Abstract
Background
Common antigens between intestinal parasites and environmental allergens may play a role in the modulation of allergic immune responses. There is a growing interest in investigating cross-reactivity between common helminths and dust mites affecting humans, particularly in the tropics.
Objective
This study examined the cross-reactivity between the human roundworm Ascaris lumbricoides (Al) and three house dust mite (HDM) species.
Methods
Specific serum IgE levels to HDM species Blomia tropicalis (Bt), Dermatophagoides pteronyssinus (Dp), and Dermatophagoides farinae (Df ); and Al extracts among allergic (n=100) and ascariasis (n=60) subjects were measured through enzyme-linked immunosorbent assay (ELISA). IgE-reactive components of HDM and Al extracts were detected through Western-Blot Analysis. Cross-reactivity between HDMs and Al was determined by ELISA inhibition using HDM and Al-specific sera from allergic (n=15) and ascariasis (n=15) subjects. The IgE-binding capacity of a recombinant paramyosin peptide (Blo t 11-fD) to allergic (n=50) and ascariasis (n=50) subjects' sera were likewise determined.
Results
Among allergic subjects, 70% exhibited Al-specific positive IgE-reactivity, while 20-28% of ascariasis subjects demonstrated HDM-specific positive IgE-reactivity. Multiple IgE-reactive components of HDM allergens (14-240 kDa) and Al antigens (15-250 kDa) were detected, indicating multi-allergen sensitization among the subjects tested. Al antigens can inhibit up to 92% of HDM-specific IgE-reactivity among allergic subjects, while up to 54% of Al-specific IgE-reactivity among ascariasis subjects was inhibited by HDM allergens. Positive rBlo t 11-fD-specific IgE reactivity was observed in 80% of the allergic subjects and 46% of the ascariasis subjects.
Conclusions
This study showed the presence of multiple cross-reactive antigens in HDM and Al extracts. Identification of these molecules may provide basis for designing novel diagnostic and therapeutic strategies. The potential role of paramyosin as a specific cross-reactive allergen present in HDMs and Al has been shown.
Keywords: Atopy, Immunity, Infection, Paramyosin, Parasitism
INTRODUCTION
Allergic diseases and helminth infections are two discrete medical conditions, but research findings for the past few decades indicate an association between them. The nature of the relationship and the mechanisms that lie behind it are still unclear and controversial. Both are immunological maladies characterized by high IgE-levels, eosinophilia, increase in mast cells, mucus hyperresponsiveness, and T-cells that preferentially secrete Th2-cytokines [1]. Inspite of parallel immune response, epidemiological studies show that the prevalence of allergic diseases and helminthiasis are inversely associated. Allergic diseases are increasing in industrialized countries and more prevalent in urban areas [2, 3], while helminth infections are decreasing in industrialized countries yet widespread in rural areas and developing countries [4].
Some evidence support protective immunity exerted by helminth infections against the development of allergic symptoms [5-7]. These and other epidemiological evidences suggest a causal relationship between allergy and helminthiasis.
Chronic helminth infections may cause IgE responses that are cross-reactive to house dust mite allergens, leading to clinically irrelevant reactions [8]. Recently, alternative Th2-responses were proposed, where the involvement of IgG4, IL-10, dendritic cells, and other downstream components of the Th2-response on the immunomodulatory ability of helminths were given emphasis.
House dust mites (HDMs) such as Blomia tropicalis (Bt), Dermatophagoides pteronyssinus (Dp) and Dermatophagoides farinae (Df) have been regarded as important causative agents of allergic diseases globally [9]. On the other hand, helminth parasites such as the roundworm Ascaris lumbricoides (Al) and hookworms are major agents of helminthiasis worldwide [1]. Elucidation of molecular and immunological mechanisms involved in the pathogenesis of both allergic diseases and helminthiasis include isolation and characterization of IgE-binding antigens from the causative agents. Thus far, certain helminth antigens like tropomyosin [10], ABA-1-like protein [11], paramyosin [12] and glutathione-S-transferase [13] have been likewise identified as allergenic proteins known to induce IgE-mediated hypersensitivity among atopic individuals.
Allergenic cross-reactivity has been described between some intestinal parasites and arthropod allergens [14]. It has been shown that shared antigens between parasites and environmental allergens have a potential role in modulating allergic immune responses [12]. The identification of molecules in intestinal parasites associated with allergy could provide basis for novel forms of treatment or prevention of these diseases. This study investigates cross-reactivity between antigens from aqueous extracts of Al, and allergens from HDMs Bt, Dp and Df.
