Abstract
Tympanic hearing is a true evolutionary novelty that appears to have developed independently in at least five major tetrapod groups—the anurans, turtles, lepidosaurs, archosaurs and mammals. The emergence of a tympanic ear would have increased the frequency range and sensitivity of hearing. Furthermore, tympana were acoustically coupled through the mouth cavity and therefore inherently directional in a certain frequency range, acting as pressure difference receivers. In some lizard species, this acoustical coupling generates a 50-fold directional difference, usually at relatively high frequencies (2–4 kHz).
In ancestral atympanate tetrapods, we hypothesize that low-frequency sound may have been processed by non-tympanic mechanisms like those in extant amphibians. The subsequent emergence of tympanic hearing would have led to changes in the central auditory processing of both high-frequency sound and directional hearing. These changes should reflect the independent origin of the tympanic ears in the major tetrapod groups. The processing of low-frequency sound, however, may have been more conserved, since the acoustical coupling of the ancestral tympanate ear probably produced little sensitivity and directionality at low frequencies. Therefore, tetrapod auditory processing may originally have been organized into low- and high-frequency streams, where only the high-frequency processing was mediated by tympanic input.
The closure of the middle ear cavity in mammals and some birds is a derived condition, and may have profoundly changed the operation of the ear by decoupling the tympana, improving the low-frequency response of the tympanum, and leading to a requirement for additional neural computation of directionality in the central nervous system. We propose that these specializations transformed the low- and high-frequency streams into time and intensity pathways, respectively.
Keywords: Middle ear, Tympanum, Lizard, Frog, Hearing, Auditory, Brain stem
1. Introduction
The emergence of a true evolutionary novelty is a rare event. By the logic of evolution, any true novelty should either arise spontaneously by random processes, for example through changes in ontogeny, or by preadaptation, co-opting structures originally used for other purposes. Obviously, preadaptation is a phenomenon much more likely to occur than the spontaneous emergence of a novel, beneficial (or at least selection neutral) structure. In this review, we outline the potential roles of the tympanic middle ear as an evolutionary novelty.
The sense of hearing is ancient in animals, stretching back to mechanoreceptors in the earliest animals. In the vertebrates, the labyrinth, i.e. the inner ear with its sensory organs, is a highly conserved structure, especially with regard to the equilibrium and gravistatic sensors. The hearing organs, however, reflect the changes from aquatic to terrestrial lifestyles in the tetrapods. Both the middle ear structures and the inner ear sensory maculae associated with perception of sound underwent dramatic changes during the water–land transition. During this transition, a true novelty emerged, the tympanic middle ear [15]. Below, we outline the likely changes from non-tympanic to tympanic hearing in the tetrapod ancestors and propose a working hypothesis for the origin of time and intensity pathways in tetrapods.
The apparent general similarity of the tympanic ears of tetrapods suggested to earlier researchers that tympanic hearing emerged early in tetrapods (or even in the tetrapod ancestors among the rhipidistian fish [37]) and that the tympanum and middle ear was therefore homologous in recent amphibians and amniotes. However, Lombard and Bolt [2,28] provided evidence from the structure of the middle ear in recent amphibians, and morphology of the ear in their tetrapod ancestors, that suggested tympanic hearing in anurans had evolved independently of tympanic hearing in the amniotes. These germinal papers were followed by the discovery of the earliest tetrapod stapes or columella [13] and a series of investigations of the middle ear of the early tetrapod, Acanthostega that suggested the columella in this species did not function in audition, and that a tympanic middle ear was not a primitive characteristic of tetrapods [14]. Furthermore, analyses of the amniote fossil record shows that even amniote ancestors did not have a tympanic ear [15,30]. These studies suggested that the tympanic ear appears to have emerged independently at least five times, i.e. in the lines leading to amphibians, turtles, lepidosaurs (lizards and snakes), archosaurs (crocodiles and birds) and mammals. The middle ear bone is homologous in the tetrapods, however, having had non-auditory function in the ancestors, either as a structural element or as an accessory in spiracular closure [15,28,31]. The consequence is that the auditory papillae and nuclei in the central auditory pathway of tetrapods, especially those nuclei involved in the processing of tympanic (i.e. high-frequency) sound, are not necessarily homologous [21].
