Synopsis
Prenatal exposure to secondhand smoke (SHS) is responsible for adverse perinatal outcomes, including preterm birth. Smoking in the home is the primary source of exposure to women during pregnancy. Hair nicotine analysis of mothers and infants was used to describe the relationship between prenatal SHS exposure and number of household smokers. Maternal hair nicotine was strongly correlated with number of household smokers, and a more sensitive measure of household smoking than infant hair. Home smoking bans and focused public media campaigns on the harmful effects of SHS exposure are necessary prevention strategies to avoid adverse perinatal outcomes.
Keywords: Pregnancy, secondhand smoke, biomarker, preterm birth, nicotine
Introduction
Primary smoking during pregnancy places a woman at greater risk for preterm delivery, preterm premature rupture of membranes, and delivering a low birth weight (LBW) or small for gestational age (SGA) infant [1]. Although there is less evidence regarding the effect of secondhand smoke on perinatal morbidity; increase risk for decreased birthweight, smaller head circumference, stillbirth and preterm birth have been consistently reported [2-5].
Secondhand smoke is defined as smoke inhaled by an individual that is not actively engaged in smoking but who is exposed to ambient tobacco smoke [6]. SHS consists of two components: sidestream smoke and mainstream smoke. Sidestream smoke refers to the smoke emitted from tobacco products, while mainstream smoke refers to the smoke exhaled by smokers. SHS contains approximately 4,000 chemicals, is responsible for nearly 3,000 cases of lung cancer deaths among nonsmokers each year, and affects more than 22 million US children annually [7].
Biomarker validation is recommended to confirm smoking and SHS due to a high deception rate for self-report status caused by the social pressures attributed to smoking during pregnancy [8]. Whether a women smokes during pregnancy or is exposed to SHS, biomarkers of exposure can be detected in both the mother and infant [9-11]. Nicotine in maternal hair is a valid biomarker strongly associated with prenatal reports of SHS exposure [12].
Research examining the association between prenatal smoking, SHS exposure and number of household smokers is limited. The purpose the study is to examine the relationship between maternal and infant hair nicotine levels and number of household smokers in women who smoke and who are exposed to SHS during pregnancy. The study aims propose to: 1) examine the difference in maternal hair nicotine in smoking and SHS-exposed women in homes with one, or two or more additional smoker(s) in the home; 2) to examine the difference in infant hair nicotine in smoking and SHS-exposed women in homes with one, or two or more additional household smokers; and 3) describe the relationship between hair nicotine levels and preterm birth in homes with multiple smokers.
METHODS
Study Design and Population
A correlational, cross-sectional study design was used to determine the association between perinatal exposure to home SHS and hair nicotine levels. Prior to data collection, the study was approved the University of Kentucky Institutional Review Board, and all participants provided informed consent. Potential study participants were identified between December 2006 and April 2007 at the University Hospital. To be included in the study women had to be at least 18 years of age, singleton gestation, and have maternal scalp hair of at least 2 cm in length. Women were excluded if their pregnancy resulted in stillbirth, neonatal death, and/or delivery of an infant with severe congenital anomalies. Women with documented use any drugs of abuse during pregnancy were also excluded. In the four month data collection period, there were 656 births, with nearly 85% meeting eligibility requirements. Quota sampling was used to ensure a representative distribution of mothers who were smokers, nonsmokers/SHS exposed, and nonsmokers/nonexposed during pregnancy.
A total of 210 women were consented to participate within three days of their infant’s birth. Mothers were identified via the Labor and Birth daily census report and approached about participating while in the postpartum unit. After obtaining written consent, mothers were asked to complete a questionnaire. After collection of urine and hair samples by trained research assistants, participants were offered a choice of two incentives: payment of $25, or the equivalent of $25 in diapers and wipes.
Classification of smoking and SHS exposure
A woman was classified as a self-reported smoker if she responded “yes” to the question, “Have you smoked a cigarette, even a puff, in the past 7 days.” Mothers who smoked were asked to classify their daily smoking consumption over the last 30 days as: <1 cigarette, 1-5, 6-10, 11-15, 16-20, 21-25, 31-35, 36-40, and > 40. Classification of SHS exposure was based on self-report after confirmation of nonsmoking status. Nonsmokers were defined by urine cotinine ≤ 99 ng/mL and current smokers were defined by urine cotinine ≥ 100 ng/mL. Previous reports on classification of smoking status using urine cotinine have yielded sensitivity and specificity of 88% and 92%, respectively [13]. Number of home SHS exposure sources was defined by number of persons living in participant’s home (> one month) that smoked tobacco products. If the participant did not report any prenatal exposures to SHS in home, vehicle or work they were classified as nonsmoking, nonexposed. If a participant answered “yes” or quantified exposure (hours or days) to any of the smoking exposure questions, they were classified as nonsmoking/SHS exposed.
