Abstract
Glioblastomas are among the most vascular tumors due to the expression of a variety of pro-angiogenic factors. New drug regimens are being developed to target angiogenesis in an attempt to arrest tumor growth. In particular, the vascular endothelial growth factor (VEGF) pathway has been a prime drug target. Preliminary results with anti-VEGF agents have been promising with prolonged progression-free survival reported. In addition, the anti-permeability effects of anti-VEGF agents have important consequences for tumor imaging and for patient quality of life by decreasing corticosteroid dependence. Unfortunately, the response to anti-VEGF therapy is transient and the majority of patients eventually relapse so more work is needed to understand mechanisms of tumor escape.
Keywords: antiangiogenic therapy, treatment, VEGF, small molecule inhibitors, antibodies, glioblastoma
Introduction
Despite aggressive treatment with surgery, radiation, and chemotherapy, glioblastoma (GBM) remains the most aggressive primary brain tumor with a 5-year survival rate under 5% and median overall survival of only 15 months.1 Researchers are actively seeking new therapeutic options and current efforts have exploited the fact that GBMs are highly vascularized tumors characterized by activation of multiple pro-angiogenic signaling pathways. Antiangiogenic agents, and particularly drugs that target the VEGF pathway, are increasingly being incorporated into drug regimens. This review will focus on antiangiogenic therapy for GBM.
Angiogenesis in gliomas
A thorough review of angiogenesis in GBM is beyond the scope of this review so only a brief overview will be outlined here. Growing tumors reach a point at which the existing blood supply can no longer support the needs of the tumor leading to areas of hypoxia. In response to this hypoxia, GBMs undergo an “angiogenic switch” and increase secretion of various growth factors to promote new blood vessel formation. VEGF is one of the most critical growth factors and plays a central role in GBM angiogenesis by interacting with a number of pathways to promote GBM growth.2–3 These pathways include PTEN/pI3-kinase/Akt4, MAPK/ERK5, nitric oxide, 6, Notch-Deltalike ligand 4 (DLL4) 7, platelet-derived growth factor-B (PDGF- B)8, epidermal growth factor (EGF)9–10, tumor necrosis factor α (TNF-α), and basic fibroblast growth factor (bFGF) 8, 11. In addition, the angiopoietins, Ang-1 and Ang-2, have a complex interaction with VEGF through their tyrosine kinase receptors, Tie1 and Tie2. In the presence of VEGF, Ang-2 promotes vessel sprouting but in the absence of VEGF, Ang-2 causes vessel regression.12 Due to this complex redundancy, selective inhibition of the VEGF signaling axis, which has been the principal therapeutic target to date, has proven insufficient for a sustained antiangiogenic effect and sustained anti-tumor effect.
Debate continues over the exact mechanism of action of antiangiogenic drugs and whether or not these drugs have a direct anti-tumor effect. Initially, antiangiogenic agents were hypothesized to work by preventing new blood vessel formation and pruning existing tumor vessels leading to tumor deprivation of oxygen and nutrients.13 More recently, Jain hypothesized a process of vascular normalization.14 Tumor blood vessels, and particularly GBM blood vessels, are highly abnormal and characterized by enlarged vessel caliber, increased permeability, lack of adequate pericyte coverage and abnormally thickened basement membranes. The result is a heterogeneous, disorganized tumor vascular network with areas of increased perfusion and areas of decreased perfusion leading to hypoxia and, potentially, inefficient or inhomogeneous delivery of chemotherapy drugs or oxygen needed in order for radiation to be effective. Antiangiogenic agents are thought to revert this abnormal vascular network to a more normalized state.15 Normalization may improve tumor perfusion and delivery of cytotoxic therapies, thus, enhancing the efficacy of concomitantly administered drugs.14 As opposed to pruning or destroying blood vessels, vascular normalization induces structural and functional changes in the abnormal tumor vasculature that transforms these vessels to a more “normal” morphological state. Finally, a third possible mechanism of action of antiangiogenic drugs is disruption of the perivascular-cancer stem cell niche. GBM stem cells, the self-renewable cells that potentially give rise to gliomas, exist in a highly specialized stem cell-vascular niche that allows these cells to remain in a self-renewing state.16 Antiangiogenic agents may disrupt the critical interaction between these GBM stem cells and endothelial cells, thus, contributing to their death.17
Recently, more evidence has been accumulating about the important interplay between angiogenesis and bone marrow derived cells. Monocytes and macrophages express VEGFR1 so are recruited to tumors with high VEGF expression and contribute to angiogenesis and tumor growth.18–19 Suppression of VEGFR-1 signaling in bone marrow derived cells can decrease tumor growth.19 These myeloid cells may also mediate resistance to antiantgiogenic therapies so likely will become another therapeutic target.