2. How and when did tympanic hearing emerge?
The anurans, the only amphibians with a tympanic membrane, probably emerged in the Triassic, although no true anuran fossils are known from this period. Furthermore, at present the origins of anurans are debated. Earlier studies proposed that anurans, together with other recent amphibians (collectively termed the lissamphibia), were descended from temnospondyls that may have developed tympanic hearing as early as in the Carboniferous [14]. Recent cladistic analyses suggest, however, that lissamphibia belong to the lepospondyls, and that temnospondyls represent a separate emergence of a tetrapod tympanic ear [25]. In that case, the earliest tympanic ear in the line leading to anurans is found in the lower Triassic proanuran Triadobatrachus, which is not an anuran, but has some anuran features. The head region of this proanuran and of the oldest fossil anuran, the early Jurassic, Vieraella herbstii, is too damaged to reveal auditory structures, but the middle Jurassic species Notobatrachus degiustoi shows an essentially modern anuran ear with a well-developed columella and probably also an operculum [1]. Therefore, it is reasonable to suppose that tympanic hearing is a primitive characteristic in anurans. The frog inner ear contains two hearing organs (review in [27]). The low-frequency hearing organ, the amphibian papilla, covers the frequency range of 10–1200 Hz and is unique to recent amphibians. This papilla is probably derived from a specialization of a region of the sacculus, an otolithic organ that in recent amphibians responds to substrate vibrations, and also to low-frequency sound [12]. The anuran high-frequency hearing organ, the basilar papilla, may be homologous to the hearing organ of the other tetrapods. The sensory epithelium of the basilar papilla is located in the lagenar recess, as is the basilar papilla of amniotes [35]. It has been suggested that the basilar papilla has retained its function as auditory organ since the crossopterygian ancestors [20], although this view may be difficult to reconcile with the view of independent origin of tympanate hearing in the anurans and the other tetrapod lineages. In recent frogs, the basilar papilla is a high-frequency hearing organ stimulated exclusively via the tympanum. Furthermore, the homology of the basilar papilla in amphibians and amniotes has been disputed, since the morphology is very different in the two groups, reflecting an underlying and fundamental difference in the mechanisms generating shear [38]. Alternatively, the basilar papillae of amphibians and amniotes may have been independently derived, but in both cases from regions of the lagena, and the similarity reflects the common lagenar origin. The function of the lagena is variously gravistatic, vibration-sensitive or auditory in the recent tetrapods, so the ancestral lagena has not necessarily been an auditory organ, and the reason for this common origin could be that access to the lagenar cavity could be important for the efficiency of early sound pressure receivers [35].
Tympanic hearing in amniote lineages also emerged during the Triassic (see [31] and [16] for reviews). The fossil evidence for the independent emergence of tympanic hearing in amniote lineages was reviewed by Clack [15]. In this study tympanic hearing was linked to morphological characters such as oval and round windows, ossification of the otic capsule and a non-structural, vibratory stapes, and she proposed that the ancestral amniotes had a non-tympanic ear like the extant Sphenodon (the tuatara). Surprisingly, all amniote lineages (and probably also the anurans) seem to have developed tympanic hearing independently during the Triassic. These events may be convergent evolution caused by the increased importance of detection of airborne sound of biotic origin, either from predators or from prey, leading to increased selection pressures for auditory sensitivity. The emergence of tympanic hearing roughly coincides with the emergence of sound-producing insects [23].
3. Hearing before the tympanic ear
What was tetrapod hearing like in the 100 million years before the emergence of the eardrum in the Triassic? The most parsimonious assumption is that originally, the tetrapod ear functioned like that of the Crossopterygian ancestors and responded to sound-induced vibrations of the skull (i.e. by mechanisms similar to bone conduction or extratympanic hearing in recent vertebrates). Hearing in Crossopterygians has not yet been studied, but in recent actinopterygian fish, one or more of the otolithic inner ear organs respond to whole-body motion along all three dimensions, usually with maximal sensitivity to frequencies below 200 Hz and lowest displacement thresholds at 0.1 nm [32]. Since the hair cells in the otolithic sensory macula are directionally sensitive, the direction of body motion can be processed from the excitation pattern of the macula. Each VIIIth nerve fiber has a well-defined preferred direction [17]. The subsequent processing of this directional information in the CNS of recent teleosts is largely unknown, but binaural interactions, probably inhibitory, may sharpen the directional response [18]. We assume that the organization of hearing in Crossopterygians would be similar to that of unspecialized actinopterygians.
