Abstract
AIM
After in utero exposure to tricyclic antidepressants, neonatal withdrawal symptoms have been reported with an estimated incidence between 20 and 50%; however, few data are available for clomipramine. This could also be the case for neonatal pharmacokinetic clomipramine parameters and so this study was set up.
METHODS
Babies exposed to clomipramine in utero were included in an observational study, approved by the local ethics committee, after written informed consent. Withdrawal symptoms were scored at 12, 24 and 48 h after birth using the Finnegan score. Plasma concentrations were determined using an in-house-developed, validated liquid chromatography with mass detection (LC-MSMS) method at 0, 12, 24 and 48 h after birth.
RESULTS
We found that three of 11 pregnancies were complicated with pre-eclampsia. Ten neonates were observed for clomipramine withdrawal symptoms. The observed withdrawal symptoms were too short a period of sleep after feeding (6), poor feeding (3), mild to severe tremors (6), hyperactive Moro reflex (3) and respiratory rate >60 breaths min−1. Serious withdrawal reactions, such as tachycardia and cyanosis, were seen. We calculated a half-life value of 42 ± 16 h for clomipramine in neonates. Only a weak correlation was found between withdrawal reactions and clomipramine plasma concentration or desmethylclomipramine plasma concentration.
CONCLUSIONS
In neonates, clomipramine is eliminated with a half-life value of 42 h, compared with 20 h in adults. In two of 10 neonates, tachycardia and cyanosis were seen as serious withdrawal symptoms after maternal use of clomipramine.
Keywords: clomipramine, neonatal abstinence syndrome, newborn, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Antidepressants are associated with withdrawal symptoms after in utero exposure.
Half-life of clomipramine in neonates is prolonged compared with that in adults.
WHAT THIS STUDY ADDS
We present 10 cases of neonates exposed in utero to clomipramine, with detailed information about withdrawal symptoms and pharmacokinetics.
There is a high and severe complication rate in our cohort regarding the mother and the neonate.
Introduction
Severe depression during pregnancy is a serious risk for maternal and neonatal complications. Untreated maternal depression may lead to premature delivery, lower birth weight and disturbed motor, social, cognitive, emotional and neural development of the neonate [1–5]. Drugs of choice for maternal depression are tricyclic antidepressants (TCAs) and fluoxetine, a serotonin reuptake inhibitor (SSRI) [6–10]. Following recent discussions about the efficacy of SSRIs in general, the use of SSRIs during pregnancy should be reconsidered [11]. In recent years, serious neonatal outcomes, such as septal defects, neonatal convulsions and neonatal pulmonary hypertension shortly after delivery, have been reported following the use of paroxetine [12–14].
Tricyclic antidepressants are transported through the placental barrier by passive diffusion. Owing to the lipophilic properties of TCAs, the mechanism of this placental passage of TCAs is probably passive diffusion [15]. Neonatal withdrawal symptoms have been reported to result from in utero exposure to TCAs. It has been estimated that 20–50% of neonates might develop TCA withdrawal reactions due to maternal use [16–18]. Neonatal TCA withdrawal symptoms have previously been reviewed and include one or more of the following symptoms: sleeplessness, temperature instability, convulsions, tachypnoea, dyspnoea, restlessness, arrhythmias, emesis, tachycardia, aberrant stool, urine retention, decreased tone, cyanosis, apathy, unstable blood pressure and agitation [19]. For clomipramine, withdrawal symptoms were seen within 24 h after birth [20–22].
Pharmacokinetic parameters of neonatal clomipramine elimination are hardly available. In one study, clomipramine was eliminated from plasma in a neonate after 10–22 days [22]. However, pharmacokinetic data are still required to establish a safety period where extra professional observations can be made before safe discharge of the neonate from the hospital.