Collection of maternal and newborn hair
Maternal hair samples were collected by cutting a proximal segment of hair (approximately 20-25 strands) from the posterior vertex nearest the scalp and placed in a paper envelope. Collection of newborn hair involved cutting a pencil-width segment of hair behind the ear, placed in a paper envelope and stored until being mailed to Wellington Hospital, Wellington, New Zealand for analysis. Additional information regarding maternal and infant hair nicotine collection and analyses were previously reported [14].
Statistical Analysis
With an effective sample size of 210 mothers and an alpha level of .05, the power of Spearman rank correlation to detect a significant association as small as .2 was calculated to be at least 80%. Univariate analyses were used to summarize demographic and socioeconomic characteristics of the participants (see Table 1). Age and number of smokers in the home are presented as mean values. The distribution of hair nicotine levels was positively skewed, thus data were log transformed. Hair nicotine levels are presented as geometric means and 95% CIs. Data were analyzed using SAS version 9.3; an alpha level of .05 was used throughout.
Table 1.
Demographics and Smoking Classification (n = 210)
| Smoker | NonSmoker/Passive | NonSmoker/NonExposed | |
|---|---|---|---|
| Ethnicity/Race | n=53 (%) | n=66 (%) | n=91 (%) |
| White | 41 (19.5) | 33 (15.7) | 45 (21.4) |
| African American | 10 (4.8) | 14 (6.7) | 8 (3.8) |
| Hispanic | 0 | 18 (8.6) | 35 (16.7) |
| Marital Status | |||
| Single | 37 (17.7) | 40 (18.9) | 7 (13) |
| Married | 15 (7.2) | 26 (12.4) | 64 (30.6) |
| Highest Grade Completed | |||
| Less than High School | 22 (11.1) | 13 (6.2) | 23 (11.0) |
| High School/GED | 17 (8.1) | 25 (12) | 20 (9.6) |
| Some College and above | 14 (6.7) | 27 (12.9) | 48 (23) |
RESULTS
The character demographics of participants are displayed in Table 1. Mean age for the participants who smoked is 24.3 years compared to 24.8 year for those who were SHS exposed. Fifty three of the 210 participants were categorized as smokers and 157 as nonsmokers. Of the nonsmokers, 66 were classified as SHS exposed; all of which reported their primary exposure site as their home. Women who smoked during pregnancy had a mean of 2.4 additional smokers in the home, compared to 1.2 for women who were SHS-exposed.
Maternal and infant hair samples were collected in nearly all of the participants (99% mothers, 98% infants). There was no significant difference in demographics between mothers who smoked and those who were exposed to SHS with the exception of college education. Significant differences in maternal hair nicotine existed between SHS-exposed and women who smoked during pregnancy when comparing hair nicotine with one, or two or more smokers in the home (one smoker in home: 0.0942, 1.9279; and two or more smokers in the home: 0.1094, 1.0501, respectively). Women who smoked during pregnancy had nearly twice the exposure time (hours) compared to women were exposed to secondhand smoke (79.8 and 45, respectively). See Figures 1 and 2 for the associations between household smoking and mean maternal-infant hair nicotine levels.
Figure 1.
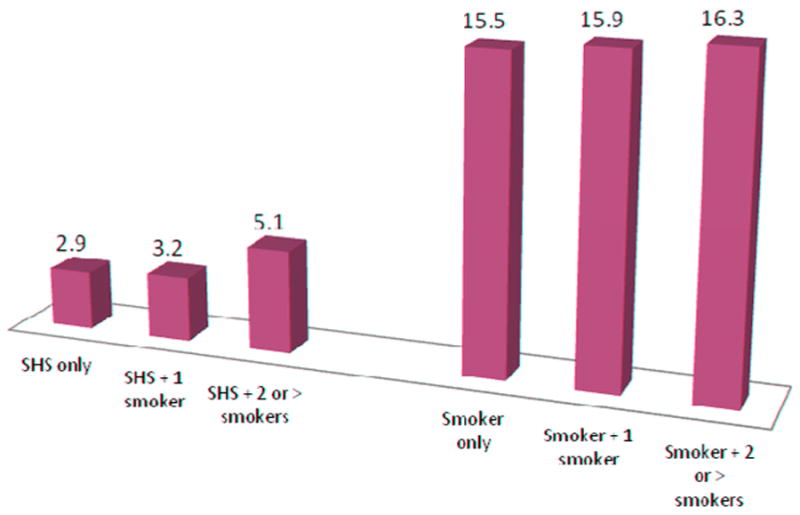
Mean Nicotine Levels (ng/ml) for Maternal Hair (n=201)
Figure 2.