Although this has not been measured, an unspecialized fish ear may respond fairly well to airborne sound. This may be understood in terms of sound energies. In water, the acoustical impedance of the medium and of the tissue is similar, so sound energy is absorbed relatively effectively by the inner ear. Since water is relatively incompressible, however, the motion component of sound, i.e., the water particle movement, is small. By comparison, in air a considerable amount of sound energy is lost by reflection because the impedance of the air and the tissue is very different. On the other hand, at similar sound pressures the motion component of sound in air is much higher than in water, so there might not be a substantial difference in sensitivity between an unspecialized fish ear in water and on land. A measure of the importance of non-tympanic auditory sensitivity can be gauged from the substantial number of anuran species that have secondarily lost their middle ear (reviewed in [24]). Most of these species use vocal communication and have reduced sensitivity, but mostly to high-frequency sounds. At low frequencies (below 400 Hz), the sensitivity is only about 20 dB less than in ‘eared’ species. Furthermore, all anurans may have ‘extratympanic’ directional sensitivity at low frequencies, where the eardrum shows very little sensitivity and directionality. The extratympanic sensitivity may be generated by sound-induced vibrations of the skull (review in [7]). The existence of extratym-panic directional sensitivity is supported by well-defined best axes for vibrations of the skull in frog auditory nerve fibers, very similar to that observed for different fish species [4]. It therefore seems likely that the atympanate ancestors had relatively crude, non-tympanic hearing, mainly restricted to low frequencies and with sensitivities at least 20–30 dB less than their tympanate descendants. We also propose that they had directional hearing based on sensitivity to skull vibration direction, sharpened by binaural interaction in the brain stem.
4. Structure and function of the early tympanic ear
The early tympanic ear was probably formed when the spiracular opening was covered by a layer of tissue contacted by the columella. The function and sensitivity of this ear would have depended on many parameters, such as the thickness of the tissue forming the membrane, the mass of the middle ear bone, its mode of attachment to the inner ear, and the pressure release windows in the inner ear. By adjusting these parameters over the course of evolution, the sensitivity of the tympanic ear would have increased compared to the non-tympanic ear. Additionally, the spiracle opened into the mouth cavity, forming a second important feature of the early tetrapod ear by acoustically coupling the eardrums. This ear would have been inherently directional, because it would function as a pressure difference receiver. The directionality would have depended on the strength of acoustical coupling, as well as on the general sensitivity of the ear. Note, however, that another effect of the acoustical coupling of the pressure difference receiver was that the ear would be less sensitive to low-frequency sound. The phase difference of sound reaching the external and internal surface of the eardrum is the arrival time difference divided by the cycle time. At low frequencies this phase difference would be minimal. For example, an arrival time difference of 100 μs produces a phase difference of 10° at 1 kHz, but only 1° at 100 Hz. Therefore, the external and internal sound component would have nearly identical phases, largely cancelling the eardrum motion. Low-frequency sounds, however, have still been perceived by the ancestral, extratympanic pathways.
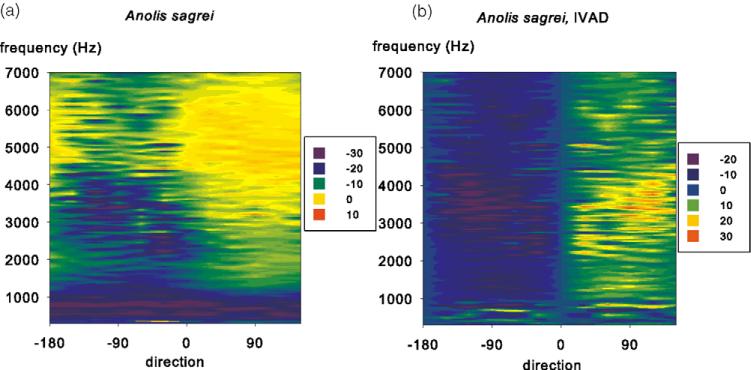
The ears of recent lizards show how strong directionality can be generated from a very simple configuration of the ear. Lizards have very sensitive ears, and thin eardrums. The eardrums are connected through the mouth cavity, and since the middle ear is not enclosed in a tympanic cavity, the head is acoustically transparent [9] with unattenuated transmission of sound from the contralateral eardrum [10,11]. An example of the directionality of a lizard ear is shown in Fig. 1. The directional difference is up to 35 dB (50-fold) at frequencies above 1 kHz. Note also that the eardrum is unresponsive at very low frequencies, and that the directionality shows a steep gradient across the mid-line. This gradient allows the sharpening of the directionality by binaural comparisons such as simple inhibitory interactions, in effect a neural subtraction of the contralateral from the ipsi-lateral response. This comparison can be modelled simply by subtracting the response by its mirror image (Fig. 1B). Note the very strong directionality resulting from this operation with a nearly perfect demarcation of the midline.
