Abstract
Glioblastoma remains one of the deadliest forms of cancer. Infiltrating cancer cells in the surrounding brain prevent complete resection and tumor cell resistance to chemoradiation results in the poor prognosis of the glioblastoma patient. Much research has been devoted over the years to the pathogenesis and treatment of glioblastoma. The tumor stem cell hypothesis, which was initially described in hematopoietic cell malignancies, may explain the resistance of these tumors to conventional therapies. In this model, a certain subset of tumor cells, with characteristics similar to normal neural stem cells, is capable of producing the variety of cell types, which constitute the bulk of a tumor. As these tumor cells have properties distinct from those constituting the bulk of the tumor, a different approach may be required to eradicate these residual infiltrating cells from the brain. Here we outline the history behind the theory of glioblastoma cancer stem-like cells, as they are now referred to. We will also discuss the implications of their existence on commonly held beliefs about glioblastoma pathogenesis and how they might influence future treatment strategies.
Keywords: Glioblastoma, stem cells, gliomagenesis, brain tumors, GBM, cancer stem cells, CNS tumors
Glioblastoma (GBM) is the most common primary malignancy of the brain, as well as its most malignant, and is classified as Grade IV on the World Health Organization (WHO) scale [1]. Despite years of advances in therapy, the median survival after radiation and chemotherapy ranges from 12 to 15 months [2]. While recent advances in therapeutics for GBM have shown some limited promise [3–5], these tumors inevitably recur in the majority of patients and are nearly uniformly fatal. Despite surgery, infiltrating cancer cells that reside away from the main tumor mass are thought to be responsible for tumor recurrence as well as radiation and chemotherapy resistance [6, 7]. These residual infiltrating GBM cells were initially felt to be biologically identical to the cells in the main tumor mass; however, the tumor stem cell hypothesis now challenges prior GBM dogma. Clearly, there exists a strong need to understand this mechanism of GBM recurrence and resistance to common treatment modalities.
Recent research efforts hypothesize that much of the therapy resistance and ability to regenerate when the bulk of the tumor mass has been treated may rest within a small population of cells, known as cancer stem-like cells (CSCs) [8, 9]. Much of our knowledge of the existence of such cells arises from knowledge developed in the discovery of and understanding of hematopoietic cancer stem cells [10]. Using lessons from the hierarchical organization of the proliferative cells within the bone marrow of mice and humans, a model of hematopoiesis emerged which relied on a small population of self-renewing hematopoietic stem cells (HSCs). These multi-potent cells were able to give rise to progressively more lineage restricted, partially differentiated progenitors with reduced self-renewal capacity but increased proliferative activity, giving rise to mature blood cells. This led to a search for a multi-potent cell, which would serve as the origin of hematopoietic cancers, and the first “cancer stem cells” were thus identified [11, 12].
Recent studies have provided supporting evidence for the existence of CSCs in glioblastoma [13]. In addition to their tumor regenerating capacity, these cells have also been shown to be chemoresistant [14, 15] and radioresistant [16, 17]. Consequently, much of the ongoing GBM research is centered on better understanding how these cells contribute to the genesis of these tumors, recurrence, and how they might be specifically targeted.
Definition and source of neural stem cells and neural progenitor cells
The search for glioblastoma cells with stem-like properties built upon the knowledge gleaned from the study of neural stem cells (NSCs). NSCs are self-renewing multipotent cells with astrocytic features [18] that can generate most differentiated tissue components of the brain [19]. These cells were first discovered in the subventricular zone (SVZ) in mice [20]. When cultured in serum-free media supplemented with defined growth factors, epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), NSCs grow in suspended cell aggregates called neurospheres, and self-renew. Upon exposure to differentiation signals such as serum, they can generate all of the different cell types within the adult brain. NSCs have been demonstrated in the human dentate gyrus [21], subcortical white matter [22] and the subventricular white matter [18, 23], which is the largest of these regions. Estimates number these cells as 0.77% of those within the SVZ, using Ki67 staining to identify mitotically active cells [23].
While it has been shown in vitro that NSCs can directly generate differentiated cells in the brain, these cells can also indirectly give rise to neurons, astrocytes and oligodendrocytes by creating fast cycling transit-amplifying progenitor cells. These progenitor cells were first demonstrated in the spinal cord of mice [24, 25]. Progenitor cells maintain proliferative ability similar to that of their precursor NSCs, but are committed to produce offspring of a neuronal or glial lineage only. There may be as many as ten million of these cells distributed throughout the brain, providing an ample reservoir of immature cells, which may be capable of malignant transformation (Figure 1, map of NSC and progenitor cell locations) [26].
Figure 1.