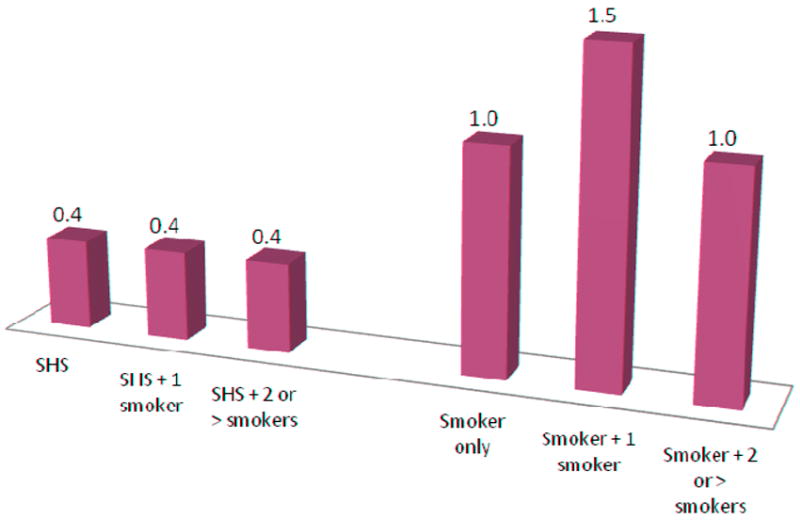
Mean Nicotine Levels (ng/ml) for Infant Hair (n=178)
Maternal hair significantly correlated with all measured smoking behaviors and had stronger association with smoking behavior than infant hair (Table 2). Infant hair demonstrated weaker but significant association in the reported smoking behaviors. Number of cigarettes smoked by other household members in the home ranged between 1- 40 cigarettes per day; with a mean of 12.5 cigarettes per day. Half of the mothers who smoked during pregnancy reported smoking 1 – 10; 28% reported smoking 11 to 20; and 22% reported smoking greater than 20 cigarettes per day. Total number of cigarettes smoked per day strongly correlated with both maternal and infant hair nicotine levels [14].
Table 2.
Spearman rank correlations among hair nicotine and smoking variables (p < .05)
| Self-reported Smoking Variables | Hair Nicotine | |
|---|---|---|
| Maternal hair | Infant hair | |
| Smoking status | .74 | .39 |
| Number of cigarettes per day | .68 | .45 |
| Total SHS exposure* | .68 | .28 |
| Two or more adults smoking in home | .61 | .23 |
Note: Total SHS exposure accounts for home, work and vehicle
Over two-thirds of the preterm infants were born from women who smoked (36%) or who were exposed to SHS (33%) during pregnancy. Infants whose mother lived with two or more smokers in the home were nearly twice as likely to be transferred to the neonatal intensive care unit (NICU) compared those with one smoker in the home. There were no significant differences in demographics in regard to NICU admission when comparing smoking to SHS exposed women.
Discussion
Maternal and infant hair nicotine levels increased as number of smokers in the home increased. Evidence continues to stress the strong association between secondhand smoke exposure and poor birth outcomes [5] [15], however measurement of number of household smokers is seldom reported and/or related with perinatal outcomes. Consistent with a previous report, this study demonstrated a significant association between maternal hair nicotine level and number of smokers in the home [16]. However, the association with infant hair nicotine and number of smokers in the home was weaker than the association with maternal hair.
Other perinatal exposure considerations include dose-response relationships and hair nicotine reference values. Dose response relationships between urine cotinine and home exposure in children and non-pregnant adults have been widely reported [17, 18]. Dose-response relationship between number of smokers in the home and perinatal outcomes is less clear. Fantuzzi et al. (2007) [16] recently noted the significant association between number of home smokers and early preterm birth (< 35 weeks gestation). Previous examination of prenatal segmental hair nicotine analysis reported proximal mean concentrations in three smoking behavior classifications: nonsmoking: 2.8 ng/mL; exposed: 4.3 ng/mL; and 11.08 ng/mL) [19]. Similar trends in the nicotine concentrations (ng/mL) of maternal hair segments were noted in our study (0.33, 1.02, and 9.6, respectively). Hair sampling size may account for lesser nicotine concentrations in the present study. In both studies, maternal hair nicotine (unlike infant hair) was able to significantly differentiate between the three smoking classifications. Also noted are recommended reference values for prenatal hair cotinine levels to distinguish active smokers from passive or unexposed is 0.2 ng/ml and 0.03 ng/ml to distinguish between those exposed and nonexposed ng/mg also exist [20].