Antiangiogenic Agents
A number of drugs that block angiogenesis are in clinical development. The majority target the VEGF pathway but as more evidence accumulates that just targeting VEGF will be inadequate, newer agents are being explored that target other pro-angiogenic molecules. Early clinical studies have employed antibodies or small molecule tyrosine kinase inhibitors (TKIs). Antibodies have the advantage of being highly selective for their target with limited cross reactivity and fewer “off target” side effects as compared to small molecule TKIs. The half-life of an antibody is generally longer than TKIs allowing for less frequent dosing. Conversely, antibodies are costly to produce, must be administered by intravenous infusion, and are large molecular weight protein molecules with limited penetration of the intact blood brain barrier (BBB). However, when targeting endothelial cells that line tumor blood vessels or in the setting of a disrupted BBB, this may be less of a concern. TKIs have the advantage of being orally active and most lack specificity for one tyrosine kinase receptor, thus, allowing for broader inhibition of a variety of growth factor pathways. This broad inhibition, though, increases the risk of side effects. A number of TKIs are in clinical development and the primary distinguishing characteristics are their selectivity and potency at different receptors.
Bevacizumab (Avastin)
Bevacizumab is the most commonly used antiangiogenic agent for GBM and received accelerated FDA approved for use in patients with recurrent GBM in 2009. It is a recombinant, humanized monoclonal antibody that inhibits VEGFR-mediated cell signaling by sequestration of its ligand VEGF-A. The half-life of bevacizumab is approximately 20 days so it is administered every 2 weeks and sometimes every 3 weeks. Initial studies with bevacizumab in recurrent GBM showed radiographic response rates of 28–40% and 6-month progression free survival (APF6) rates of 40–50%.20–22 This compared favorably to prior studies in recurrent GBM in which the median APF6 was only 15%.23
Based on these promising results, a randomized, non-comparative phase II trial of patients with recurrent GBM was completed. One hundred and sixty-seven patients were randomized to bevacizumab alone (85 patients) or bevacizumab plus irinotecan (82 patients).24 The combination arm was postulated to be more effective than bevacizumab alone because of the possible normalizing effects on tumor vasculature resulting in improved delivery of a cytotoxic chemotherapy. The APF6 was 35.1% in the bevacizumab alone arm and 50.2% in the combination therapy arm. Median overall survival was 9.7 months in the bevacizumab arm and 8.9 months in the combination arm with more frequent CTCAE Grade 3 toxicities in the bevacizumab and irinotecan arm (67% versus 48%). Consequently, although APF6 was better in the combination arm, the increased rate of toxicity and equivalent median overall survival raise questions as to whether or not there is a significant advantage to combining bevacizumab with irinotecan over bevacizumab alone.
Antiangiogenic agents may improve response to radiation and a small pilot study determined that bevacizumab was safe in newly diagnosed GBM patients who were also receiving radiation and temozolomide.25 Two randomized Phase III clinical trials are exploring bevacizumab in combination with radiation and temozolomide in newly diagnosed GBM but results from these studies are not yet available.