Fig. 1.
Eardrum directionality in the lizard species Anolis sagrei. (A) The normalized vibration velocities (colour scale, in dB re 1 mm/(s Pa)) are plotted as a function of direction (x-axis, contralateral angles on the left and ipsilateral angles on the right) and frequency (y-axis). Each horizontal line corresponds to a polar plot. (B) Interaural difference plot modelling the output of a binaural difference (EI) neuron in Anolis sagrei. The eardrum vibration data set is subtracted from its reflection along the body axis. The colour scale is relative interaural differences in dB. From Christensen-Dalsgaard and Manley [10,11].
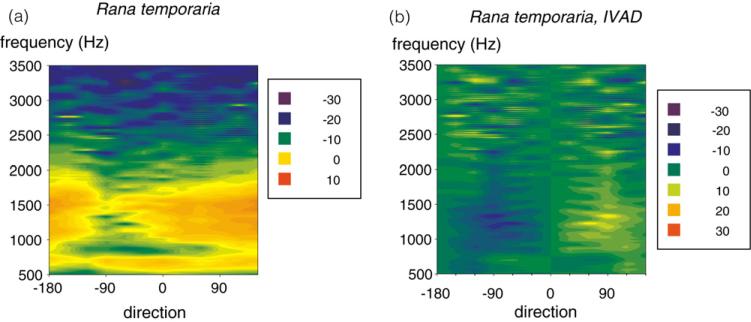
Frog eardrums are also coupled acoustically, but the coupling is generally weaker than in the lizards [22], since the middle ear cavities are connected to the mouth cavity by narrower Eustachian tubes, reducing the interaural transmission by approximately 10 dB [22]. Nevertheless, in the anurans this coupling generates directionality in a certain frequency band (Fig. 2A) with maximal directional differences of 10 dB [9] that also can be sharpened by simple binaural inhibitory interactions (Fig. 2B).
Fig. 2.
Eardrum directionality in the grass frog, Rana temporaria. Other details same as Fig. 1. From Christensen-Dalsgaard and Manley [9].
Given the data from frogs and lizards, it seems that processing of directional information in the auditory pathway may be relatively simple for an ear like the frog or lizard ear, and perhaps also for the tympanate tetrapod ancestors, since the input from the auditory nerve itself should be directional at frequencies at which the tympanum is responsive. It should be noted that phase locking is effectively non-existent at frequencies above 1.2 kHz in the gecko [34], so binaural comparisons of a directional signal from the tympanum should have been based on interaural level differences. At lower and perhaps extratympanic frequencies, however, the neural input would be strongly phase locked, and the binaural response would likely be influenced by both interaural time and intensity differences.
We therefore propose that the low and high frequencies would be processed in different streams. We will define the border between low and high frequencies at approximately 400 Hz. The low-frequency stream would be the ancestral pathway, whereas the high-frequency stream would have emerged with tympanic hearing. As a corollary, the nuclei in the low-frequency stream should be more similar across diverse animal groups than nuclei in the high-frequency stream.
In the frogs, the low-frequency stream (frequencies below 400 Hz) originates in the rostral patch of the amphibian papilla in the inner ear. The hair cells in this part of the papilla are oriented in a complex, three-dimensional pattern, are sensitive to both sound and vibration, and have well-defined vibration axes, as described above [27,4]. Furthermore, the rostral patch is clearly separate from the other part of the amphibian papilla macula, the caudal extension, which covers the higher frequencies in the amphibian papilla. The rostral patch is also conserved among all lissamphibia, whereas the shape of the caudal extension is much more variable [26]. In lizards, it is proposed that the low-frequency population of hair cells in the basilar papilla macula (tectorial hair cells) is ancestral and generally similar to hair cells in other amniotes, whereas the high-frequency population of free-standing hair cells may be derived [29]. The tectorial and free-standing hair cells in the alligator lizard differ in their phase locking properties, reflecting adaptation for temporal processing in the tectorial hair cells [33].