MATERIALS AND METHODS
Serum samples
The study utilized 260 serum samples from Filipino subjects living in Metro Manila and nearby provinces of Laguna, Cavite, Bulacan and Nueva Vizcaya, Philippines. A total of 100 allergic subjects were selected using questionnaires based on the criteria set by the International Study of Asthma and Allergies in Childhood and the International Primary Care Airways Group. Allergic individuals with current or recent intestinal parasite infection were excluded. The control group for the IgE-reactivity against HDMs consisted of 40 non-atopic subjects. A total of 60 subjects with Al infections were selected through microscopic stool examination. Subjects with other intestinal infestation and reported past experience of allergic disease or symptoms were excluded. Sixty subjects with negative stool analysis and no reported experience of allergic disease or symptoms served as control group for the IgE-reactivity against Al. All the allergic and control groups came from urban areas. Among ascariasis patients, 43 came from rural areas while 17 came from city slum areas. Control groups were used to set cut-off value for IgE-positive reactions. Written informed consents were obtained from all of the study participants. The study proposal has been approved by review boards from city health offices and the University of Santo Tomas Graduate School prior to blood collection.
Preparation of HDM and Al extracts
HDMs from pure cultures of Bt, Dp, and Df, and Al worms cut into 1×1 mm pieces, were homogenized separately for 30 min at 4℃ using phosphate-buffered saline with 2 mM Aprotinin (Sigma, USA). The suspensions were incubated at 4℃ with horizontal shaking for 16 h and centrifuged at 10,000 rpm for 10 min using Sorvall® SuperT21 Tabletop Superspeed Centrifuge (Kendro Laboratory Products, USA). Aqueous extracts were transferred in 1-mL aliquots, quantitated by Bio-Rad Protein Assay (Bio-Rad, USA), analyzed by SDS-PAGE using Mini-Protean® System (Bio-Rad, USA) of 15% Tris-glycine denaturing gel and stored at -80℃.
Human IgE enzyme-linked immunosorbent assay (ELISA)
IgEs specific to Bt, Dp, Df and Al or rBlo t 11-fD were measured through ELISA. Aqueous extracts (10 µg/mL) or rBlo t 11-fD in 0.1 M NaHCO3 buffer were coated onto 96-well EIA/RIA plates (Corning Inc., USA) for 16 h at 4℃. Plates were blocked with 1% Bovine Serum Albumin (Sigma-Aldrich, USA) and incubated with 5' dilution of serum samples in blocking buffer for 16 h at 4℃. Biotinylated anti-human IgE (diluted 1:1,00; BD Biosciences, USA) were added, followed by ExtrAvidin-Alkaline Phosphatase conjugate (diluted 1:2,000; Sigma-Aldrich, USA). Finally, colorimetric development was performed using p-Nitrophenyl Phosphate (Sigma-Aldrich, USA). Plates were washed with 200 µL of PBST in between steps. Absorbance at 405 nm was determined using Bio-Tek ELX800 ELISA reader (Tecan, Austria). Serial dilutions of 2 µg/mL semi-purified Human IgE (BD Biosciences, USA) incubated in anti-Human IgE (BD Biosciences, USA) were used as standards.
Western blot analysis
Specific IgE-binding proteins present in HDM and Al extracts were determined by Western blot analysis. IgE-positive sera from 14 allergic and 12 ascariasis subjects were selected. The aqueous extracts and Invitrogen® Magic-Mark Western Standard were subjected to SDS-PAGE as described above. Resolved proteins were transferred onto Hybond-C nitrocellulose membrane (Amersham BioSciences, UK) using MiniproteanII® Cell chamber (Bio-Rad, USA) for 1 h at 110 V. Membranes were blocked with skimmed milk (5%) in 1 PBST and incubated with serum samples (diluted 1:5 in blocking buffer) for 16 h. Allergens were visualized by incubating with Biotinylated anti-human IgE (diluted 1:1,000) followed with ExtrAvidin-Alkaline Phosphatase (diluted 1:5,000) and Alkaline Phosphatase (BCIP/NBT) Color Development Solution (Sigma-Aldrich, USA).
Cross inhibition assays
Absorption assay was used to determine the inhibition capacity of Bt, Dp and Df extracts to Al extract and vice-versa. Fifteen serum samples from each group of allergic and ascariasis subjects that reacted positively to both HDM and Al extracts were selected based on their IgE-reactivity and availability of sera. Allergic subjects' sera were pre-absorbed for 14-16 h with 100 µg/mL Al extract at 4℃ followed by human IgE ELISA using Bt-, Dp- and Df-coated plates as described above. On the other hand, 15 IgE-positive ascariasis subjects' sera were pre-absorbed for 14-16 h with 100 µg/mL Bt, Dp and Df extracts at 4℃ followed by human IgE ELISA using Al-coated plates.