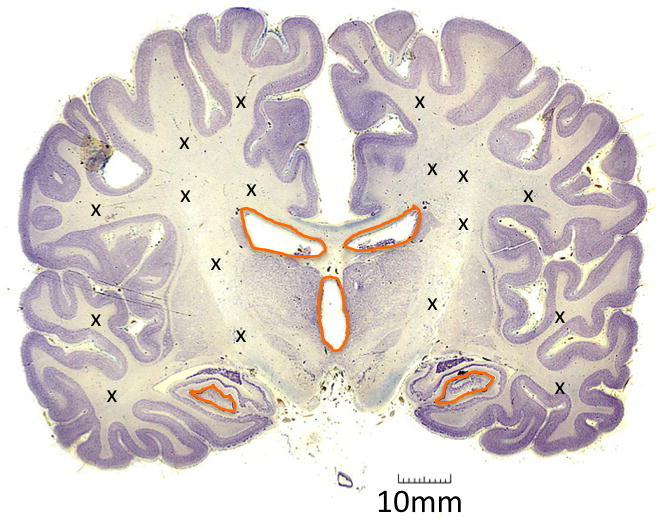
Locations of multipotent cells within the brain. Orange areas denote regions that contain NSCs. X’s show areas inhabited by glial progenitor cells (one group has also noted NSCs in these areas (22)) Coronal brain pathology slide provided by The Brain Biodiversity Bank at Michigan State University, with permission, funded by the National Science Foundation, https://www.msu.edu/~brains
Discovery and definition of GBM cancer stem-like cells
The discovery of multi-potent NSCs within the brain and their study has produced a better understanding of the possible cells of origin for GBM. It has also spurred a paradigm shift in the theories defining the mechanism for the generation and maintenance of the heterogeneous cell mass that constitutes a GBM. Since most GBM tumors occur late in life they are not considered developmental or congenital tumors; therefore, transformation of an otherwise normal adult cell must occur to create the first pathological cell or tumor-initiating cell (TIC). Conceptually, GBM could arise through dedifferentiation of mature brain cells into more primitive cells or more directly from less differentiated cells. NSCs represent a population of cells from which the heterogeneous and aberrant cell populations found within GBM could be generated [27]. Early experiments were able to determine that there were in fact cells within gliomas which exhibited characteristics of normal NSCs, e.g. the ability to self-renew and generate a variety of progeny [28]. To isolate and grow these cells from excised patient GBM tumors, serum free media with growth factors are required, creating proliferating cell suspensions known as neurospheres. Interest in these cells grew considerably when it was shown that they carried genetic aberrations and could generate orthotopic tumor xenografts upon implantation in the brains of mice. The tumors engendered displayed more phenotypic similarity to the patient tumors from which they were derived and could be initiated from as little as 100 implanted cells [29] (Figure 1). The tumor cell populations identified in this way were defined as CSCs as they were shown capable of proliferation, self-renewal, and differentiation into cells of various lineages, [30].
One of the current challenges in the study of CSCs in GBM, is to distinguish them from the rest of the tumor cell population. Current studies focus on defining specific markers that will facilitate their identification, quantification in tumors, and isolation for experimental characterization. Early studies used the marker CD133 or the “side-population” method to purify CSCs. CD133 (or prominin I) is a transmembrane protein, which has been used as a marker for NSC and CSCs [31]. Initial studies delineated clear differences between CD133 positive and negative glioma cells, including a dramatic difference in tumor-forming capacity in xenografts [32]. While these studies suggested that CD133 may be necessary for CSC designation, further studies have shown that CD133 negative stem-like cells may also be tumorigenic [33, 34]. More recent studies suggest a high level of plasticity within this stem-like cancer cell population, as CD133 negative cell populations can produce CD133 positive cells after generating tumors in mice [35].
The “side population” method of sorting hematopoietic stem cells is based upon staining with the Hoechst dye 33342, which generates fluorescence in a subset of murine bone marrow cells, and was found to predict the expression of hematopoetic stem cell markers on these cells. This method has been used to identify GBM CSCs [36, 37]; however, recent evidence suggests that using this process does not effectively identify CSCs [38]. As CSCs form a small fraction of the GBM tumor bulk, many groups use a process of enzymatic and mechanical dissociation of patient GBMs, with subsequent re-suspension in stem cell media that favors the growth of CSCs [30]. As these methods are refined, we should expect that we will continue to learn even more about this population of CSCs and their origins.
