Abstract
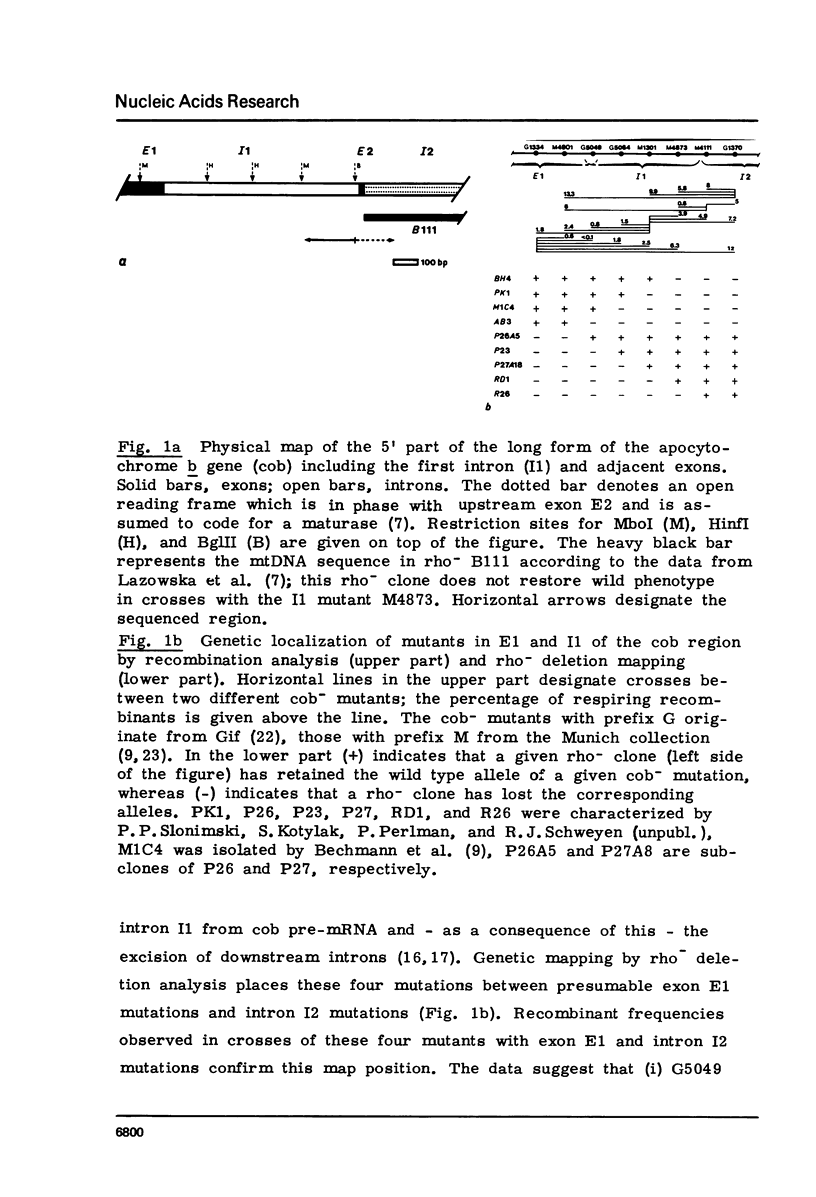
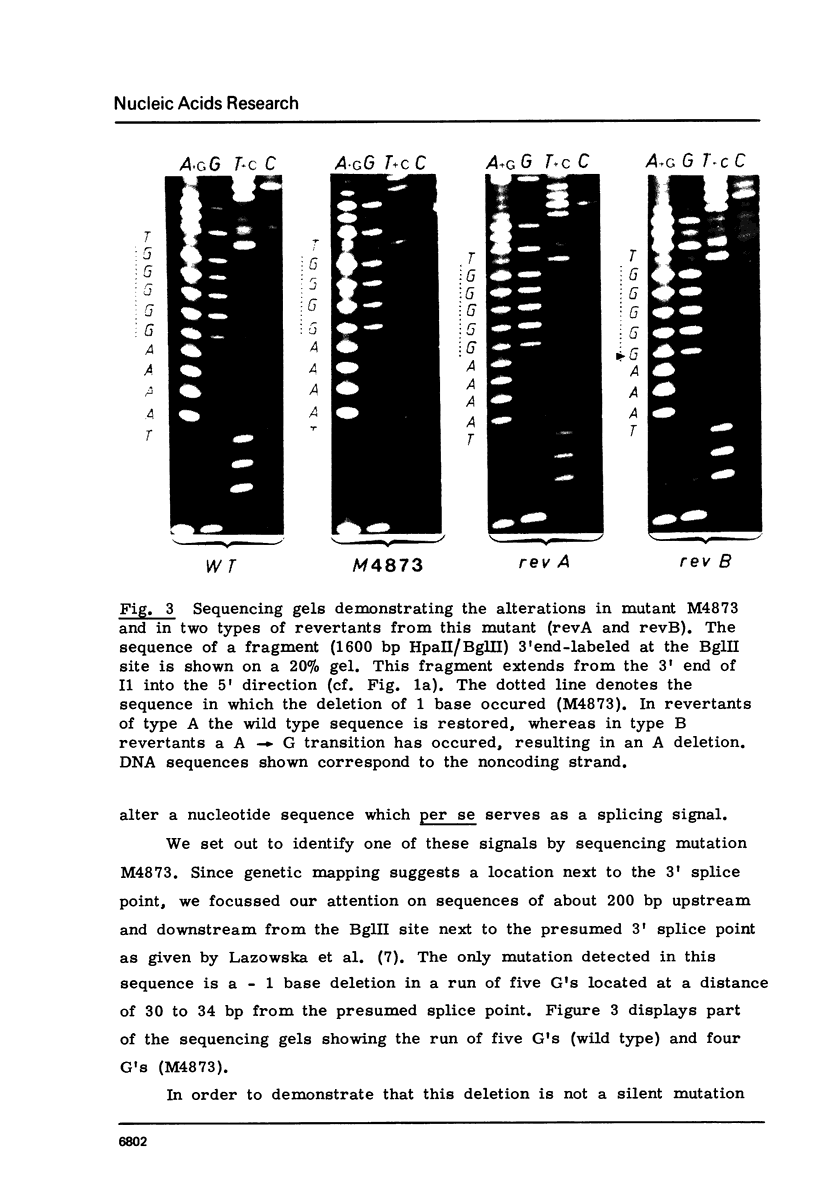
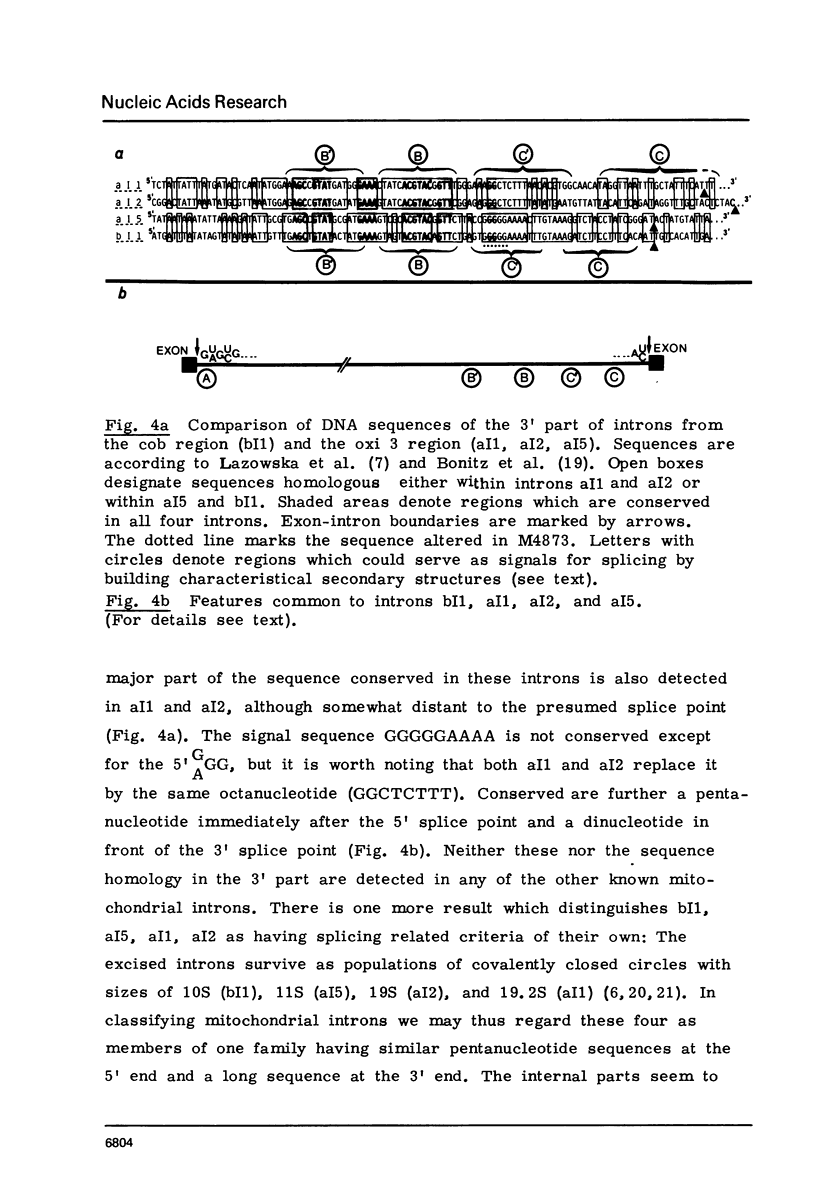
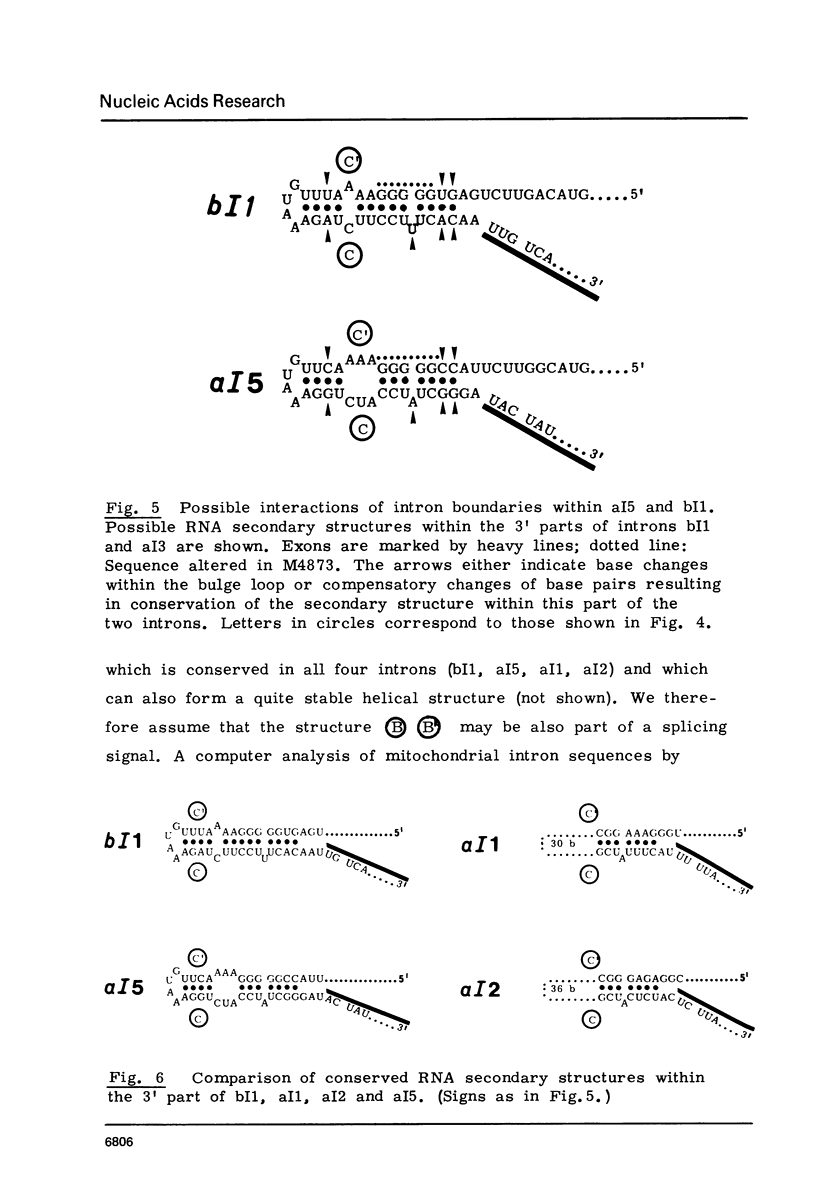
Four mitochondrial mutations are known to block excision of intron I1 of the cob gene in S.cerevisiae. The nucleotide sequence alteration of one of them, M4873, has been determined. It is a deletion of 1 bp in a run of five G's at a distance of 30 to 34 bp upstream to the 3' splice point. Reversion is found to occur by restoration of the run of five G's either by insertion of 1 G (wild type reversion) or by transition A leads to G next to this run of G's (pseudo-wild type reversion). The effect of mutation and reversion on RNA splicing indicates that the run