5. Binaural processing in the stem hindbrain auditory nuclei
Evidence for separate high- and low-frequency pathways in the hindbrain emerges from studies on the alligator lizard. Generally, vertebrate auditory nerve fibers bifurcate as they enter the medulla, terminating in an ipsilateral, dorsally located column of auditory nuclei. In the alligator lizard, however, projections from the basilar papilla are partitioned so that tectorial hair cell afferents (CFs 100–800 Hz) conform to the vertebrate pattern and project to the nucleus magnocellularis and the lateral nucleus angularis, whereas free-standing hair cell afferents (CFs 900–4000 Hz) project only to the medial nucleus angularis [36,5]. Separate high best frequency projections may characterize free-standing hair cell populations in all lizards [29].
In the gecko (Christensen-Dalsgaard, Tang and Carr, unpublished), neurons in the nucleus magnocellularis generally have low best frequencies and phase lock to auditory stimuli. Unlike magnocellularis neurons in birds, however, magnocellular neurons in the gecko are excited by inputs from both ears. Binaural first order responses are not found in birds or mammals, but in experiments on geckos where sound transmission from the contralateral ear was blocked, responses to contralateral sound stimuli were still present in nucleus magnocellularis, suggesting that there is projection to the nucleus magnocellularis from the contralateral first or second order auditory nuclei. Magnocellular responses are clearly modulated by both ITD and ILD, again reflecting the binaural and directional responses present in the auditory nerve and projections from the contralateral auditory nuclei. The processing of high frequencies is much less clear, although the phase locking of the auditory fibers is insignificant at frequencies above 1 kHz. We hypothesize that further processing of the higher best frequency binaural processing may take place in the superior olivary nuclei and depend largely upon ILD.
In anurans, auditory nerve fibers from the amphibian papilla and the basilar papilla project the dorsal medullary nucleus. A commissural tract exists between the two DMN, making this the first site along the auditory pathway in which binaural interactions occur [19,8]. Binaural neurons in the DMN are excited by monaural stimuli, and often inhibited by dichotic stimuli [8]. It is presently not clear, however, whether there is a clear partitioning of low and high frequencies, as in the lizards. Neither lizards nor anurans show evidence for segregated processing of time and intensity cues.
6. Changes in the early tympanic ear
As we have outlined above, the early tympanic ears may have been similar to the lizard ear, with substantial acoustical coupling of the ears. If this was the case, the mammalian pressure receiver ear must be derived from such an ear, raising the question of which evolutionary processes would lead to partial isolation of the middle ears. One such selective pressure could be a requirement to shield the eardrums from respiration noise [9]. Changes in buccal pressure associated with respiration in anurans or lizards cause the eardrums to move. Because of the higher respiration rate in mammals, this disturbance of their auditory function may be more serious. Another explanation could be a general protection of the middle ear bone(s) that are completely exposed in the mouth cavity of lizards, while a third explanation would be a side effect of the enlargement of the mammalian brain, specifically growth of the brain downward. Finally, pressure gradient directionality comes at the expense of sensitivity, because the subtraction inherent in the mechanism can produce both decreased low-frequency sensitivity and nulls, or directions of low sensitivity, at higher frequencies. Whatever the cause(s) of the isolation of the middle ears, their separation would lead to a non-directional ear, where sound direction processing would need additional computing in the CNS. The transformation of a pressure gradient ear into a pressure receiver ear would also lead to increased tympanic stimulation at low frequencies. The increased tympanic sensitivity and the need for additional neural computation of directionality may have transformed parts of the original low-frequency stream to a time pathway, since the low-frequency stream would already show phase locking [33]. Changes associated with the transformation into the time pathways known from recent birds and mammals include improved synaptic coupling (endbulbs of Held) in the first-order acoustic nuclei, and increased phase locking in hair cells and auditory nerve [6]. Binaural comparisons in the high-frequency pathway were originally based on ILD and would have been transformed into the intensity pathway. Once the pressure gradient mechanisms were lost or diminished, there might have been selection pressure for increased high-frequency sensitivity, and for peripheral structures (external ears, pinnae) that could increase ILD cues. On top of these peripheral changes, the neural processing would now have to compensate for the loss of directional information from the eardrum. This might have led to specialization in central time processing such as the delay lines in archosaurs and the precisely timed inhibitory–excitatory binaural processing in the gerbil MSO [3,21]. Note that even these specialized binaural neurons probably may have had counterparts in the ancestral tetrapods, since the hindbrain auditory nuclei of anurans and lizards also exhibit excitatory and inhibitory binaural connections.