Expression and purification of the rBlo t 11-fD in Escherichia coli
Recombinant Blo t 11-fD (r Blo t 11-fD) was expressed as a GST-fusion peptide in E. coli BL21 previously transformed with the plasmid pGEx-4T-1-fD [15]. The fusion peptides were produced by Isopropyl-β-D-thiogalactopyranoside induction. Bacterial cell pellets were washed with tris-buffered saline and lysed using SDS-Lysis Buffer (10% SDS, 0.25 M NaOH, 0.2 M Na2EDTA). rBlo t 11-fD-GST was isolated from the bacterial lysate by Affinity Chromatography using Glutathione Agarose Column (Sigma-Aldrich, USA). Purified protein samples were analyzed by SDS-PAGE and quantified by Bio-Rad Protein Assay as above.
RESULTS
IgE reactivity to HDM and Al extracts
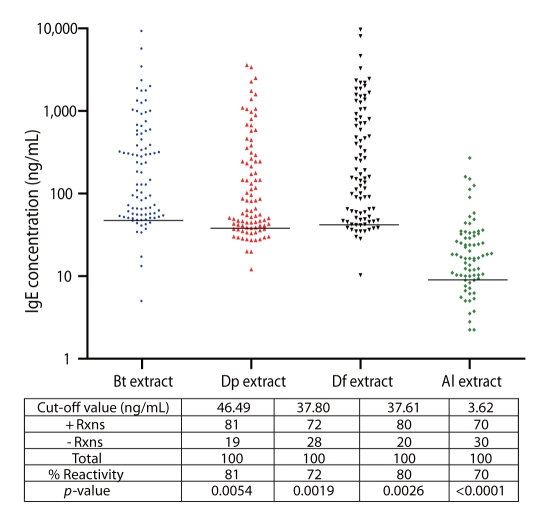
Consistently high percentage of positive reactions to Bt, Dp, and Df extracts was observed among allergic subjects (81%, 72% and 80%, respectively) (Fig. 1). Moreover, 70% of the allergic subjects reacted positively to Al, indicative of high IgE-binding antigens present in Al (Fig. 1). Unpaired t-test confirms significant difference between IgE-reactivity of allergic or ascariasis subjects and the controls. Cut-off values for Bt, Dp, Df and Al are 43.38, 35.87, 42.74, and 17.23 ng/mL, respectively. These were obtained by computing the mean+SD IgE-reactivity of the control groups. Cut-off values vary among allergens/antigens since the extracts contain different kinds and amounts of IgE-binding proteins.
Fig. 1.
IgE Reactivity of Allergic patients to Blomia tropicalis (Bt), Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df) and Ascaris lumbricoides (Al) extracts. Black horizontal lines indicate the cut-off values for positive reactions obtained by computing the mean+SD of the IgE reactivity of the control groups.
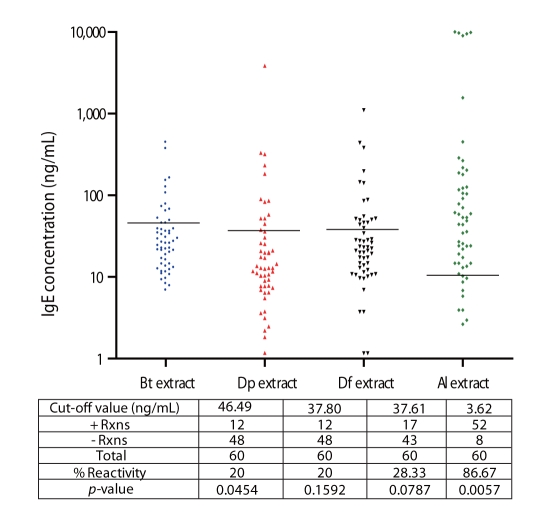
High percentage reactivity to Al extract (87%) was observed among ascariasis subjects while there was lower reactivity against HDM allergens (20% to Bt, 20% to Dp and 28.33% Df) (Fig. 2). This lower HDM sensitization among ascariasis subjects coincides with studies which determined the influence of geohelminth infection and poor sanitation in the risk of allergy and suggested that drugs derived from parasite products may be utilized as reagents to alleviate clinical allergic disease [6].
Fig. 2.
IgE-Reactivity of ascariasis patients to Blomia tropicalis (Bt), Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df) and Ascaris lumbricoides (Al) extracts. Black horizontal lines indicate the cut-off values for positive reactions obtained by computing the mean+SD of the IgE reactivity of the control groups.
Multiple sensitizations to the 4 aqueous extract preparations were observed among allergic and ascariasis subjects (Figs. 1 and 2). Relatively high percentages of allergic subjects were double positive to Bt and Dp (66%), Bt and Df (73%), and Dp and Df (69%) and overall HDM sensitization was registered at 64%.