Neonatal clomipramine pharmacokinetics are thought to be different from those in adults, because cytochromes P450 3A4, 2C19 and 1A2, which are responsible for demethylation of clomipramine in adults, are present to a lesser extent in neonates [23]. At birth, CYP3A4 has an activity of 2% compared with adults, which increases to 13% of adult activity in 7 days [24, 25]. For CYP1A2 and CYP2C19, the fraction of adult activity is 0.1 and 0.03, respectively, in the first 7 days of the neonate [25]. Furthermore, desmethylclomipramine is eliminated by glomerular filtration, which is low in newborns. Renal blood flow increases from 16% of cardiac output at term birth to 20–25% of cardiac output at 1 year of age, which affects both glomerular filtration rate and tubular drug excretion [24]. In addition to pharmacokinetics, it is not yet known how pharmacodynamics of antidepressants in neonates and in adults relate to one another [26].
These differences make interpretation of clomipramine concentrations in neonates difficult; these may be of importance to distinguish between withdrawal reactions and neonatal toxicity, together with structural assessment of neonatal behaviour.
Some researchers call for a revision of the concept of neonatal antidepressant withdrawal syndrome [27–29]. They argue that high blood concentration levels in the neonate may cause both toxicity and withdrawal reactions. Therefore, we decided to study the assessed blood concentration levels of the TCA in the neonate to investigate the relation between pharmacokinetics and neonatal behaviour.
Patients and methods
This observational study was performed in Zwolle, The Netherlands, at the Isala Clinics, a large (1000 beds) secondary teaching hospital with about 3000 deliveries each year. The study was approved by the hospital's medical ethics committee and the central medical ethics committee of The Netherlands (CCMO). In our hospital, a pregnancy consultation service team providing collaborative care with medical specialists and other healthcare professionals, including specialists in gynaecology, psychiatry, paediatrics, physiotherapy, mental health and clinical pharmacology, has developed a specific psycho-obstetric protocol for treatment of pregnant women with psychiatric diseases. Midwives and general practitioners in the region of our hospital redirect pregnant women with psychiatry disorders to the pregnancy consultation service team.
Inclusion criteria for the pregnant women were written informed consent for the study protocol, use of clomipramine during the whole pregnancy and age >18 years.
We excluded women who were mentally incapacitated, had twin pregnancies, used drugs of abuse (not nicotine or alcohol), used additional other antidepressants or antipsychotic drugs, used drugs with the same or higher teratogenic risk classification, and used any additional drugs with any influence on TCA metabolism. Compared with normal monitoring of patients at risk during pregnancy, after delivery, the patients were asked to visit the physician or primary investigator of the research team for additional blood sampling and to test the severity of the depression using CES-D scores. Demographic data (bodyweight and height, and tertiary study parameters) were collected. Once included and with a signed informed consent, the patient's GP and pharmacist were informed that his/her patient participated in this trial.
The Finnegan score, which was developed in the 1970s to diagnose neonatal opioid withdrawal symptoms, has been used frequently to diagnose neonatal antidepressant withdrawal and was used to detect clomipramine withdrawal [30]. We added the following items: urine retention, tachycardia, cyanosis, anxiety, restlessness, fluctuations in blood tension, fluctuations in temperature (0.5°C), fluctuations in cardiac rhythms, dyspnoea, excitement and apathy, based on research by Laine et al., Moses-Kolko et al. and Dilsaver et al. [29, 31, 32]. Other relevant explorative data of the neonates, such as gestational age and birth weight, were collected.
Neonatal blood sampling (0.5 ml) was performed using EDTA-containing tubes. Sampling times were immediately after delivery from the umbilical cord, and 12, 24 and 48 h postpartum. Due to the observational character of this study, we did not want to disrupt any sleeping pattern of the mother or the neonate, so sampling times were adjusted if appropriate for the situation. At each sampling point, withdrawal symptoms were scored.