According to this hypothesis, many of the features of the avian and mammalian central auditory system reflect modifications of existing structures in an already functioning system. Thus, the tympanum may be considered a novelty in the course of the evolution of the tetrapod ear.
References
- 1.Báez AM, Basso NG. The earliest known frogs of the Jurassic of South America: review and cladistic appraisal of their relationships, Münchner Geowiss. Abh. A. 1996;30:131–158. [Google Scholar]
- 2.Bolt JR, Lombard RE. Evolution of the amphibian tympanic ear and the origin of frogs. Biol. J. Linn. Soc. 1985;24:83–99. [Google Scholar]
- 3.Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- 4.Brandt C, Christensen-Dalsgaard J. Responses to three-dimensional vibrations and sound stimuli in single fibers from the 8th cranial nerve of the grass frog, Rana temporaria. In: Elsner N, Kreuzberg GW, editors. Göttingen Neurobiology Report 2001. Georg Thieme; Stuttgart: 2001. p. 386. [Google Scholar]
- 5.Carr C, Code RA. The central auditory system of reptiles and birds. In: Dooling RJ, Fay RR, Popper AN, editors. Comparative Hearing: Birds and Reptiles. Springer; New York: 2000. pp. 197–248. [Google Scholar]
- 6.Carr CE, Soares D, Parameshwaran S, Perney T. Evolution of time coding systems. Curr. Opin. Neurobiol. 2001;11:727–733. doi: 10.1016/s0959-4388(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 7.Christensen-Dalsgaard J. Directional hearing in non-mammalian vertebrates. In: Popper AN, Fay RR, editors. Sound Source Localization. Springer; New York: 2005. pp. 67–123. [Google Scholar]
- 8.Christensen-Dalsgaard J, Kanneworff M. Binaural interactions in the frog dorsal medullary nucleus. Brain Res. Bull. 2005;66:522–525. doi: 10.1016/j.brainresbull.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Christensen-Dalsgaard J, Manley GA. Directionality of the lizard ear. J. Exp. Biol. 2005;208:1209–1217. doi: 10.1242/jeb.01511. [DOI] [PubMed] [Google Scholar]
- 10.Christensen-Dalsgaard J, Manley GA. Acoustical coupling of the lizard eardrums. Soc. Neurosci. Abs. 2005:44.2. doi: 10.1007/s10162-008-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen-Dalsgaard J, Manley GA. Acoustical coupling of the lizard eardrums. JARO. doi: 10.1007/s10162-008-0130-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen-Dalsgaard J, Narins PM. Sound and vibration sensitivity in the frogs Leptodactylus albilabris and Rana pipiens pipiens. J. Comp. Physiol. A. 1993;172:653–662. doi: 10.1007/BF00195391. [DOI] [PubMed] [Google Scholar]
- 13.Clack JA. Discovery of the earliest tetrapod stapes. Nature. 1989;342:425–430. doi: 10.1038/342425a0. [DOI] [PubMed] [Google Scholar]
- 14.Clack JA. Homologies in the fossil record: the middle ear as a test case. Acta Biotheor. 1993;41:391–409. doi: 10.1007/BF00709373. [DOI] [PubMed] [Google Scholar]
- 15.Clack JA. The evolution of tetrapod ears and the fossil record. Brain Behav. Evol. 1997;50:198–212. doi: 10.1159/000113334. [DOI] [PubMed] [Google Scholar]
- 16.Clack JA, Allin E. The evolution of single- and multiple-ossicle ears in fishes and tetrapods. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. Springer; New York: 2004. pp. 128–163. [Google Scholar]
- 17.Edds-Walton PL, Fay RR. Sharpening of directional responses along the auditory pathway of the oyster toadfish, Opsanus tau. J. Comp. Physiol. A. 2005;191:1079–1086. doi: 10.1007/s00359-005-0051-z. [DOI] [PubMed] [Google Scholar]
- 18.Edds-Walton PL, Fay RR. Directional encoding by fish auditory systems. Proc. R. Soc. B. 2000;355:1281–1284. doi: 10.1098/rstb.2000.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng AS, Capranica RR. Sound localization in anurans. I. Evidence of binaural interaction in the dorsal medullary nucleus of the bullfrog (Rana catesbeiana) J. Neurophysiol. 1976;39:871–881. doi: 10.1152/jn.1976.39.4.871. [DOI] [PubMed] [Google Scholar]