Western blot analysis of HDM and Al aqueous extracts
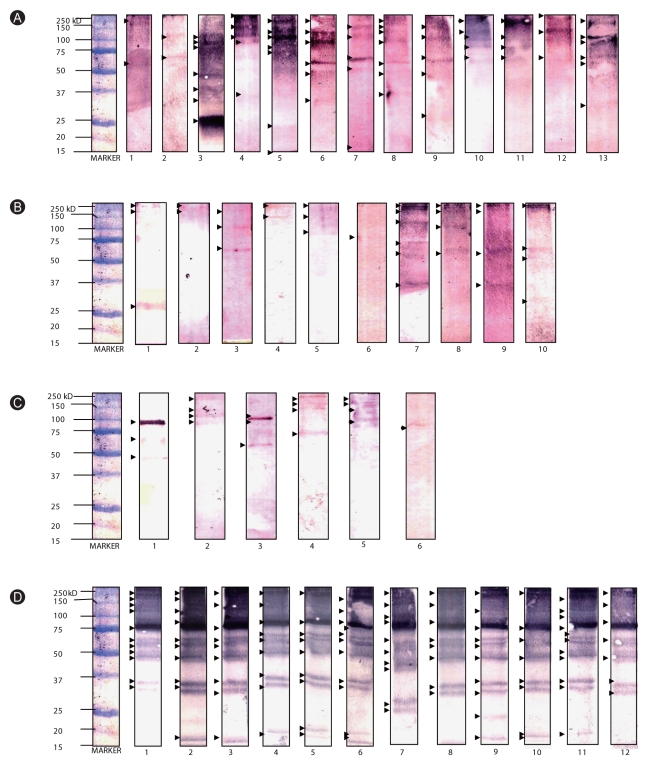
Western blot analysis was performed to determine the molecular weights of IgE-binding proteins present in extracts. Multiple sensitizations to HDM allergens were observed among allergic subjects, with molecular weights ranging from ~14-240 kD (Figs. 3A-C). Among the 14 selected allergic sera, 13 exhibited sensitization to various protein bands that migrated at approximately 250, 240, 150, 130, 110, 100, 75, 65, 60, 50, 37, 35, 27, 25, 22, and 15 kD level for the Bt extract. On the other hand, 10 exhibited sensitization to protein bands at 240, 150, 120, 100, 75, 65, 37, and 27 kD level for the Dp extract, and 6 exhibited sensitization to protein bands at 240, 150, 130, 110, 100, 75, 65, 50 kD level for the Df extract (Figs. 3A-C). Allergen groups 1, 3, and 9 may be indicated by bands at 21-30 kD level while bands at 63-65 kD, 100 kD, and 150 kD levels may specify allergen groups 15, 11, and 14, respectively.
Fig. 3.
Western-blot analysis of (A) Blomia tropicalis, (B) Dermatophagoides pteronyssinus, (C) Dermatophagoides farinae, and (D) Ascaris lumbricoides aqueous extracts to selected sera from allergic (A-C) and ascariasis (D) patients. IgE-binding proteins present in the extracts were marked with arrow heads.
On the other hand, 250, 170, 120, 100, 75, 60, 65, 50, 35, 30, 27, 25, and 15 kD-proteins are responsible for the IgE reactivity of ascariasis subjects against Al antigens (Fig. 3D). It should be noted that there is a very intense band at 75 kD level, but this is not yet identified currently since there are very limited studies regarding sensitization to Al antigens. It is notable that IgE reactivity to bands at ~35-37 and 100 kD level was observed, which accords to the molecular weights of HDM group 10 (tropomyosins, ~37 kD) and 11 (paramyosins, 98-102 kD).