During pregnancy and postpartum, a women’s primary prenatal SHS exposure locale is in her home, and primary exposure source is her spouse and/or significant other [21]. In this study, her significant other was most often defined as spouse, partner and/or infant’s father, followed by mother or mother-in-law. Home smoking restrictions or home smoking bans/policies can decrease and/or prolong relapse rates in pregnant and/or postpartum women [22] [21][23][24]. Conversely, homes that allow smoking in the home have higher prenatal smoking rates [25]. Having home smoking restrictions has also been reported to impact intention to quit smoking [26]. Although sustained smoking cessation provides the most benefits for decreasing perinatal exposure to SHS, consideration for a “reduction of smoke” could offer health benefits to the family [24]. Few studies evaluate the relationship between a maternal home smoking ban and perinatal outcomes. Yoo et al. (2010) [27]concluded that spouses’ who smoked outside the home did not reduce overall exposure to pregnant women compared to nonsmoking spouses.
Limitations in the present study exist and should be addressed. First, self report of smoking and SHS exposure during pregnancy can result in high deception rates; however only 3% of confirmed smokers via urine cotinine reported nonsmoking status. Second, all participants were recruited from a local hospital, which may limit generalizability. Third, sampling error cannot be calculated with quota sampling methods. Fourth, ethnic hair nicotine differences were not accounted for due to sample size and statistical limitations. In the present study, ethnicity would have greatly reduced group sizes when examining SHS and smoking behaviors in homes with one, or two or more smokers in the home. Finally, maternal and infant hair nicotine measurement was performed at one time point. Although, nicotine in hair is a very stable marker of long-term exposure, collection during each trimester would offer a more comprehensive and valid assessment regarding smoking and exposure status.
Strengths in the study include biochemical validation of smoking status of pregnant women; use of hair nicotine as a valid and long-term biomarker of secondhand smoke exposure; collection of both maternal and infant hair samples; and collection of several self-reported measures of home SHS exposure (hours per day, number of exposure sources, and number of household smokers).
Conclusions
Maternal hair is a more valid and sensitive measure of number of household smokers than infant hair. Pregnant women who smoke or who are exposed to SHS are more likely to experience a multitude of adverse perinatal outcomes than those who do not smoke and/or are not exposed. Furthermore, pregnant women living in households with two or more smokers are more likely to have their infant admitted to the NICU immediately after birth. Health care providers and clinical nurse leaders are strongly encouraged to educate all clients about the adverse perinatal effects of smoking and SHS exposure among pregnant women. Compromised infants face a multitude of acute and lifelong health issues; thus a significant percentage of adverse outcomes could be prevented by avoiding primary and secondary smoking during pregnancy. Prevention strategies including home smoking bans and focused public media campaigns on the harmful effects of SHS exposure are necessary to avoid the adverse perinatal outcomes associated with secondhand smoke.
Acknowledgments
FUNDING This study was funded by a University of Kentucky Faculty Research Grant and completed in part by a United States Public Health Service grant supporting the University of Kentucky General Clinical Research Center #M01RR02602. This publication was made possible by grant number K12DA14040 from the Office of Women’s Health Research and the National Institute on Drug Abuse at the National Institute of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services, P.H.S. Women and smoking: a report of the Surgeon General. Office of the Surgeon General; Washington, DC: 2001. [Google Scholar]
- 2.Ashford KB, et al. The Effects of Prenatal Secondhand Smoke Exposure on Preterm Birth and Neonatal Outcomes. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2010;39(5):525–535. doi: 10.1111/j.1552-6909.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegaard HK, et al. The effect of environmental tobacco smoke during pregnancy on birth weight. Acta Obstet Gynecol Scand. 2006;85(6):675–81. doi: 10.1080/00016340600607032. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease and Prevention and Promotion. Office of Smoking and Health; Atlanta, GA: 2006. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. [Google Scholar]