GBM cancer stem-like cell niche
As much of GBM CSC research had its basis in NSC research, the location of a putative CSC “niche” was sought, in analogy to the subventricular zone where NSCs preferentially reside and self-perpetuate. Much research has been devoted to studying the local environment of NSCs, with the underlying premise being that the microenvironment plays holds the key to understanding how these cells retain their stemness, and there is definite evidence that heterotypic cell interactions with surrounding epithelial cells and associated factors play a significant part in their proliferation [39, 40]. Similarly, studies of GBM CSCs have shown they may be influenced by interaction with endothelium [41]. Co-culture experiments have shown that GBM CSCs preferentially associate with endothelial cells, proliferate more rapidly in the presence of secreted factors from endothelial cells, and more effectively produce orthotopic brain lesions when implanted with these cells [41]. One recent study examined the histopathological differences between single CD133+ tumor cells scattered in different locations within the tumor mass versus those located in clusters in these vascular niches [42]. They found that the CD133+ cells within these vascular niches expressed more stem cell markers as compared to those single cells that expressed CD133. Nitric oxide produced by endothelial cells seems to support the stemness of CSCs in a Notch dependent manner [43] (Figure 2, Vascular niche). However, a separate body of literature suggests preferential proliferation of GBM CSCs in hypoxic environments [44]. In these studies, the hypoxia inducible factor (HIF) pathway is examined as an explanation of the resilience of the cells in these areas, with hypoxia increasing the proportion of CD133 positive cells grown in a culture of primary GBM cell lines in a HIF-2a dependent manner and HIF-1a being preferentially expressed in areas of necrosis [45, 46]. It remains unclear whether these two niches might provide similar stemness signals, or whether different populations of CSCs are present in the vascular versus the hypoxic niches, with the two areas exerting differing influences on the cells. The latter supports the idea that there may be two different stem cell niches which serve different functions for the tumor mass [42]. These niches may in fact be complimentary, in the sense that it is known that VEGF, whose production is increased in hypoxic areas in a HIF-dependent manner [47], stimulates the production of aberrant glomeruloid vessels with hyperplastic endothelial cells which may support the vascular niche.
Figure 2.
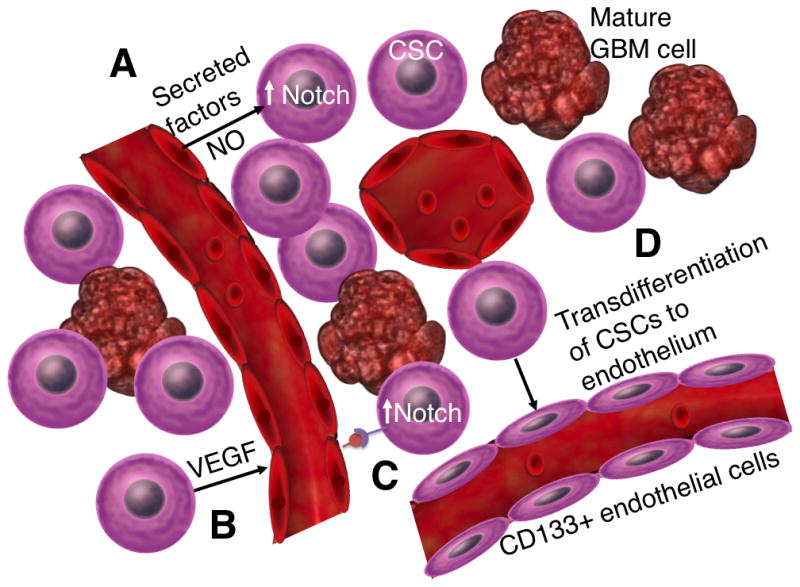
This diagram illustrates the various symbiotic relationships between CSCs and its vascular niche. A. Endothelial cells have been found to secrete Nitrous Oxide among other factors which support the stemness of CSCs in a Notch-dependent manner. B. VEGF secreted by CSCs stimulates angiogenesis C. Endothelial cell Notch ligands also support the stem cell qualities of CSCs. D. CSCs may differentiate into CD133 positive endothelial cells, further enriching the vascular niche.
Genesis of glioblastoma
The clonal origin of cancer postulates that tumors originate from the expansion of single cells having acquired sequential genetic and epigenetic alterations conferring them with the hallmarks of cancer, a series of phenotypic changes to normal cell physiology. The accumulation of multiple genetic mutations in a cell provide a survival advantage related to properties that include proliferation, evasion of growth suppression, and apoptosis [48]. Ongoing studies are focused on understanding what the inciting mutations might be in the genesis of glioblastoma, which are the cell(s) of origin and what the carcinogenic causes might be [49] (Figure 3, Cells of origin of GBM).
Figure 3.
