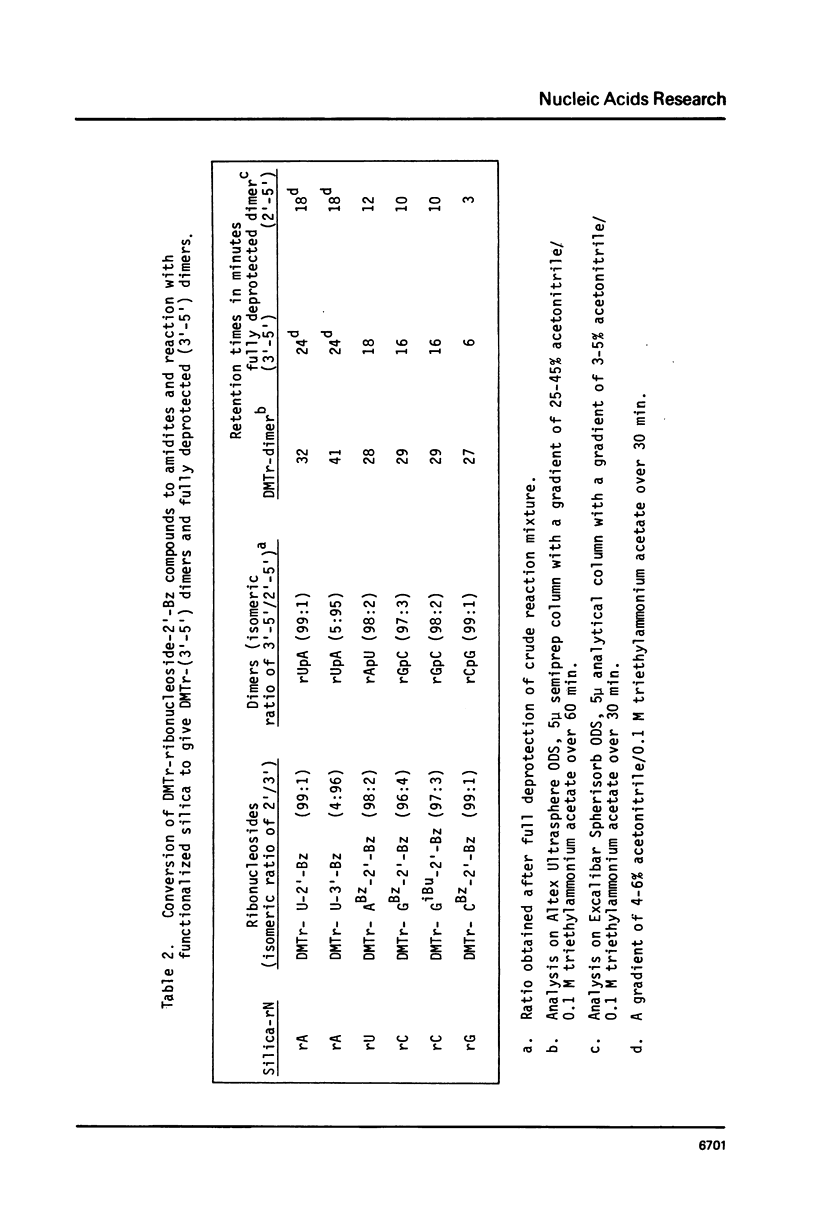









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow F., Kempe T., Palm G. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Res. 1981 Jun 25;9(12):2807–2817. doi: 10.1093/nar/9.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. F., Broom A. D. Specific chemical synthesis of ribonucleoside O-benzyl ethers. J Org Chem. 1972 Nov 3;37(22):3398–3401. doi: 10.1021/jo00795a003. [DOI] [PubMed] [Google Scholar]
- Daub G. W., van Tamelen E. E. Synthesis of oligoribonucleotides based on the facile cleavage of methyl phosphotriester intermediates. J Am Chem Soc. 1977 May 11;99(10):3526–3528. doi: 10.1021/ja00452a069. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Barrell B. G., Weith H. L. The use of primed synthesis by DNA polymerase I to study an intercistronic sequence of phiX-174 DNA. Eur J Biochem. 1975 Oct 15;58(2):383–395. doi: 10.1111/j.1432-1033.1975.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Fromageot H. P., Griffin B. E., Reese C. B., Sulston J. E. The synthesis of oligoribonucleotides. 3. Monoacylation of ribonucleosides and derivatives via orthoester exchange. Tetrahedron. 1967 May;23(5):2315–2331. doi: 10.1016/0040-4020(67)80068-1. [DOI] [PubMed] [Google Scholar]
- Fromageot H. P., Griffin B. E., Reese C. B., Sulston J. E., Trentham D. R. Orientation of ribonucleoside derivatives by proton magnetic resonance spectroscopy. Tetrahedron. 1966 Feb;22(2):705–710. doi: 10.1016/0040-4020(66)80041-8. [DOI] [PubMed] [Google Scholar]
- Gough G. R., Nadeau J. G., Gilham P. T., Singleton C. K., Weith H. L. Ribonucleoside and ribonucleotide derivatives in polynucleotide synthesis. Nucleic Acids Symp Ser. 1980;(7):99–102. [PubMed] [Google Scholar]
- Gough G. R., Singleton C. K., Weith H. L., Gilham P. T. Protected deoxyribonucleoside-3' aryl phosphodiesters as key intermediates in polynucleotide synthesis. Construction of an icosanucleotide analogous to the sequence at the ends of Rous sarcoma virus 35S RNA. Nucleic Acids Res. 1979 Apr;6(4):1557–1570. doi: 10.1093/nar/6.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B., Sulston J. E. The synthesis of oligoribonucleotides. II. Methoxymethylidene derivatives of ribonucleosides and 5'-ribonucleotides. Tetrahedron. 