Blood samples were analysed with an ultra performance liquid chromatography with mass detection technique (Acquity® UPLC system, Acquity® TQD detector and Acquity® UPLC BEH C18 column, and Masslynx v4.1 software for concentration calculations, all from Waters Chromatography, Etten-Leur, The Netherlands). Samples were processed as follows. Fifty microlitres of plasma was added to 200 µl internal standard solution (nortriptyline-D3 0.1 mg l−1 in acetonitril : methanol 2:1), then mixed thoroughly and frozen for 10 min at −20°C, after which 150 µl of the supernatant was added to 600 µl of Millipore water. Injection volume was 20 µl. Samples were eluted with a gradient elution system containing 200 ml Millipore water added to 200 µl formic acid (A) and 200 ml acetonitril added to 200 µl formic acid (B). During elution, the ratio A : B changed from 75:25 during the first 30 s to 60:40 at 2.5 min. Thereafter, a 0.5 min column-cleaning procedure was performed using 100% acetonitril to remove interfering phospholipid structures from the plasma. The linearity of our developed method was between 5 and 400 µg l−1 for both clomipramine and desmethylclomipramine, which seems suitable for the expected plasma concentration range (50–450 µg l−1). Within-day variations for three concentrations (75, 150 and 225 µg l−1) and between-day variations for three concentrations (75, 150 and 225 µg l−1) were lower than 4.8%, and the limit of quantification was 5 µg l−1 for both components. All reagents were of analytical grade. Acetonitril (UV grade) and methanol were purchased from Labscan® (Cuijck, The Netherlands), nortriptyline-D3 was purchased from LGC (Wessel, Germany), and formic acid was purchased from Sigma-Aldrich Chemicals (Zwijndrecht, The Netherlands).
Pharmacokinetic calculations were performed using Microsoft Excel (MS-Excel 2002, Microsoft Corperation USA).
Results
From 2004 until 2009, 11 mother–child pairs were included in this study. Demographic data and drug information are presented in Table 1. Three of 11 pregnancies were complicated by pre-eclampsia, and one pregnancy was complicated by diabetes mellitus. Four neonates were born by caesarean section. Due to protocol violation in the delivery room, data from one neonate (no. 10) were not collected; this infant was excluded from the study. In eight neonates, clomipramine levels and in seven neonates, desmethylclomipramine levels above the limit of quantification (LOQ) (5 µg l−1 for both) could be determined.
Table 1.
Patient characteristics and pregnancy outcomes
| Mother | Neonate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age (years) | PPW (kg) | DD (mg) | DDD (mg) | TF | Indication | Compliance* | Complications | Sex | GA | BW (g) | Breast feeding |
| 1 | 36 | 90 | 37.5 | 37.5 | IR | PD | 110% | – | M | 37 + 5 | 3520 | No |
| 2 | 41 | 84 | 75 | 75 | SR | MDD | 97% | – | F | 37 + 5 | 2560 | Yes |
| 3 | 36 | 64 | 75 | 113 | SR | PD | 101% | Caesarean section due to presence of amniotic fluid containing meconium, diabetes gravidarum, pregnancy-related hypertension | M | 38 + 4 | 2890 | No |
| 4 | 40 | 81 | 75 | 100 | SR | PD | 100% | Pre-eclampsia; caesarean section due to delayed delivery | F | 38 + 1 | 4020 | No |
| 5 | 32 | 80 | 60 | 60 | IR | MDD | 96% | Caesarean section due to delayed delivery | M | 37 + 1 | 2700 | No |
| 6 | 30 | 63 | 50 | 50 | IR | MDD | 96% | Diabetes, not pregnancy related | F | 38 + 1 | 3230 | Yes |
| 7 | 34 | 60 | 100 | 125 | IR | OCD | 97% | Caesarean section due to unsuccessful vacuum extraction and delayed delivery | M | 39 + 3 | 3490 | Yes |
| 8 | 26 | 50 | 50 | 50 | IR | PD | 101% | Pre-eclampsia | F | 35 + 5 | 2515 | Yes |
| 9 | 26 | 50 | 50 | 50 | IR | PD | 101% | Pre-eclampsia | F | 34 + 2 | 2365 | Yes |
| 10 | 31 | 125 | 20 | 25 | IR | PD | 101% | – | F | 38 + 1 | 4300 | Yes |
| 11 | 29 | 52 | 125 | 125 | IR | OCD | 76% | – | M | 36 + 6 | 2740 | No |
Abbreviations:. BW, birth weight (in grams); DD, daily dose at time of inclusion (in milligrams); DDD, daily dose at delivery (in milligrams); F, female; GA, gestational age (weeks + number of days); IR, immediate release; M, male; MDD, major depressive disorder; OCD, obsessive compulsive disorder; PD, panic disorder; PPW, prepregnancy weight (in kilograms); SR, sustained release; and TF, tablet formulation.