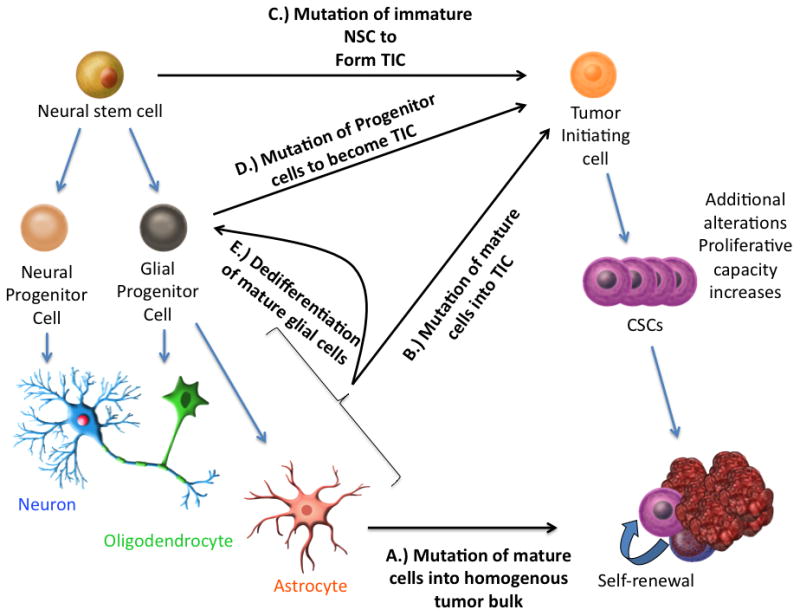
An overview of the various theories of the genesis of GBM. Initially, it was thought that mature glial cells mutated to form the various equivalent tumor cells of the GBM A.), any of which would be capable of regenerating the mass. More recent theories for inciting events include B.) the mutation of mature cells into the TIC, C. the mutation of immature stem cells into a multipotent tumor initiating cell, which then develops into CSCs, D.) the mutation of the numerous glial progenitor cells into the TIC or even E.) the dedifferentiation of mature glial cells into a less differentiated state (progenitor cells) which then transforms into a TIC
Under the stem cell hypothesis, the tumor-initiating cell (TIC) is the first genetically aberrant cell that can initiate the process of tumor development. The TIC may be responsible for the development of the bulk of tumor cells and over time can acquire sufficient alterations to engender a cancer stem-like cell (CSC). If we accept the tumor stem cell hypothesis, then there must be inciting events that generate these unique cells with significant expansion capacity and the ability to generate new tumors. To better understand the tumor initiating process, it is important to identify the cell(s) of origin for GBM subtypes and to define which are the early transformation steps that lead to the formation of TICs and their progression to CSCs (Figure 2). To address these important questions, two main approaches have been taken. First, it was hypothesized that comparison of gene expression signatures between CSCs and neural stem or progenitor cells in the brain might reveal similarities that may indicate cell lineage. A microarray study, which subdivided GBM into prognostic subgroups, showed that tumors with worst prognosis expressed markers of NSCs [50] such as Y-box protein 1 (YB-1) which is essential for normal brain development. YB-1 is highly expressed in the SVZ, is present in both NSCs and CSCs, and silencing YB-1 suppresses neurosphere growth and promotes differentiation [51]. Second, functional studies focus on experimentally testing the transformation potential of different genetic alterations on different cell types. The presence of a precursor lesion in a WHO Grade II astrocytoma of a patient with a germ-line p53 mutation was demonstrated using a p53 transcriptional assay in yeast [52]. One of the earlier studies linking neural progenitor cells to gliomagenesis demonstrated that rat oligodendrocyte-type-2 astrocyte progenitor cells, when transformed by c-myc and H-ras, created tumors which closely approximated human GBM when injected into rats [53]. Inducing the loss of p16 and p19 tumor suppressors in mice and transducing constitutively active EGFR into their neural stem cells enables these cells to form GBM-resembling tumors when implanted in immunodeficient mice. Mature astrocytes with knockdown of p16 and p19 can also be dedifferentiated in serum free media with EGF, and these immature cells are also tumorigenic[54]. EGFR drives the acquisition of stem-cell like characteristics in these cells by inducing inhibitor of differentiation 3 (ID3)[55]. Another group was able to infuse PDGF into the subventricular zone of adult mice and induce what appeared to be early glioma formation with proliferation of the PDGFR-alpha positive NSCs located there [56]. Mice which lack p53 and have a conditional allele of the NF1 tumor suppressor gene that negatively regulates Ras signaling produce tumors with pathological features consistent with glioblastoma in regions of the brain containing NSCs [57]. We now have evidence that NSCs, glial progenitor cells, and mature astrocytes by dedifferentiation, could be the cell of origin of GBM.
New approaches to GBM treatment exploiting the CSC theory (Figure 4, Treatment strategies)
Figure 4.