Cross-inhibition of HDM allergens and Al antigens
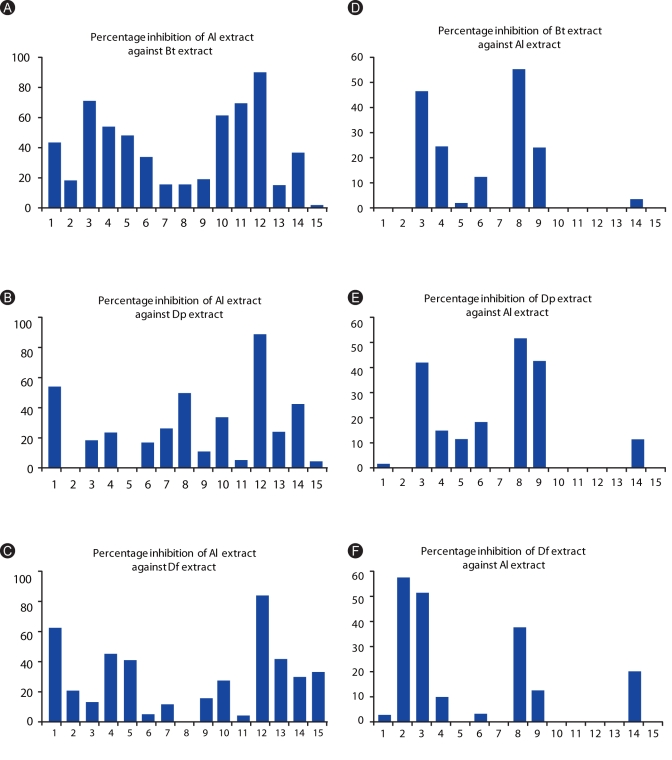
Al extract was able to inhibit the binding of HDM-specific IgEs present in the sera of allergic subjects (Figs. 4A-C). Inhibitions of Al against Bt extract ranges from 1.4-89.6% and registered a mean of 39.43%. Average inhibition against Dp extract was 27.03% and ranges from 3.5-92.2%. For the Df extract, inhibition ranges from 4.8-88.9 with an average of 30.64%.
Fig. 4.
Percentage inhibition of Al to the IgE-reactivity of allergic patients against (A) Blomia tropicalis (Bt), (B) Dermatophagoides pteronyssinus (Dp), (C) Dermatophagoides farinae (Df), and (D) Ascaris lumbricoides (Al); and percentage inhibition of Bt (D), Dp (E), and Df (F) to the IgE reactivity of ascariasis patients against HDM extracts. Percentage Inhibitions are computed as: ([absorbance of unincubated serum (at 405 nm) - absorbance of pre-incubated serum]/ absorbance of unincubated serum × 100).
Inhibition by HDM extracts against the IgE-reactivity of ascariasis subjects was also observed (Figs. 4D-F), however, it was lower than the allergic subjects. Percentage inhibitions only ranged from 1.4-54.4 for Bt extract, 1-53.3 Dp extract, and 2.3-82.3 for Df extract while mean inhibitions registered at 10.9%, 13.14%, and 14.95%, respectively.
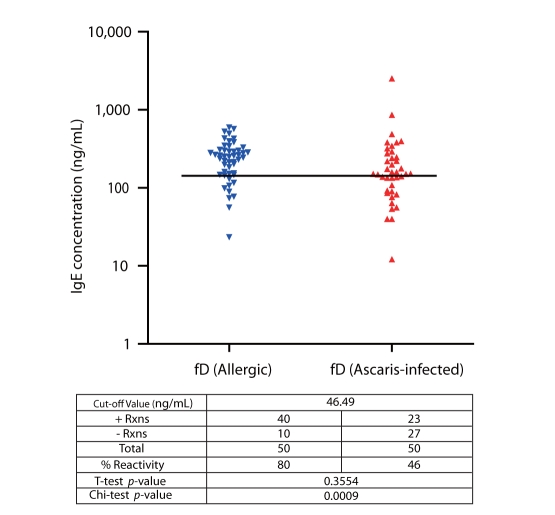
IgE reactivity of selected allergic and ascariasis subjects to rBlo t 11-fD
Results show that sensitization to rBlo t 11-fD among allergic subjects was 80% and accords to previous studies performed (Fig. 5) [15]. Cut-off value for positive reactions was 140.97 ng/mL obtained from the mean+SD IgE concentration of the controls. It is interesting to note that 46% of the ascariasis subjects exhibited positive reactions to rBlo t 11-fD.
Fig. 5.
IgE Reactivity to rBlo t 11-fD of Allergic and Al-infested subjects. Black horizontal lines indicate cut off values for positive reactions obtained by getting the mean+SD of the IgE reactivity of non-allergic, non-ascariasis controls against rBlo t 11-fD.
DISCUSSION
The determination of IgE concentration against crude extracts is an important means to establish sensitization profile of a certain population and an initial step in the identification of specific allergens. To date, there have only been a few studies on the sensitization profile of the general populations in Southeast Asian countries to house dust mites. In the Philippines particularly, there is limited information regarding the sensitization profiles of the general population to HDM allergens based on IgE reactivity [16]. The consistently high reactivity to HDM allergens (81, 72, and 80%) exhibited by the allergic volunteers in this study confirms clinical importance of Bt, Dp and Df allergens as major causative agents of allergic sensitization among Filipinos. The results obtained herein agree with initial sensitization studies previously done which demonstrated that Bt, Dp and Df are major allergens that cause sensitization among allergic Filipino individuals [16]. Moreover, the occurrence of Al-specific IgE among individuals with respiratory allergy (73%) was also reported in another tropical area [17].