Calculated with medication event monitoring system-recorded administration times.
The courses of clomipramine blood levels in the first 60 h after birth are shown in Figure 1. For most neonates, clomipramine levels decreased slowly after birth. Two neonates (nos 3 and 4) showed an increase in clomipramine levels after 24 h. The clomipramine concentration of neonate no. 2 reached the LOQ after 24 h. Furthermore, when we assumed first-order kinetics, we could calculate a half-life of 42 ± 16 h (mean ± SD) for five neonates with three data points (see Table 2). In Figure 2, the ratio of desmethylclomipramine to clomipramine is plotted. The small positive slope of the graphs indicates an increase of metabolic capacity.
Figure 1.
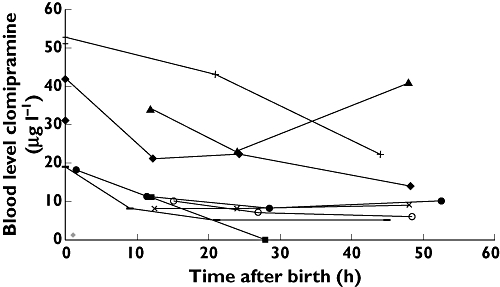
Blood levels of clomipramine after birth (0 = umbilical cord blood)
Table 2.
Calculated half-life values
| Neonate ID | Half-life (h) |
|---|---|
| 6 | 68 |
| 7 | 34 |
| 8 | 27 |
| 9 | 48 |
| 11 | 35 |
Figure 2.

Ratio of desmethylclomipramine to clomipramine after birth (0 = umbilical cord blood)
Clomipramine withdrawal symptoms
Ten neonates could be evaluated for clomipramine withdrawal symptoms. The observed withdrawal reactions are presented in Figure 3. Mean Finnegan scores were 5.6, 2.3 and 2.0 at 12, 24 and 48 h postpartum, respectively. One neonate had nine withdrawal symptoms, one had seven symptoms, three had five symptoms, one had four symptoms, one had one symptom and one showed no withdrawal symptoms.
Figure 3.

Withdrawal reactions due to in utero exposure to clomipramine. Abbreviation: pp, postpartum
The most frequently observed withdrawal symptoms were too short a period of sleep after feeding in six of 10 cases, poor feeding in three of 10 cases, mild to severe tremors when disturbed in six of 10 cases, hyperactive Moro reflex in three of 10 cases and respiratory rate >60 breaths min−1 with or without trembling in three of 10 cases. Serious withdrawal reactions were seen in two neonates: tachycardia and cyanosis. No differences were seen in the frequency of withdrawal symptoms or half-life values between neonates who were breastfed and neonates who were bottle fed. All withdrawal symptoms are presented in Figure 3. Besides the withdrawal reactions, we did not find any structural anomaly with the 20 weeks of pregnancy structured ultrasound procedure nor immediately after delivery. A weak correlation was found between the rate of blood level decrease and the Finnegan score, as shown in Figure 4. The decrease of clomipramine or desmethylclomipramine plus clomipramine levels account for the higher Finnegan score, though correlation coefficients are low (0.2144 and 0.1078, respectively).
Figure 4.