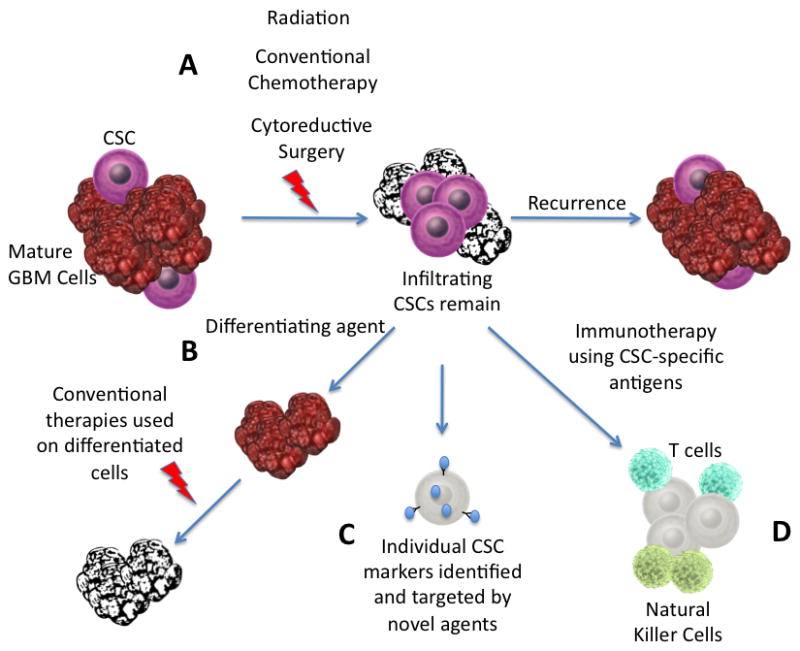
Therapies targeting CSCs. A. Conventional therapies target the mature GBM cells, leaving the multipotent, self-renewing CSCs within the brain to cause recurrence. B. Differentiating the CSCs may enable conventional therapies to be more effective. C. Developing therapies targeted toward unique CSC markers may prevent these cells from evading treatment. D. Immunotherapy targeted at CSC specific surface antigens can use the body’s innate defense mechanisms to kill these invasive cells
Current standard of care for GBM involves aggressive surgery, radiation and chemotherapy, yet provides only a modest survival benefit [2]. Most patients will eventually succumb to local recurrence of their tumor even if the patient undergoes a gross total resection. Making large inroads into the treatment of GBM will require new therapies designed to destroy the residual infiltrating tumor cells, which have the ability to reconstitute the entire initial tumor. Many in the neuro-oncology community believe the key to effective treatment of GBM will be directly targeting the CSCs within the tumor bulk which are resistant to standard therapies (Figure 3). Studies of neurosphere cultures and orthotopic xenograft tumors generated from these neurospheres have shown that these CSCs are radioresistant due to increased activation of DNA damage checkpoints [58, 59]. Enrichment of CD133+ cells was shown with radiation treatment, and inhibition of the Chk1 and Chk2 DNA checkpoint kinases removed the CD133+ survival benefit. In studying the HEDGEHOG-GLI1 signaling pathway, it was discovered that the most commonly used adjuvant chemotherapeutic agent, temozolomide does not block glioma CSC self-renewal [60], and this mirrors findings of multi-drug resistance in CSCs across tumor types [61]. Studies such as these highlight the need to develop new strategies, which target CSCs preferentially over therapies targeting the tumor bulk in order to prevent recurrence. To achieve this goal, a number of approaches are currently being tested.
Differentiation of GBM cancer stem-like cells (Figure 5, Differentiation pathways)
Figure 5.