Despite the improved response rate and PFS with bevacizumab, all patients eventually relapse so different drug combinations with bevacizumab have been explored. Erlotinib, cetuximab, etoposide, and histone deactylase inhibitors are currently being explored or have been explored in patients with recurrent GBM. Unfortunately the results so far have not suggested any additional benefit over bevacizumab alone. 26–27
Other Antibodies
Aflibercept (VEGF-Trap) is a soluble decoy molecule that binds to VEGF-A with higher affinity than bevacizumab but also binds VEGF-B and placental growth factor (PlGF), another ligand for VEGFR-1.28 Preliminary data from a Phase II trial of aflibercept in recurrent malignant glioma patients demonstrated a 30% radiographic response rate in the subset of patients with GBM.29 In a mouse xenograft model, aflibercept combined with radiation was shown to be more effective than radiation alone, suggesting that it might be beneficial in combination with radiation and temozolomide in newly diagnosed GBM patients so a trial was recently completed in this patient population but the results are pending.30 IMC-3G3, a monoclonal antibody to PDGFRα, and ramucirumab (IMC-1121B), a monoclonal antibody to VEGFR2 are both being evaluated in clinical trials for recurrent GBM. Antibodies to Ang2 are also being developed.
Cediranib (Recentin®)
Cediranib has been the most studied TKI in GBM. It blocks all VEGFRs (−1/−2/−3), c-kit, and PDGFR-α/β and can be administered once daily. In 30 patients with recurrent GBM treated with cediranib alone, the radiographic response proportion was 56%, median PFS was 117 days, median overall survival was 227 days, and APF6 was 25.8%.31 As part of this study, patients underwent specialized MRI scans to study the tumor vasculature and a transient window of normalization was identified. Vessel size and permeability rapidly decreased suggesting normalization of the abnormal GBM vascular network. A positive consequence of reduced vascular permeability in these patients was reduction of vasogenic cerebral edema, a cause of major morbidity in this patient population. The anti-edema effect of cediranib also resulted in a steroid-sparing effect with most subjects able to reduce or discontinue corticosteroids as the cerebral edema resolved. Based on these promising results, an international randomized phase III trial of cediranib versus cediranib with lomustine, an oral alkylating agent that has been a standard therapeutic in patients with recurrent GBM, was completed. Unfortunately, preliminary results from this study did not demonstrate a survival benefit of cediranib alone or in combination with lomustine versus lomustine alone. Trials of cediranib in newly diagnosed GBM patients also receiving radiation and temozolomide are ongoing.
Other TKIs
There are several other broad spectrum TKIs that target VEGFRs currently under study in the GBM patient population including sorafanib, sunitinib, vandetanib, cabozantinib, pazopanib, AEE788, E7080, and CT-322 (Table 1). Results from these trials are preliminary but generally disappointing.32–35 Toxicty with TKIs is often greater than with bevacizumab. TKIs that target other angiogenesis pathways such as inhibitors of PDGF, hepatocyte growth factor/c-MET, FGF, or PI3 kinase are in clinical trials.
Table 1.
Select antiangiogenic agents in trial for GBM.