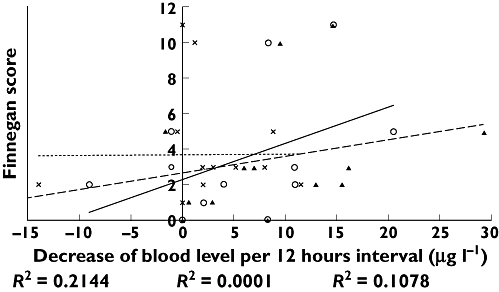
Relation of blood level decrease with Finnegan score
Discussion
In this study, we found the half-life value of clomipramine in neonates who were exposed in utero to be 42 ± 16 h (mean ± SD). Most frequently observed withdrawal symptoms were a shortened period of sleep after feeding, poor feeding, mild to severe tremors, hyperactive Moro reflex and a respiratory rate >60 breaths min−1 with or without trembling. Furthermore, three of 11 pregnancies were complicated by pre-eclampsia. A relation between withdrawal symptoms and blood levels of clomipramine and desmethylclomipramine was not found to be statistically significant.
The half-life of clomipramine in neonates we found (42 h) is roughly twice as high as adult values (20 h) [33, 34]. This was to be expected because of functional immaturity of physical processes and organ functioning in neonates [25]. Schimmel et al. reported a substantially higher neonatal half-life for clomipramine of 93 h [35]. However, analysis of clomipramine in this specific case was done by a nonselective method of concentration measurement, which cannot exclude attributable effects of the metabolite, desmethylclomipramine [35]. Loughhead et al. presented a study in which analysis of clomipramine and desmethylclomipramine was performed on maternal and umbilical cord samples [36]. The ratio of the concentrations of clomipramine and its active metabolite, desmethylclomipramine, between umbilical cord blood and maternal blood, were 0.60 ± 0.50 and 0.80 ± 0.60, respectively. However, in their study no data were available of neonatal clomipramine or desmethylclomipramine concentrations [36].
For calculating half-life values, we used formulae for first-order kinetics. It was not possible with our sparse data set to distinguish between first-order kinetics and zero-order Michaelis–Menten kinetics. For convenience, we used first-order kinetics; however, we hypothesized that physiologically a saturated condition of enzymatic breakdown of clomipramine would more accurately reflect the situation.
Previous research has shown that the fetal liver contains a very small amount of CYP450 iso-enzymes and that delivery triggers development of enzymatic activity, which is very limited immediately after birth [25]. The ratio of desmethylclomipramine to clomipramine increases in the first days of life, suggesting some metabolism of clomipramine into its demethylated metabolite by CYP3A4, CYP2C19 and CYP1A2. The large interindividual differences may be explained by large variation in maturation of processes and growth.
The withdrawal symptoms observed in the present study are in line with previously described neonatal symptoms after maternal clomipramine use [19, 35]. It has been estimated that 20–50% of neonates might develop TCA withdrawal reactions as a result of maternal use [16–18]. However, specifications for clomipramine and its active metabolite, desmethylclomipramine, are not available. Most withdrawal reactions (28 of 39 scored) were registered in the first 24 h. Given the delayed elimination, it is not likely that the observed symptoms are attributable to a genuine withdrawal syndrome, but they are probably better described as a kind of neonatal adaptation syndrome, in the absence of specific data regarding whether excessive or reduced serotonin concentrations are present resulting from the exposure to clomipramine. While a substantial number of reactions were seen at 48 h after birth, it is doubtful whether clomipramine-exposed neonates could be safely discharged from the hospital 48 h after birth. Studies with SSRIs found percentages of 15–30% for neonatal withdrawal symptoms in the first days after delivery [37, 38]. However, most data were derived from case reports and not, as in the present study, with structured observations of every clomipramine-complicated pregnancy. Some neonatal symptoms, such as feeding problems, were also reported in non-exposed neonates of depressed mothers [19];;; therefore, in judging neonatal symptoms, it has to be taken into account that some symptoms may be caused by other factors, such as the transition from intrauterine to extrauterine life. Whether the fact that three of 11 pregnancies were complicated by pre-eclampsia could be attributable to the use of clomipramine is not clear, but in our opinion deserves further attention. Furthermore, we did not find a difference in frequency of withdrawal symptoms between breastfed and bottle-fed infants. This is in contrast with previous studies showing that breastfed neonates had significantly fewer withdrawal symptoms than bottle-fed neonates [19]. This might be explained by minimal transfer of clomipramine from the mother's milk to the newborn, as found previously [35, 39]. The discussion about the safety of antidepressants during pregnancy for mother and child is complicated by observations that untreated depression and stress symptoms during pregnancy may impede fetal growth, lead to smaller head circumference at delivery, increase the risk of obstetrical and postnatal complications, i.e. preterm delivery, low birth weight, spontaneous abortion, pre-eclampsia and substance abuse, and precipitate long-standing behavioural changes in the offspring [40–42]. To decide whether or not to treat depression with any antidepressant, only randomized trials can give the definite answers. Until then, the drugs of choice for pharmacological treatment of maternal depression are TCAs and fluoxetine [6–10].