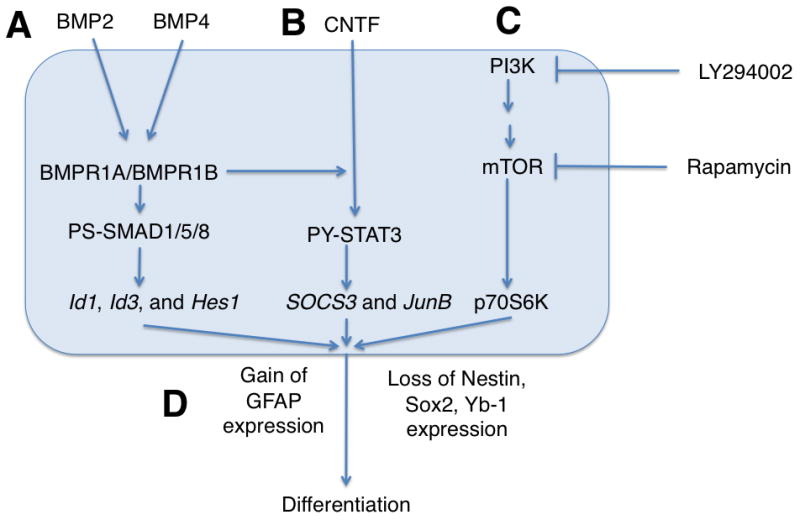
Differentiation pathways of CSCs. A. The Bone morphogenic protein pathway, through the activation of the various bone morphogenic protein receptors increases the activity of PS-SMAD1/5/8, leading to an increase in Id1, Id3 and Hes1 expression, ultimately leading to differentiation. B. The STAT3 pathway is similarly activated by CNTF in a manner mediated by BMPR1B, also leading to differentiation. C. Inhibition of the mTOR pathway, either by direct inhibition with Rapamycin or by inhibition of the upstream PI3K pathway leads to decreased phosphorylation of p70S6K, also driving differentiation. D. As a result of the above pathways, the CSC differentiates, losing Nestin, Sox2 and Yb-1 expression and gaining GFAP
Bone morphogenic proteins (BMPs) are a group of growth factors, which have been shown to play an integral role in the differentiation of normal NSCs [62, 63]. This has led to efforts to understand the differences between the activity of BMPs on normal NSCs and CSCs. One such study showed that defects in the BMP receptor 1B function impaired astroglial differentiation [64]. As differentiation can reduce the self-renewing capacity of the CSCs, many studies have investigated various ways to affect this BMP-mediated pathway to induce CSC differentiation as a therapeutic approach. BMP4 was shown to reduce the ability of GBM neurosphere-derived cells to form xenografts in mice and, furthermore, infusion of BMP4 was able to prevent GBM cell growth and increased survival [65]. As noted above, YB-1 is highly expressed in the subventricular zone of mouse fetal brain tissues, and silencing YB-1 expression reduced growth of neurospheres derived from GBM patients by triggering differentiation [51]. Rapamycin triggers differentiation in GBM CSCs through mTOR inhibition, thus increasing the radiosensitivity of the CSCs and reducing their ability to form tumors in murine brains[66]. Rapamycin cotreatment with a PI3K inhibitor was similarly found to promote differentiation and have a significant treatment effect [67]. In these ways and others, forcing differentiation of these CSCs may reduce their pathological potential.
GBM Stem-like cell Niche
Since the vascular niche may support the “stemness” of CSCs, many therapies aim to disrupt this relationship. Endothelial cells within the vascular niche express Notch ligands, which support the self-renewal capacity of CSCs. Inducing knockdown of these ligands from the endothelial cells decreases the propagation of CSCs in co-culture, so attacking these ligands could be a therapeutic option [68]. Bevacizumab, an anti-VEGF monoclonal antibody, has been shown to reduce aberrant blood vessel production in GBM and has been FDA-approved for the treatment of recurrent GBM [5]. Reducing the availability of supporting vessels could reduce the population of CSCs as well. While anti-angiogenic therapy has shown some promise, the success has been limited, and this may be due to the CSCs ability to create their own vascular niche. Recent studies have shown that many of the endothelial cells within GBM tumors contain the same genetic alterations as the malignant lesion, and orthotopic implantation of neurospheres in mice produced tumor xenografts with vessels composed of human endothelial cells [69]. It has been shown that treatment with bevacizumab or shRNA knockdown of VEGFR2 inhibits the maturation of tumor endothelial progenitors into vessels but does not prevent the differentiation of CD133+ cells into endothelial progenitors. However, inhibition of NOTCH1 signaling does prevent this vascular mimicry, which may lead the way toward future joint treatment [17].
Targeting Cancer Stem Cells
Ongoing attempts are underway to find signal transduction pathways that influence the tumorigenicity of CSCs that are unique to these cells, allowing treatments to be specifically targeted to these pathways [70]. IL-6, long believed to be implicated in multiple disease processes [71] and shown to be over-expressed in GBM [72] has led researchers to be interested in the signal transducer and activator of transcription 3 (STAT3), as this transcription factor can be activated by IL-6. STAT3 is constitutively active in some GBM cells, helping tumor cells resist apoptosis and promote local immunosuppression [9, 73]. The ubiquitous nature of STAT3 has made it a difficult target for therapeutics, but the bone marrow x-linked kinase (BMX), which associates with and activates STAT3, does show significantly increased expression in CSCs as compared to the bulk of GBM tumor cells. Furthermore, BMX shows minimal expression in normal tissues, making it an attractive therapeutic target [31].
Many groups are making use of nanotechnology to target CSCs. One group has used a virus-free, nanoparticle method of transfection to demonstrate significantly increased plasmid uptake in GBM CSCs as compared to normal fetal NSCs as a potential method of delivering therapeutic genes to these tumors [74]. Another group has treated GBM CSCs with iron oxide nanoparticles conjugated to an antibody against the glioblastoma specific target of EGFRvIII, promoting apoptosis in those cells [17], and increasing animal survival after convection-enhanced delivery (CED) treatment. Others have used curcumin nanoparticles to treat GBM CSC neurosphere cultures in vitro and demonstrated a reduction in clonogenicity and CD133+ cells [62].