of five G's is of critical importance for intron I1 excision, possibly in participating in the formation of a splice signal with a helical structure. This presumption is confirmed by the observation that this sequence is part of a larger sequence of some 80 bp next to the 3' splice point which is conserved to some extend in the four mitochondrial introns (bI1, aI1, aI2, aI5) that survive after excision as circular RNAs. Most striking is the conservation of this sequence at the level of secondary structure.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnberg A. C., Van Ommen G. J., Grivell L. A., Van Bruggen E. F., Borst P. Some yeast mitochondrial RNAs are circular. Cell. 1980 Feb;19(2):313–319. doi: 10.1016/0092-8674(80)90505-x. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bechmann H., Haid A., Schweyen R. J., Mathews S., Kaudewitz F. Expression of the "split gene" COB in yeast mtDNA. Translation of intervening sequences in mutant strains. J Biol Chem. 1981 Apr 10;256(7):3525–3531. [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- De La Salle H., Jacq C., Slonimski P. P. Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell. 1982 Apr;28(4):721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Heyting C., Meijlink F. C., Verbeet M. P., Sanders J. P., Bos J. L., Borst P. Fine structure of the 21S ribosomal RNA region on yeast mitochondria DNA. I. Construction of the physical map and localization of the cistron for the 21S mitochondrial ribosomal RNA. Mol Gen Genet. 1979 Jan 11;168(3):231–246. doi: 10.1007/BF00271496. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Schmelzer C., Schweyen R. J. Evidence for ribosomes involved in splicing of yeast mitochondrial transcripts. Nucleic Acids Res. 1982 Jan 22;10(2):513–524. doi: 10.1093/nar/10.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweyen R. J., Weiss-Brummer B., Backhaus B., Kaudewitz F. The genetic map of the mitochondrial genome in yeast: map positions of drug' and mit- markers as revealed from population analyses of rho- clones in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Feb 16;159(2):151–160. doi: 10.1007/BF00270888. [DOI] [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]