- 20.Fritzsch B. Hearing in two worlds. Theoretical and actual adaptive changes of the aquatic and terrestrial ear. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. Springer; New York: 1999. pp. 15–42. [Google Scholar]
- 21.Grothe B, Carr CE, Casseday JH, Fritzsch B, Köppl C. The evolution of central pathways and their neural processing patterns. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. Springer; New York: 2004. pp. 289–359. [Google Scholar]
- 22.Ho CCK, Narins PM. Directionality of the pressure-gradient receiver ears in the Northern leopard frog Rana pipiens pipiens. J. Comp. Physiol. A. 2006;192:417–429. doi: 10.1007/s00359-005-0080-7. [DOI] [PubMed] [Google Scholar]
- 23.Hoy R. The evolution of hearing in insects as an adaptation to predation from bats. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. Springer; New York: 1992. pp. 115–129. [Google Scholar]
- 24.Jaslow AP, Hetherington TE, Lombard RE. Structure and function of the amphibian middle ear. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. Wiley; New York: 1988. pp. 69–91. [Google Scholar]
- 25.Laurin M, Reisz R. A new perspective on tetrapod phylogeny. In: Sumida SS, Martin KLM, editors. Amniote Origins. Completing the Transition to Land. Academic Press; San Diego: 1997. pp. 9–59. [Google Scholar]
- 26.Lewis ER, Lombard RE. The amphibian inner ear. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. Wiley; New York: 1988. pp. 93–123. [Google Scholar]
- 27.Lewis ER, Narins PM. The acoustic periphery of amphibians: anatomy and physiology. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. Springer; New York: 1999. pp. 101–154. [Google Scholar]
- 28.Lombard RE, Bolt J. Evolution of the tetrapod ear: an analysis and reinterpretation. Biol. J. Linn. Soc. 1979;11:19–76. [Google Scholar]
- 29.Manley GA. The lizard basilar papilla and its evolution. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. Springer; New York: 2004. pp. 200–223. [Google Scholar]
- 30.Manley GA, Clack J. An Outline of the evolution of vertebrate hearing organs. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. Springer; New York: 2004. pp. 1–26. [Google Scholar]
- 31.Manley GA, Köppl C. Phylogenetic development of the cochlea and its innervation. Curr. Opin. Neurobiol. 1998;8:468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- 32.Popper AN, Fay RR. The auditory periphery in fishes. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. Springer; New York: 1999. pp. 43–100. [Google Scholar]
- 33.Rose C, Weiss TF. Frequency dependence of synchronization of cochlear nerve fibers in the alligator lizard: evidence for a cochlear origin of timing and non-timing pathways. Hear. Res. 1988;33:151–166. doi: 10.1016/0378-5955(88)90028-7. [DOI] [PubMed] [Google Scholar]
- 34.Sams-Dodd F, Capranica RR. Representation of acoustic signals in the eighth nerve of the Tokay gecko: I. Pure tones. Hear. Res. 1994;76:16–30. doi: 10.1016/0378-5955(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 35.Smotherman M, Narins PM. Evolution of the amphibian ear. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the Vertebrate Auditory System. Springer; New York: 2004. pp. 164–199. [Google Scholar]
- 36.Szpir MR, Sento S, Ryugo DK. Central projections of cochlear nerve fibers in the alligator lizard. J. Comp. Neurol. 1990;295:530–547. doi: 10.1002/cne.902950403. [DOI] [PubMed] [Google Scholar]
- 37.van Bergeijk WA. Evolution of the sense of hearing in vertebrates. Am. Zool. 1966;6:371–377. doi: 10.1093/icb/6.3.371. [DOI] [PubMed] [Google Scholar]
- 38.Wever EG. The Reptile Ear. Princeton University Press; Princeton: 1978. [Google Scholar]