Immunotherapy
Several groups are investigating both cell-based and antibody-mediated methods of treatment of GBM [75]. Given the tumor stem cell hypothesis, more investigators are turning these immune based therapies toward CSCs specifically. Recently, one group was able to show killing of CSCs by lectin-activated NK cells and furthermore showed sensitivity of these cells to antibody-mediated cytotoxicity using cetuximab [76]. Another group has demonstrated that SOX6 is an effective human glioma antigen, capable of inducing SOX6 peptide-specific cytotoxic T lymphocytes, which showed efficacy in killing GBM CSCs [77]. The difficulty in advancing this treatment strategy will be continuing to identify cell-surface antigens which are specific to CSCs. Given the similarities between NSCs and CSCs, any treatments must be rigorously tested for toxicity to NSCs before they can be widely used.
Conclusion
The pathogenesis of GBM and the role of CSCs remain a controversial area in neuro-oncology. Fundamental questions remain as defining what it is, precisely, that defines a CSC, and defining once and for all which normal cell(s) within the brain is its precursor, which may vary based on GBM subtype, and determining the specific nature of the insults that induce the transformation process. In order to further target these cells effectively, we will need to determine what separates these cells from normal neural stem/progenitor cells within the brain, so that we can treat these cells without causing undue toxicity. As optimization of the retrieval and culture of these cells continues to evolve [30], along with the reliable creation of phenotypically accurate GBM orthotopic xenografts using neurospheres, we can expect that our understanding of and ability to treat these cells will grow significantly. It is our hope that making significant strides in the treatment of this small population of cells may make the difference that greatly increases the rates of progression free survival and overall survival in GBM patients.
Acknowledgments
The authors would like to acknowledge The National Institutes of Health, The National Institute of Neurological Disorders and Stroke, The Dana Foundation and The Georgia Cancer Coalition for their support.
Contributor Information
Edjah Kweku-Ebura Nduom, Department of Neurosurgery, Emory University School of Medicine.
Costas George Hadjipanayis, Department of Neurosurgery, Emory University School of Medicine; Georgia Cancer Coalition Distinguished Scholar; Director, Emory Brain Tumor Nanotechnology Laboratory, Winship Cancer Institute of Emory University.
Erwin G. Van Meir, Departments of Neurosurgery and Hematology and Medical Oncology, Emory University School of Medicine; Leader, Winship Cancer Institute Cancer Cell Biology Program; Director, Emory Graduate Program in Cancer Biology; Director, Laboratory of Molecular Neuro-Oncology.
References
- 1.Brat DJ, Prayson RA, Ryken TC, et al. Diagnosis of malignant glioma: role of neuropathology. J Neurooncol. 2008;89(3):287–311. doi: 10.1007/s11060-008-9618-1. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91(16):1382–90. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–12. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 5.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 6.Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–74. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 7.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70(2):217–28. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 8.Hadjipanayis CG, Van Meir EG. Brain cancer propagating cells: biology, genetics and targeted therapies. Trends Mol Med. 2009;15(11):519–30. doi: 10.1016/j.molmed.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatoo A, Nanaszko MJ, Allen BB, et al. Understanding the role of tumor stem cells in glioblastoma multiforme: a review article. J Neurooncol. 2011;103(3):397–408. doi: 10.1007/s11060-010-0406-3. [DOI] [PubMed] [Google Scholar]
- 10.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 11.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197(4302):461–3. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 12.Bruce WR, Van Der Gaag H. A Quantitative Assay for the Number of Murine Lymphoma Cells Capable of Proliferation in Vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 14.Salmaggi A, Boiardi A, Gelati M, et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia. 2006;54(8):850–60. doi: 10.1002/glia.20414. [DOI] [PubMed] [Google Scholar]
- 15.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16(5):837–47. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 16.Facchino S, Abdouh M, Chatoo W, et al. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30(30):10096–111. doi: 10.1523/JNEUROSCI.1634-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–12. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2(4):287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12(11):4565–74. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 22.Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9(4):439–47. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 23.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 24.Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95(7):3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer-Proschel M, Kalyani AJ, Mujtaba T, et al. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19(4):773–85. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 26.Rhee W, Ray S, Yokoo H, et al. Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia. 2009;57(5):510–23. doi: 10.1002/glia.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 28.Ignatova TN, V, Kukekov G, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Gursel DB, Beyene RT, Hofstetter C, et al. Optimization of glioblastoma multiforme stem cell isolation, transfection, and transduction. J Neurooncol. 2011;104(2):509–22. doi: 10.1007/s11060-011-0528-2. [DOI] [PubMed] [Google Scholar]