Multiple sensitization to the HDM allergens suggest possible cross-reactivity among them and coincides with various studies worldwide. Over 30 different proteins can induce sensitization or IgE response among HDM allergic subjects [18]. These proteins have been classified into different allergen groups. Of the 19 denominated allergens, groups 1, 2, 3, 9, 11, 14 and 15 allergens have been reported for major IgE-binding. Sensitization to multiple HDM allergens is commonly observed among allergic individuals since different HDM species usually co-inhabit their immediate environment (parallel sensitization) and cross-reactive allergens among dust mites are known to be present. On the other hand, dual sensitization to HDM and Al extracts imply the possibility of cross-reaction between HDM allergens and Al antigens.
There is limited information with regards to sensitization of ascariasis subjects to HDM allergens and no previous studies have yet compared HDM and Ascaris-specific IgE reactivities between allergic and ascariasis populations. The data presented here indicate that HDM sensitization is predominantly observed among allergic subjects and is reduced in ascariasis subjects although a few (20-28%) possess HDM-specific antibodies. Moreover, Al sensitization is exhibited both by ascariasis and allergic subjects, and interestingly a number of subjects exhibited sensitization to both HDM and Al (64% positive reactions among allergic and 30% among ascariasis subjects).
Results of the cross-inhibition assay performed in this study sugests that cross-reactivity exists between the allergens from HDMs Bt, Dp, and Df and antigens from Al. This clearly indicates that Al and the three HDM species have specific allergens that share homology or at least similarity in epitope recognition sites. However, since Al extracts exhibited stronger inhibition capacity than HDM extracts, these cross-reactive proteins could be present in high amounts in Al and in lesser amounts in HDMs. The identities of these proteins are not yet known and further analyses such as characterization of IgE-binding proteins present in the extracts needs further confirmation.
Allergenic components among dust mites have been analyzed and IgE antibodies from mite-allergic subjects have been found to cross-react in different dust mite species as measured by percentage inhibitions [18-21]. Cross-reactivity between Dermatophagoides spp. and Blomia spp. using crude extracts and specific recombinant allergens has been determined [20, 21]. Allergen groups 10 (tropomyosin) and 11 (paramyosin) are probably the main allergens involved in HDM cross-reactivity [21]. Antigenic cross-reactivity between helminths and other intestinal parasites, such as Anisakis simplex [22], Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus [23], Necator americanus and Al [24] have also been reported. Cross-reactive antigens among allergens and among helminths are being identified and characterized for their potential in serodiagnosis and vaccination [25-27]. The nematode Anisakis simplex cross-reacts with the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dp [14] as well as with German cockroach and chironomids [28]. Observed inhibitions were reported to involve various allergens with different molecular weights [6].
Structural proteins in invertebrate muscles such as tropomyosin and paramyosin [29] were demonstrated responsible for the observed cross-reactions between nematode and arthropod allergens. Tropomyosin from Al [12] and paramyosin from Schistosoma [27] and Anisakis [30] are currently considered as potential vaccine targets specific for each nematode infection. These proteins, as previously stated, are also responsible for the cross-reactivity among HDM allergens. Isolation, characterization and immunological analyses of these proteins from nematodes can be very promising in the production of novel diagnostic and therapeutic reagents for allergic diseases. Conversely, HDM homologs of these proteins that were previously characterized and produced recombinantly can be tested as prospective vaccine candidates for helminth infections. Tropomyosin and paramyosin have been previously isolated, molecularly cloned, and characterized as important IgE-binding proteins present in Al [10] and Anisakis simplex [30], respectively. Al tropomyosin was found to have a high degree of sequence identity to tropomyosin in other parasites, mites and cockroaches, and a significant correlation of IgE reactivity with Periplaneta americana [10]. The use of recombinant tropomyosin from cockroach and ascaris to investigate IgE antibody responses is also being studied.
No previous studies, however, have yet investigated correlation between Al and HDM paramyosins. Blo t 11, a paramyosin homolog in Bt, was previously isolated and characterized [15]. The presence IgEs specific to rBlo t 11-fD among ascariasis subjects indicates a potential similarity between the epitopes of Bt paramyosin and Al allergens, probably paramyosin. This coincides with the Western-blot analysis of the Al extracts that showed positive reactions in protein bands that migrated at approximately 100 kD, which falls in the MW range of paramyosin (98-102 kD). Paramyosin, therefore, is potentially one of the specific allergens present in HDM and Al responsible for the observed cross-reactivity among the extracts.
These findings may have a clinical impact and simplify diagnostic procedures and therapeutic regimens. The potential of Al antigens as diagnostic reagent or vaccine for allergic diseases may be very promising and needs further investigation. The identification of specific allergenic components that are possibly responsible for the demonstrated cross-reactions is also necessary.