1967 May;23(5):2301–2313. doi: 10.1016/0040-4020(67)80067-x. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B. The synthesis of oligoribonucleotides. IV. Preparation of dinucleoside phosphates from 2',5'-protected ribonucleoside derivatives. Tetrahedron. 1968 Jan;24(2):639–662. doi: 10.1016/0040-4020(68)88015-9. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Reese C. B., Stephenson G. F., Trentham D. R. Oligoribonucleotide synthesis from nucleoside 2'-O-benzyl ethers. Tetrahedron Lett. 1966 Sep;36:4349–4354. doi: 10.1016/s0040-4039(00)76063-1. [DOI] [PubMed] [Google Scholar]
- Kikugawa K., Sato F., Tsuruo T., Imura N., Ukita T. On the benzylation of nucleosides. II. A novel synthesis of 2'-o-benzyluridine. Chem Pharm Bull (Tokyo) 1968 Jun;16(6):1110–1115. doi: 10.1248/cpb.16.1110. [DOI] [PubMed] [Google Scholar]
- McFarland G. D., Borer P. N. Separation of oligo-RNA by reverse-phase HPLC. Nucleic Acids Res. 1979 Oct 25;7(4):1067–1080. doi: 10.1093/nar/7.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Pon R. T. The chemical synthesis of oligoribonucleotides VII. A comparison of condensing agents in the coupling of silylated ribonucleosides. Nucleic Acids Res. 1980 May 10;8(9):2105–2115. doi: 10.1093/nar/8.9.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Theriault N., Sadana K. L. Synthesis of oligoribonucleotides. J Am Chem Soc. 1977 Nov 9;99(23):7741–7743. doi: 10.1021/ja00465a073. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. IX. Ribooligonucleotide synthesis using a photosensitive o-nitrobenzyl protection at the 2'-hydroxyl group. Nucleic Acids Res. 1974 Oct;1(10):1351–1357. doi: 10.1093/nar/1.10.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. B., Trentham D. R. Acyl migration in ribonucleoside derivatives. Tetrahedron Lett. 1965 Jul;(29):2467–2472. doi: 10.1016/s0040-4039(01)84008-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Donelson J. E., Coulson A. R., Kössel H., Fischer D. Use of DNA polymerase I primed by a synthetic oligonucleotide to determine a nucleotide sequence in phage fl DNA. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1209–1213. doi: 10.1073/pnas.70.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]