| Drug | Mechanism | Most Advanced Phase | Results |
|---|---|---|---|
|
Antibodies
| |||
| bevacizumab | VEGF antibody | Phase II-recurrent GBM | Bevacizumab alone: 29–35 % APF6 |
| Phase III- newly diagnosed | Bevacizumab + irinotecan: 50.2% APF6 | ||
|
| |||
| aflibercept | VEGF-A & B, PlGF “receptor decoy” | Phase II- recurrent GBM | 30% response rate |
| Phase I- newly diagnosed | ongoing | ||
|
| |||
| IMC-3G3 | PDGFR α antibody | Phase I/II in recurrent GBM | ongoing |
|
| |||
| ramucirumab | VEGFR2 antibody | Phase I/II in recurrent GBM | ongoing |
|
| |||
| Small Molecule Inhibitors | |||
|
| |||
| cediranib | VEGFR-1/2/3, c-kit, PDGFR- α/β inhibitor, weak FGFR-1, EGFR inhibitor | Phase III- recurrent GBM | no improvement in OS compared to lomustine alone |
| Phase II- newly diagnosed GBM | ongoing | ||
|
| |||
| E7080 | VEGFR-2/3, FGFR1, PDGFR-β | Phase II- recurrent GBM | ongoing |
|
| |||
| sunitinib | VEGFR-2, PDGFR, c-kit inhibitor | Phase I- recurrent GBM | APF6 24% |
|
| |||
| cabozantinib | VEGFR-2, c-met, Tie-2, c-kit inhibitor | Phase II- recurrent GBM | ongoing |
|
| |||
| pazopanib | VEGFR-1/2/3, PDGFR-α/β, c-kit inhibitor | Phase I/II- recurrent GBM | APFS6 3% |
|
| |||
| AEE788 | VEGFR-1/2, EGFR inhibitor | Phase I/II- recurrent GBM | results pending |
|
| |||
| sorafenib | VEGFR-2/3, PDGFR-β, Flt-3, Raf inhibitor | Phase I/II- recurrent and newly diagnosed GBM | results pending |
|
| |||
| vandetanib | VEGFR-1/2, EGFR, Ret kinases inhibitor | Phase I/II- newly diagnosed GBM | study halted because failed to meet interim analysis goals |
Please see the National Cancer Institute website for up-to-date information on ongoing trials (www.clinicaltrials.org). VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor; PlGF: placental growth factor; EGFR: epidermal growth factor receptor; PDGFR: platelet derived growth factor receptor; FGFR: fibroblast growth factor receptor
Toxicities of Antiangiogenic Agents
Although generally well tolerated, antiangiogenic agents have several rare but serious complications (Table 2).36 Hypertension has been the most notable side effect because VEGF blocks nitric oxide and prostacyclin synthesis, impairs baroreceptor response, and perturbs endothelial cell function. As many as 60% of patients, particularly those with borderline hypertension, will require treatment with anti-hypertensive agents and early intervention is important to pre-empt development of severe hypertension. There is a low incidence (<5%) of intratumoral hemorrhage and only minimal increase in thromboemolic events. Poor wound healing because of angiogenic blockade is particularly concerning in patients who have recently undergone craniotomy for resection of their tumor. Bowel perforation, proteinuria, kidney damage and reversible posterior leukoencephalopathy have also been reported. Injury to the kidney may also influence the development of hypertension. For specific small molecule inhibitors, the toxicity profile varies depending on the agent but fatigue and diarrhea are especially common.
Table 2.
Possible toxicities of antiangiogenic agents
| Toxicity | Possible Mechanism |
|---|---|
| Hypertension | blockade of NO and prostacyclins, decreased capillary density, impaired baroreceptor response, kidney damage |
| Thrombotic events | EC apoptosis, platelet activation |
| Bleeding, Impaired wound healing | platelet dysfunction |
| Proteinuria | podocyte dysfunction in kidney glomeruli |
| Hand-foot syndrome | epidermal cell apoptosis, EC dysfunction |
| GI perforation | mucosal cell apoptosis, EC dysfunction |
| Hypothyroidism | decreased thyroid vascularity |
| Fatigue | hypothyroidism- unclear |
| PRES | BBB dysregulation, hypertension |
EC- endothelial cell; NO- nitric oxide; GI- gastrointestinal; PRES- posterior reversible encephalopathy syndrome
Antiangiogenic Agents for the Treatment of Cerebral Edema
Vasogenic edema is a common feature of malignant gliomas and obligates patients to chronic corticosteroid use. Unfortunately, this usually leads to steroid-related toxicities including osteoporosis, weight gain, insomnia, infection, and psychiatric effects- all of which impair the quality of life for these patients. Vasogenic edema is the result of disruption of the BBB and increased vascular permeability from over-secretion of VEGF. By blocking VEGF, anti-VEGF agents restore the integrity of the BBB and decrease vasogenic edema. Vatalanib, cediranib, and bevacizumab have all been observed to decrease vasogenic edema as measured by serial MRI scans and patients have been able to decrease or stop steroids completely.31, 37–40