Cross-reactivity between HDM allergens and helminth antigens, specifically Al, (as presented in this study) plays a role in the complex immunological relationship between allergy and helminthiasis. To date, whether helminth infections confer protection against or serve as risk to the development of allergic diseases and vice versa, and the mechanisms on how these occur are still controversial. It can be suggested that cross-reactivity is responsible for this complexity. First, since certain helminth antigens are cross-reactive with allergens, helminthic infections may serve as initial exposure to the cross-reactive allergen and trigger sensitization to genetically predisposed atopic individuals. Moreover, non-specific potentiation of IgE synthesis may also occur. This may explain why certain studies find helminth infections as risk to the development of allergic disease, such as the occurrence of a positive association between Al infection and increased risk of childhood asthma and atopy in rural China [31]. Second, helminth infections may confer protection against atopy [1], probably since chronic infections bring about repeated exposure to the antigen (which is cross-reactive to the allergen) and thus provides mild sensitization to the individual which may lead to tolerance, similar to the purposes of allergen immunotherapy. Third, allergic diseases may serve as risk to helminth-infested individuals when IgE concentrations are further elevated due to cross-reactive antigens and leads to increased burden of the patients. Lastly, elevated IgE levels in allergic diseases may confer protection [32] when it leads to the immediate expulsion of the parasites.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Chua Kaw Yan of the National University of Singapore and the Philippine Council for Health Research and Development-Department of Science and Technology (PCHRD-DOST), Philippines.
References
- 1.Cooper PJ. Intestinal worms and human allergy. Parasite Immunol. 2004;26:455–467. doi: 10.1111/j.0141-9838.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- 2.Flohr C. Dirt, worms and atopic dermatitis. Br J Dermatol. 2003;148:871–877. doi: 10.1046/j.1365-2133.2003.05403.x. [DOI] [PubMed] [Google Scholar]
- 3.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 4.Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedón JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. 2007;119:654–661. doi: 10.1016/j.jaci.2006.12.609. [DOI] [PubMed] [Google Scholar]
- 5.Dagoye D, Bekele Z, Woldemichael K, Nida H, Yimam M, Hall A, Venn AJ, Britton JR, Hubbard R, Lewis SA. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med. 2003;167:1369–1373. doi: 10.1164/rccm.200210-1204OC. [DOI] [PubMed] [Google Scholar]
- 6.Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, Liem HT, Campbell J, Pritchard D, Hien TT, Farrar J, Williams H, Britton J. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: A cross-sectional study. J Allergy Clin Immunol. 2006;118:1305–1311. doi: 10.1016/j.jaci.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Schäfer T, Meyer T, Ring J, Wichmann HE, Heinrich J. Worm infestation and the negative association with eczema (atopic/nonatopic) and allergic sensitization. Allergy. 2005;60:1014–1020. doi: 10.1111/j.1398-9995.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 8.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 9.Arlian LG, Morgan MS, Neal JS. Dust mite allergens: ecology and distribution. Curr Allergy Asthma Rep. 2002;2:401–411. doi: 10.1007/s11882-002-0074-2. [DOI] [PubMed] [Google Scholar]
- 10.Arruda LK, Santos AB. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr Opin Allergy Clin Immunol. 2005;5:399–402. doi: 10.1097/01.all.0000182536.55650.d0. [DOI] [PubMed] [Google Scholar]
- 11.Muto R, Imai S, Tezuka H, Furuhashi Y, Fujita K. The biological activity of ABA-1-like protein from Ascaris lumbricoides. J Med Dent Sci. 2001;48:95–104. [PubMed] [Google Scholar]
- 12.Pearce EJ, James SL, Hieny S, Lanar DE, Sher A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc Natl Acad Sci U S A. 1988;85:5678–5682. doi: 10.1073/pnas.85.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebau E, Eckelt VH, Wildenburg G, Teesdale-Spittle P, Brophy PM, Walter RD, Henkle-Dührsen K. Structural and functional analysis of a glutathione S-transferase from Ascaris suum. Biochem J. 1997;324:659–666. doi: 10.1042/bj3240659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson E, Aponno M, Lundberg M, Van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001;56:660–666. doi: 10.1034/j.1398-9995.2001.00798.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramos JD, Cheong N, Lee BW, Chua KY. Peptide mapping of immunoglobulin E and immunoglobulin G immunodominant epitopes of an allergenic Blomia tropicalis paramyosin, Blo t 11. Clin Exp Allergy. 2003;33:511–517. doi: 10.1046/j.1365-2222.2003.01623.x. [DOI] [PubMed] [Google Scholar]