- 5.Salmasi G, et al. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(4):423–441. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 6.Klerman L. Protecting children: reducing their environmental tobacco smoke exposure. Nicotine Tob Res. 2004;6(Suppl 2):S239–53. doi: 10.1080/14622200410001669213. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. Involuntary exposure to tobacco smoke: a report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center on Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 8.Webb DA, et al. The discrepancy between self-reported smoking status and urine continine levels among women enrolled in prenatal care at four publicly funded clinical sites. J Public Health Manag Pract. 2003;9(4):322–5. doi: 10.1097/00124784-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Klein J, Blanchette P, Koren G. Assessing nicotine metabolism in pregnancy--a novel approach using hair analysis. Forensic Science International. 2004;145(2-3):191–4. doi: 10.1016/j.forsciint.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos C, et al. Hair concentrations of nicotine and cotinine in women and their newborn infants. JAMA. 1994;271(8):621–3. [PubMed] [Google Scholar]
- 11.Jacqz-Aigrain E, et al. Maternal smoking during pregnancy and nicotine and cotinine concentrations in maternal and neonatal hair. BJOG. 2002;109(8):909–11. doi: 10.1111/j.1471-0528.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen M, et al. Biomarkers of exposure to environmental tobacco smoke in infants. Biomarkers: Biochemical Indicators Of Exposure, Response, And Susceptibility To Chemicals. 2007;12(1):38–46. doi: 10.1080/13547500600943148. [DOI] [PubMed] [Google Scholar]
- 13.Bernert JT, et al. TECHNICAL NOTE: Use of Cotinine Immunoassay Test Strips for Preclassifying Urine Samples from Smokers and Nonsmokers Prior to Analysis by LCMSMS. Journal of Analytical Toxicology. 2005;29:814–818. doi: 10.1093/jat/29.8.814. [DOI] [PubMed] [Google Scholar]
- 14.Ashford KB, et al. Measuring prenatal secondhand smoke exposure in mother–baby couplets. Nicotine & Tobacco Research. 2010;12(2):127–135. doi: 10.1093/ntr/ntp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khader Y, et al. The Association Between Second Hand Smoke and Low Birth Weight and Preterm Delivery. Maternal and Child Health Journal. 2011;15(4):453–459. doi: 10.1007/s10995-010-0599-2. [DOI] [PubMed] [Google Scholar]
- 16.Fantuzzi G, et al. Preterm delivery and exposure to active and passive smoking during pregnancy: a case–control study from Italy. Paediatric & Perinatal Epidemiology. 2007;21(3):194–200. doi: 10.1111/j.1365-3016.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 17.Yvonne KY. Household Characteristics, Smoking Bans, and Passive Smoke Exposure in Young Children. Journal of Pediatric Health Care. 2006;20(2):98–105. doi: 10.1016/j.pedhc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56(1):66–71. doi: 10.1136/jech.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichini S, et al. Assessment of chronic exposure to cigarette smoke and its change during pregnancy by segmental analysis of maternal hair nicotine. Journal of Exposure Analysis & Environmental Epidemiology. 2003;13(2):144. doi: 10.1038/sj.jea.7500264. [DOI] [PubMed] [Google Scholar]
- 20.Florescu A, et al. Reference values for hair cotinine as a biomarker of active and passive smoking in women of reproductive age, pregnant women, children, and neonates: systematic review and meta-analysis. Ther Drug Monit. 2007;29(4):437–46. doi: 10.1097/FTD.0b013e318074df6e. [DOI] [PubMed] [Google Scholar]
- 21.Sockrider MM, et al. An exploratory study of control of smoking in the home to reduce infant exposure to environmental tobacco smoke. Nicotine Tob Res. 2003;5(6):901–10. doi: 10.1080/14622200310001615240. [DOI] [PubMed] [Google Scholar]
- 22.Yunsheng M, et al. Predictors of Smoking Cessation in Pregnancy and Maintenance Postpartum in Low-Income Women. Maternal & Child Health Journal. 2005;9(4):393–402. doi: 10.1007/s10995-005-0020-8. [DOI] [PubMed] [Google Scholar]
- 23.McBride CM, et al. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89(5):706–11. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Severson HH, et al. Reducing maternal smoking and relapse: long-term evaluation of a pediatric intervention. Prev Med. 1997;26(1):120–30. doi: 10.1006/pmed.1996.9983. [DOI] [PubMed] [Google Scholar]
- 25.Orr ST, et al. Factors associated with prenatal smoking among black women in eastern North Carolina. Maternal & Child Health Journal. 2005;9(3):245–252. doi: 10.1007/s10995-005-0010-x. [DOI] [PubMed] [Google Scholar]
- 26.Gilpin EA, et al. Home smoking restrictions: which smokers have them and how they are associated with smoking behavior. Nicotine Tob Res. 1999;1(2):153–62. doi: 10.1080/14622299050011261. [DOI] [PubMed] [Google Scholar]
- 27.Yoo S-H, et al. Hair nicotine levels in non-smoking pregnant women whose spouses smoke outside of the home. Tobacco Control. 2010;19(4):318–324. doi: 10.1136/tc.2009.033134. [DOI] [PubMed] [Google Scholar]