The anti-permeability effect of anti-VEGF therapies may also be beneficial for radiation necrosis. Radiation necrosis occurs several months to years after therapeutic doses of radiation and is thought to be mediated by endothelial cell damage and the release of VEGF.41–42 Patients with cerebral radiation necrosis develop progressive vasogenic cerebral edema and typically require long-term corticosteroid use to control swelling. Several small studies have suggested that bevacizumab can ameliorate radiation necrosis based on improvement in MRI scans (decreased T2 signal and contrast enhancement) and decreased requirement for corticosteroids.40, 43–44
Resistance to Antiangiogenic Agents
Although antiangiogenic agents have been associated with prolonged progression-free and overall survival, most if not all GBM patients treated with these drugs eventually relapse. Additionally, there appears to be a subset of patients who do not respond to bevacizumab. These patients may have a tumor that is not dependent on VEGF for growth and have an intrinsic resistance to antiangiogenic therapy. For patients who initially responded, tumors may develop evasive/adaptive resistance by upregulation of alternate pro-angiogenic pathways, improved protection of tumor neovasculature by increased pericyte coverage, and increased invasiveness of tumor cells that co-opt native brain blood vessels.45 Hypoxic tumors can recruit vascular progenitor cells and pro-angiogenic monocytes from the bone marrow that differentiate into endothelial and pericyte progenitors.46 Support for these mechanisms has predominantly come from pre-clinical models and there are increasing efforts to identify tumor escape mechanisms in humans using blood or MRI biomarkers. For example, elevated stromal derived growth factor-1α (SDF1α), bFGF, PDGF, DLL-4/notch, and Tie-2 have been implicated in the upregulation of alternate pro-angiogenic signaling pathways.31, 47 Genetic alteration in the VEGF molecule itself or its receptor, a commonly cited mechanism for acquired drug resistance in traditional chemotherapeutics, has not been clearly demonstrated.
Determining Glioblastoma Response to Antiangiogenic Agents
An increasing concern with antiangiogenic therapies has been how to assess tumor radiographic response and tumor progression. Reduction in contrast enhancement on post-contrast T1-weighted MRI images has been the conventional methods to determine GBM response to treatment. GBMs enhance after the administration of intravenous contrast agents because the tumor vasculature has a dysfunctional, permeable BBB that enables leakage of contrast from the intravascular space into the brain parenchyma. Decreasing contrast enhancement with treatment has been interpreted as a decrease in tumor burden. However, in the setting of antiangiogenic agents, the association between reduction in contrast enhancement and decreased tumor burden is less clear. Antiangiogenic agents restore vascular integrity, which results in a reduction in BBB permeability and contrast leakage independent of any effect on the underlying tumor. If only change in contrast enhancement determines tumor status, clinicians will not accurately measure tumor response since the tumor may still be growing behind the now intact BBB. Thus, alternative methods to measure tumor response and progression need to be developed for antiangiogenic drugs. Diffusion imaging has been one such tool that may prove helpful.48–49 Preclinical models and small human studies have suggested that blocking angiogenesis may lead to the tumor co-opting native brain blood vessels as an alternate way of maintaining an adequate blood supply.50–52 Histological sections from gliomas in rats treated with a VEGF murine antibody demonstrated tumor cells infiltrating into normal surrounding brain and tracking along native brain blood vessels. These blood vessels maintain an intact BBB so the perivascular tumor will not be visible on contrast-enhanced MRI studies. There is a concern that observed changes on fluid attenuation inversion recovery (FLAIR) sequences or diffusion imaging represent tumor growth without an associated increase in contrast enhancement.48 Therefore, new radiographic criteria have been proposed to assess glioma response in the setting of antiangiogenic therapy.53 The new Response Assessment in Neuro-Oncology (RANO) criteria incorporate change in FLAIR hyperintensity as a metric for tumor progression. These criteria will need to be validated and refined but represent an important update to response assessment.