- 31.Guryanova OA, Wu Q, Cheng L, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19(4):498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh SK, I, Clarke D, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- 33.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 34.Sakariassen PO, Prestegarden L, Wang J, et al. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci U S A. 2006;103(44):16466–71. doi: 10.1073/pnas.0607668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122(4):761–8. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 36.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 37.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broadley KW, Hunn MK, Farrand KJ, et al. Side population is not necessary or sufficient for a cancer stem cell phenotype in glioblastoma multiforme. Stem Cells. 2011;29(3):452–61. doi: 10.1002/stem.582. [DOI] [PubMed] [Google Scholar]
- 39.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9(3):331–9. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 41.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Christensen K, Schroder HD, Kristensen BW. CD133+ niches and single cells in glioblastoma have different phenotypes. J Neurooncol. 2011;104(1):129–43. doi: 10.1007/s11060-010-0488-y. [DOI] [PubMed] [Google Scholar]
- 43.Charles N, Ozawa T, Squatrito M, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141–52. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129(3):465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seidel S, Garvalov BK, Wirta V, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133(Pt 4):983–95. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 46.Pistollato F, Abbadi S, Rampazzo E, et al. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28(5):851–62. doi: 10.1002/stem.415. [DOI] [PubMed] [Google Scholar]
- 47.Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research. Am J Pathol. 2001;158(3):789–96. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 50.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Fotovati A, Abu-Ali S, Wang PS, et al. YB-1 bridges neural stem cells and brain tumor-initiating cells via its roles in differentiation and cell growth. Cancer Res. 2011;71(16):5569–78. doi: 10.1158/0008-5472.CAN-10-2805. [DOI] [PubMed] [Google Scholar]
- 52.Fulci G, Ishii N, Maurici D, et al. Initiation of human astrocytoma by clonal evolution of cells with progressive loss of p53 functions in a patient with a 283H TP53 germ-line mutation: evidence for a precursor lesion. Cancer Res. 2002;62(10):2897–905. [PubMed] [Google Scholar]
- 53.Barnett SC, Robertson L, Graham D, et al. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells transformed with c-myc and H-ras form high-grade glioma after stereotactic injection into the rat brain. Carcinogenesis. 1998;19(9):1529–37. doi: 10.1093/carcin/19.9.1529. [DOI] [PubMed] [Google Scholar]
- 54.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–77. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 55.Jin X, Yin J, Kim SH, et al. EGFR-AKT-Smad Signaling Promotes Formation of Glioma Stem-like Cells and Tumor Angiogenesis by ID3-Driven Cytokine Induction. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-1330. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Buylla A, Jackson EL, Garcia-Verdugo JM, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–30. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67(19):8980–4. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 59.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 60.Clement V, Sanchez P, de Tribolet N, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45(8):872–7. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 62.Eberhart CG, Lim KJ, Bisht S, et al. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biology & Therapy. 2011;11(5):464–473. doi: 10.4161/cbt.11.5.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehler MF, Mabie PC, Zhu G, et al. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22(1–2):74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Son MJ, Woolard K, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13(1):69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 66.Zhuang W, Li B, Long L, et al. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int J Cancer. 2011 doi: 10.1002/ijc.25975. [DOI] [PubMed] [Google Scholar]
- 67.Sunayama J, Sato A, Matsuda K, et al. Dual blocking of mTor and PI3K elicits a prodifferentiation effect on glioblastoma stem-like cells. Neuro Oncol. 2010;12(12):1205–19. doi: 10.1093/neuonc/noq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu T, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing Notch ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 70.Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano T, Akira S, Taga T, et al. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11(12):443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 72.Van Meir E, Sawamura Y, Diserens AC, et al. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990;50(20):6683–8. [PubMed] [Google Scholar]
- 73.Rahaman SO, Harbor PC, Chernova O, et al. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21(55):8404–13. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 74.Tzeng SY, Guerrero-Cazares H, Martinez EE, et al. Non-viral gene delivery nanoparticles based on poly(beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–10. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow KH, Gottschalk S. Cellular immunotherapy for high-grade glioma. Immunotherapy. 2011;3(3):423–34. doi: 10.2217/imt.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Avril T, Vauleon E, Hamlat A, et al. Human glioblastoma stem-like cells are more sensitive to allogeneic NK and T cell mediated killing compared to serum-cultured glioblastoma cells. Brain Pathol. 2011 doi: 10.1111/j.1750-3639.2011.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ueda R, Ohkusu-Tsukada K, Fusaki N, et al. Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. Int J Cancer. 2010;126(4):919–29. doi: 10.1002/ijc.24851. [DOI] [PubMed] [Google Scholar]