- 16.Salvador-Tayag F, Sumpaico MR. Aeroallergen sensitization and serum immunoglobulin levels of Filipino children with chronic and recurrent otitis media. Philippine J Allergy Asthma Immunol. 2003;9:8–16. [Google Scholar]
- 17.Medeiros D, Silva A, Rizzo J, Motta M, Oliveira F, Sarinho E. Total IgE level in respiratory allergy: study of patients at high risk for helminthic infection. J Pediatr (Rio J) 2006;82:255–259. doi: 10.2223/JPED.1503. [DOI] [PubMed] [Google Scholar]
- 18.Thomas WR, Smith WA, Hales BJ, Mills KL, O'Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- 19.Johansson E, Schmidt M, Johansson SG, Machado L, Olsson S, van Hage-Hamsten M. Allergenic crossreactivity between Lepidoglyphus destructor and Blomia tropicalis. Clin Exp Allergy. 1997;27:691–699. [PubMed] [Google Scholar]
- 20.Simpson A, Green R, Custovic A, Woodcock A, Arruda LK, Chapman MD. Skin test reactivity to natural and recombinant Blomia and Dermatophagoides spp. allergens among mite allergic patients in the UK. Allergy. 2003;58:53–56. doi: 10.1034/j.1398-9995.2003.23354.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas WR, Hales BJ, Smith W. Blomia tropicalis: more than just another source of mite allergens. Clin Exp Allergy. 2003;33:416–418. doi: 10.1046/j.1365-2222.2003.01634.x. [DOI] [PubMed] [Google Scholar]
- 22.Lozano MJ, Martín HL, Díaz SV, Mañas AI, Valero LA, Campos BM. Cross-reactivity between antigens of Anisakis simplex s.l. and other ascarid nematodes. Parasite. 2004;11:219–223. doi: 10.1051/parasite/2004112219. [DOI] [PubMed] [Google Scholar]
- 23.Ishida MM, Rubinsky-Elefant G, Ferreira AW, Hoshino-Shimizu S, Vaz AJ. Helminth antigens (Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus) and cross-reactivities in human infections and immunized animals. Acta Trop. 2003;89:73–84. doi: 10.1016/j.actatropica.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard DI, Quinnell RJ, McKean PG, Walsh L, Leggett KV, Slater AF, Raiko A, Dale DD, Keymer AE. Antigenic cross-reactivity between Necator americanus and Ascaris lumbricoides in a community in Papua New Guinea infected predominantly with hookworm. Trans R Soc Trop Med Hyg. 1991;85:511–514. doi: 10.1016/0035-9203(91)90239-u. [DOI] [PubMed] [Google Scholar]
- 25.Slater JE. Characterization of allergen extracts. Dev Biol (Basel) 2005;122:145–152. [PubMed] [Google Scholar]
- 26.Winter JA, Davies OR, Brown AP, Garnett MC, Stolnik S, Pritchard D. The assessment of hookworm calreticulin as a potential vaccine for necatoriasis. Parasite Immunol. 2005;27:139–146. doi: 10.1111/j.1365-3024.2005.00756.x. [DOI] [PubMed] [Google Scholar]
- 27.Nara T, Iizumi K, Ohmae H, Sy OS, Tsubota S, Inaba Y, Tsubouchi A, Tanabe M, Kojima S, Aoki T. Antibody isotype responses to paramyosin, a vaccine candidate for schistosomiasis, and their correlations with resistance and fibrosis in patients infected with Schistosoma japonicum in Leyte, The Philippines. Am J Trop Med Hyg. 2007;76:384–391. [PubMed] [Google Scholar]
- 28.Pascual CY, Crespo JF, San Martin S, Ornia N, Ortega N, Caballero T, Muñoz-Pereira M, Martin-Esteban M. Cross-reactivity between IgE-binding proteins from Anisakis, German cockroach, and chironomids. Allergy. 1997;52:514–520. doi: 10.1111/j.1398-9995.1997.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 29.Hooper SL, Thuma JB. Invertebrate muscles: muscle specific genes and proteins. Physiol Rev. 2005;85:1001–1060. doi: 10.1152/physrev.00019.2004. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Pérez J, Fernández-Caldas E, Marañón F, Sastre J, Bernal ML, Rodríguez J, Bedate CA. Molecular cloning of paramyosin, a new allergen of Anisakis simplex. Int Arch Allergy Immunol. 2000;123:120–129. doi: 10.1159/000024442. [DOI] [PubMed] [Google Scholar]
- 31.Palmer LJ, Celedón JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 32.Black PN. Does atopy protect against enteric infections? Allergy. 2005;60:30–34. doi: 10.1111/j.1398-9995.2005.00697.x. [DOI] [PubMed] [Google Scholar]