Conclusion
Tumor angiogenesis is a pathophysiological process characterized by redundant pro-angiogenic signaling pathways and multiple escape mechanisms. To date, results of antiangiogenic therapies for GBM have been modestly encouraging with some positive impact on progression-free survival in patients with GBM. Salvage therapies are needed and combination strategies will be necessary for a sustained anti-angiogenic effect. Novel agents such as microRNAs are also being explored to target angiogenesis but have not yet reached the clinic. Improved understanding of the mechanism of action of antiangiogenic therapies will be vital to optimize the use of these agents. Specifically, identification of the normalization window in GBM by non-invasive serial imaging may shed light on when to combine antiangiogenic drugs and cytotoxic therapies. In addition, antiangiogenic therapies may prove effective in the treatment of conditions associated with increased vascular permeability such as vasogenic cerebral edema related either to the tumor itself of radiation-induced inflammation and cerebral radiation necrosis.
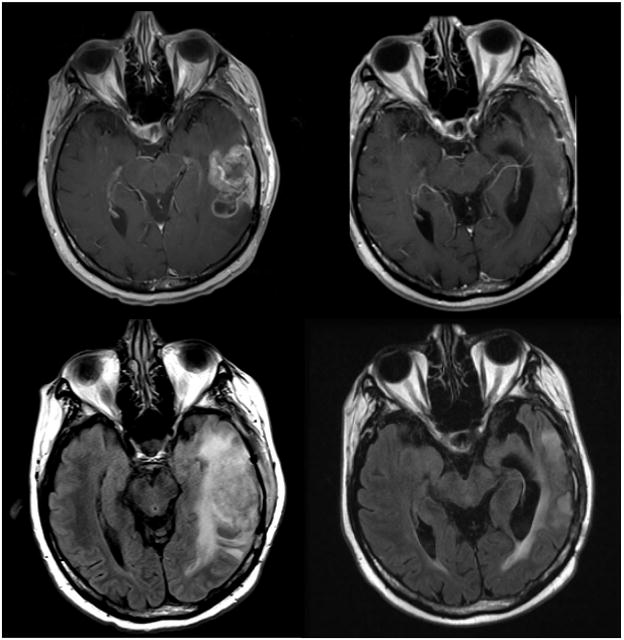
Figure 1.
MRI scan of a patient with a recurrent left temporal GBM. Top row: Post-contrast T1 MRI prior to treatment with bevacizumab and irinotecan (left) and 6 months later (right). Second row: Fluid attenuation inversion recovery (FLAIR) prior to therapy with bevacizumab and irinotecan (left) and 6 months later (right).
Footnotes
Conflicts of Interest and Source of Funding: DR. Batchelor has received honoraria from MERCK, ROCHE, SPECTRUM, MILLENNIUM. DR. BATCHELOR HAS RECEIVED GRANTS FROM PFIZER, ASTRAZENECA AND MILLENNIUM. For the remaining authors none were declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78:233–47. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- 3.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–8. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Manzano C, Fueyo J, Jiang H, et al. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann Neurol. 2003;53:109–17. doi: 10.1002/ana.10396. [DOI] [PubMed] [Google Scholar]
- 5.Yoshino Y, Aoyagi M, Tamaki M, Duan L, Morimoto T, Ohno K. Activation of p38 MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int J Oncol. 2006;29:981–7. [PubMed] [Google Scholar]
- 6.Saino M, Maruyama T, Sekiya T, Kayama T, Murakami Y. Inhibition of angiogenesis in human glioma cell lines by antisense RNA from the soluble guanylate cyclase genes, GUCY1A3 and GUCY1B3. Oncol Rep. 2004;12:47–52. [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82:864–73. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- 9.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryuto M, Ono M, Izumi H, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–8. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 11.Morrison RS, Gross JL, Herblin WF, et al. Basic fibroblast growth factor-like activity and receptors are expressed in a human glioma cell line. Cancer Res. 1990;50:2524–9. [PubMed] [Google Scholar]
- 12.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–7. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 15.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 18.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–43. [PubMed] [Google Scholar]
- 19.Kerber M, Reiss Y, Wickersheim A, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342–51. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 20.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 21.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 22.Kreisl TN, Kim L, Moore K, et al. Phase II Trial of Single-Agent Bevacizumab Followed by Bevacizumab Plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–8. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 24.Cloughesy TFPM, Wen PY, et al. A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) J Clin Oncol. 2008 May 20;26 suppl; abstr 2010b. [Google Scholar]