This study has some limitations. First, the Finnegan score as a measure for the severity of withdrawal has been used in most studies about antidepressant withdrawal so far, but has been developed for opioid withdrawal [30]. A major problem for detecting withdrawal is the lack of appropriate diagnostic instruments. However, Moses-Kolko et al. proposed a symptom list for neonatal SSRI withdrawal [31], Dilsaver et al. proposed a withdrawal syndrome in adults [32], and Laine et al. proposed a symptom list derived from the symptoms associated with the serotonin syndrome [29]. The diagnosis of withdrawal symptoms could be biased by the following factors: metabolic diseases (hypoglycaemia or low blood concentrations of calcium, magnesium or sodium), endocrine diseases (hyperthyroidism), diseases of the central nervous system (hypoxic–ischaemic encephalopathy, periventricular haemorrhage or meningitis) and other diseases (hyperviscosity and excitation in small-for-gestational-age infants) [43]. However, in our study population, these diseases were not found.
Second, as only 11 mother–baby pairs were included, it was not possible to find a strong relation between withdrawal reactions and blood concentrations of clomipramine and desmethylclomipramine; therefore, revision of the neonatal antidepressant withdrawal syndrome concept seems unsuitable. However, as we did not find high clomipramine and desmethylclomipramine concentrations (therapeutic window for the sum concentration of clomipramine and desmethylclomipramine in adults, 175–450 µg l−1[44]), but still found withdrawal reactions in the presence of a long half-life, these data seem to support the hypothesis that the phenomena observed can better be described as a neonatal adaptation syndrome. However, we did not analyze free-fraction concentrations of clomipramine, which could be increased compared with those in adults [45]. Symptoms of withdrawal were seen independent of blood (concentration) levels, even when blood levels of clomipramine and desmethylclomipramine were below 5 µg l−1. A weak correlation was seen between the rate of decrease of the sum concentration of clomipramine and desmethylclomipramine and the Finnegan score. This was hypothesized earlier, where a relation was assumed between half-life values and appearance of withdrawal reactions [13, 46].
Third, it is hard to draw any conclusions about the safety for the newborn of treatment with clomipramine of maternal depression during pregnancy, owing to the small sample size and lack of reference data. Compared with the serious and irreversible adverse effects seen with the use of SSRIs, it seems that clomipramine is relatively safe.
In order to make comparisons possible, data from this study suggest the need for structured surveillance in the first days after birth of neonates who were exposed to clomipramine and other depressants in utero. It is to be expected that after 8 days (five times half-life) clomipramine levels would be negligible in the neonate, so the period of observation in our study might have been too short for safe discharge from the hospital; however, most withdrawal reactions (28 of 39) were in the first 24 h.