- 25.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–80. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 26.Sathornsumetee S, Vredenburgh J, Rich J, et al. Phase II study of bevacizumab and erlotinib in patients with recurrent glioblastoma multiforme. J Clin Oncol. 2008 May 20;26 suppl; abstr 13008. [Google Scholar]
- 27.Rich J, Desjardins A, Sathornsumetee S, et al. Phase II study of bevacizumab and etoposide in patients with recurrent malignant glioma. J Clin Oncol. 2008;26 (May 20 suppl; abstr 2022) [Google Scholar]
- 28.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Groot J, Wen P, Lamborn K, et al. Phase II single arm trial of aflibercept in patients with recurrent temozolomide-resistant glioblastoma: NABTC 0601. J Clin Oncol. 2008;26 (May 20 suppl; abstr 2020) [Google Scholar]
- 30.Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–37. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nabors L, Rosenfeld M, Chamberlain M, et al. A phase I trial of sorafenib (BAY 43–9006) for patients with recurrent or progressive malignant glioma (NABTT 0401). ASCO Annual Meeting Proceedings Part I; 2007. p. 2058. (June 20 Supplement) [Google Scholar]
- 33.Sandstrom M, Johansson M, Bergstrom P, Bergenheim AT, Henriksson R. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol. 2008;88:1–9. doi: 10.1007/s11060-008-9527-3. [DOI] [PubMed] [Google Scholar]
- 34.Reardon D, Cloughesy T, Conrad C, et al. A phase I study of AEE788, a novel multitargeted inhibitor of ErbB and VEGF receptor family tyrosine kinases, in recurrent GBM patients. ASCO Annual Meeting Proceedings; 2005. p. Abst 3063. [Google Scholar]
- 35.Quant EC, Batchelor T, Lassman AB, Schiff DTJK, Wong E, Mikkelsen T, Drappatz J, Norden AD, Beroukhim R, Weiss SE, Alexander BM, Sceppa C, Gerard M, Hallisey SD, Bochacki CA, Smith KH, Muzikansky A, Wen PY. Preliminary results from a multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma (GBM) J Clin Oncol. 2011;29 doi: 10.1158/1078-0432.CCR-14-3220. (suppl; abstr 2069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475–85. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 37.Conrad C, Friedman H, Reardon D, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). ASCO Annual Meeting Proceedings; 2004. p. Abstr 1512. [Google Scholar]
- 38.Drevs J, Muller-Driver R, Wittig C, et al. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–22. [PubMed] [Google Scholar]
- 39.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–60. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–6. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Chung YG, Kim CY, Kim HK, Lee HK. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19:879–86. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nonoguchi N, Miyatake SI, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 2011 doi: 10.1007/s11060-011-0610-9. [DOI] [PubMed] [Google Scholar]
- 43.Sanborn MR, Danish SF, Rosenfeld MR, O’Rourke D, Lee JY. Treatment of steroid refractory, Gamma Knife related radiation necrosis with bevacizumab: Case report and review of the literature. Clin Neurol Neurosurg. 2011;113:798–802. doi: 10.1016/j.clineuro.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–95. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.di Tomaso E, Snuderl M, Kamoun WS, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerstner ER, Chen PJ, Wen PY, Jain RK, Batchelor TT, Sorensen G. Infiltrative patterns of glioblastoma spread detected via diffusion MRI after treatment with cediranib. Neuro Oncol. 2010;12:466–72. doi: 10.1093/neuonc/nop051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–9. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 50.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–14. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–8. [PubMed] [Google Scholar]
- 52.Gomez-Manzano C, Holash J, Fueyo J, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–5. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]