Conclusion
We found that clomipramine is eliminated with an estimated half-life of 42 h in neonates exposed in utero. Tachycardia and cyanosis were seen as serious withdrawal reactions due to maternal use of clomipramine. Of the 11 observed pregnancies, three were complicated with pre-eclampsia, three with a caesarean section and one with diabetes mellitus.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Depression during pregnancy: overview of clinical factors. Clin Drug Investig. 2004;24:157–79. doi: 10.2165/00044011-200424030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Eberhard-Gran M, Eskild A, Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation: practical recommendations. CNS Drugs. 2006;20:187–98. doi: 10.2165/00023210-200620030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Boyd RC, Zayas LH, McKee MD. Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Matern Child Health J. 2006;10:139–48. doi: 10.1007/s10995-005-0042-2. [DOI] [PubMed] [Google Scholar]
- 5.Mian AI. Depression in pregnancy and the postpartum period: balancing adverse effects of untreated illness with treatment risks. J Psychiatr Pract. 2005;11:389–96. doi: 10.1097/00131746-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153:592–606. doi: 10.1176/ajp.153.5.592. [DOI] [PubMed] [Google Scholar]
- 7.Grover S, Avasthi A, Sharma Y. Psychotropics in pregnancy: weighing the risks. Indian J Med Res. 2006;123:497–512. [PubMed] [Google Scholar]
- 8.Marcus SM, Barry KL, Flynn HA, Tandon R, Greden JF. Treatment guidelines for depression in pregnancy. Int J Gynaecol Obstet. 2001;72:61–70. doi: 10.1016/s0020-7292(00)00318-0. [DOI] [PubMed] [Google Scholar]
- 9.National collaborating centre for mental health. NICE Clinical Guideline 45: Antenatal and Postnatal Mental Health. Midplace, UK: National Institute for Health and Clinical excellence; 2007. pp. 1–48. [Google Scholar]
- 10.Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128–37. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teratology of paroxetine: evidence for cardiovascular malformations. 2005. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm152310.htm (last accessed 17 August 2011)
- 13.Sanz EJ, las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482–7. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 14.Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:579–87. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 15.Unadkat JD, Dahlin A, Vijay S. Placental drug transporters. Curr Drug Metab. 2004;5:125–31. doi: 10.2174/1389200043489171. [DOI] [PubMed] [Google Scholar]
- 16.Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27:1068–72. doi: 10.1177/106002809302700912. [DOI] [PubMed] [Google Scholar]
- 17.Withdrawing patients from antidepressants. Drug Ther Bull. 1999;37:49–52. doi: 10.1136/dtb.1999.37749. [DOI] [PubMed] [Google Scholar]
- 18.Diamond BI, Borison RL, Katz R, DeVeaugh-Geiss J. Rebound withdrawal reactions due to clomipramine. Psychopharmacol Bull. 1989;25:209–12. [PubMed] [Google Scholar]
- 19.Ter Horst PG, Jansman FG, van Lingen RA, Smit JP, de Jong-van den Berg LT, Brouwers JR. Pharmacological aspects of neonatal antidepressant withdrawal. Obstet Gynecol Surv. 2008;63:267–79. doi: 10.1097/OGX.0b013e3181676be8. [DOI] [PubMed] [Google Scholar]
- 20.Cowe L, Lloyd DJ, Dawling S. Neonatal convulsions caused by withdrawal from maternal clomipramine. Br Med J (Clin Res Ed) 1982;284:1837–8. doi: 10.1136/bmj.284.6332.1837-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musa AB, Smith CS. Neonatal effects of maternal clomipramine therapy. Arch Dis Child. 1979;54:405. doi: 10.1136/adc.54.5.405-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boringa JB, de Jong GM, Touw DJ. [Neonatal withdrawal symptoms following the use of clomipramine during pregnancy] Ned Tijdschr Geneeskd. 1992;136:1473–5. [PubMed] [Google Scholar]
- 23.Myllynen P, Immonen E, Kummu M, Vahakangas K. Developmental expression of drug metabolizing enzymes and transporter proteins in human placenta and fetal tissues. Expert Opin Drug Metab Toxicol. 2009;5:1483–99. doi: 10.1517/17425250903304049. [DOI] [PubMed] [Google Scholar]
- 24.Ward RM. Drug disposition in the late preterm (‘near-term’) newborn. Semin Perinatol. 2006;30:48–51. doi: 10.1053/j.semperi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–86. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 26.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 27.Koren G. A new indication for therapeutic drug monitoring in the neonate. Ther Drug Monit. 2006;28:1. doi: 10.1097/01.ftd.0000187973.02624.67. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DJ. Effects of third trimester fluoxetine exposure on the newborn. J Clin Psychopharmacol. 1995;15:417–20. doi: 10.1097/00004714-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–6. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- 30.Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2:141–58. [PubMed] [Google Scholar]
- 31.Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–83. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 32.Dilsaver SC, Greden JF. Antidepressant withdrawal phenomena. Biol Psychiatry. 1984;19:237–56. [PubMed] [Google Scholar]
- 33.Balant-Gorgia AE, Gex-Fabry M, Balant LP. Clinical pharmacokinetics of clomipramine. Clin Pharmacokinet. 1991;20:447–62. doi: 10.2165/00003088-199120060-00002. [DOI] [PubMed] [Google Scholar]
- 34.Gelman CR, Rumack BH. MICROMEDEX® Healthcare Series. 3. Vol. 93. Greenwood Village, CO: MICROMEDEX; 2000. [Google Scholar]
- 35.Schimmell MS, Katz EZ, Shaag Y, Pastuszak A, Koren G. Toxic neonatal effects following maternal clomipramine therapy. J Toxicol Clin Toxicol. 1991;29:479–84. doi: 10.3109/15563659109025744. [DOI] [PubMed] [Google Scholar]
- 36.Loughhead AM, Stowe ZN, Newport DJ, Ritchie JC, DeVane CL, Owens MJ. Placental Passage of Tricyclic Antidepressants. Biol Psychiatry. 2005;59:287–90. doi: 10.1016/j.biopsych.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 37.Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65:230–7. doi: 10.4088/jcp.v65n0214. [DOI] [PubMed] [Google Scholar]
- 38.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 39.Wisner KL, Perel JM, Findling RL. Antidepressant treatment during breast-feeding. Am J Psychiatry. 1996;153:1132–7. doi: 10.1176/ajp.153.9.1132. [DOI] [PubMed] [Google Scholar]
- 40.Newport DJ, Wilcox MM, Stowe ZN. Antidepressants during pregnancy and lactation: defining exposure and treatment issues. Semin Perinatol. 2001;25:177–90. doi: 10.1053/sper.2001.24901. [DOI] [PubMed] [Google Scholar]
- 41.Koren G, Matsui D, Einarson A, Knoppert D, Steiner M. Is maternal use of selective serotonin reuptake inhibitors in the third trimester of pregnancy harmful to neonates? CMAJ. 2005;172:1457–9. doi: 10.1503/cmaj.1041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001–20. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- 43.Leeuwenburgh-Pronk WG, de Vries MC, Clement-de BA. A multidisciplinary approach is necessary in the neonatal withdrawal syndrome. Ned Tijdschr Geneeskd. 2006;150:761–5. [PubMed] [Google Scholar]
- 44.Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, Kuss HJ, Laux G, Muller-Oerlinghausen B, Rao ML, Riederer P, Zernig G. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37:243–65. doi: 10.1055/s-2004-832687. [DOI] [PubMed] [Google Scholar]
- 45.Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–34. doi: 10.2165/00003088-200645100-00005. [DOI] [PubMed] [Google Scholar]
- 46.Vlaminck JJ, van Vliet IM, Zitman FG. [Withdrawal symptoms of antidepressants] Ned Tijdschr Geneeskd. 2005;149:698–701. [PubMed] [Google Scholar]


