Abstract
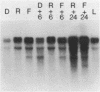
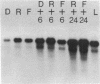
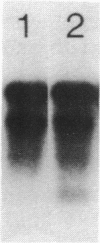
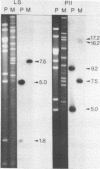
We have found major evolutionary changes in the types of transcripts produced by specific chloroplast genes, in particular those encoding the large subunit (LS) of ribulose-1,5-bisphosphate carboxylase and a photosystem II polypeptide (PII). Two distinct patterns of LS gene transcripts are revealed by hybridizing an LS gene probe to electrophoretically separated RNA from 19 angiosperms. Most species, including pea, contain the single transcript of approximately 1.6 kb previously observed in corn, spinach and mustard. However, in mung bean and other members of the legume genera Vigna and Phaseolus, the 1.6 kb transcript represents only a minor fraction of LS transcripts, and instead, two larger LS transcripts of approximately 2.4 and 2.6 kb predominate. The PII gene produces a single transcript in pea and most other species examined, while members of the related legume genera Vigna, Phaseolus and Glycine contain two additional transcripts which are smaller in size and probably represent specific RNA breakdown products. A single species, sweet pea (Lathyrus odoratus), contains a second PII transcript which is 0.2 kb larger than the approximately 1.2 kb transcript found in all species. The LS and PII genes map to the same 5 kb region in both pea and mung bean and are transcribed off the same DNA strand. In contrast, published studies indicate that the two genes are approximately 50 kb apart and are transcribed off opposite DNA strands in five other chloroplast genomes. These differences are probably the consequence of an approximately 50 kb inversion which distinguishes the pea and mung bean genomes from those of most other angiosperms (1).
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Coen D. M., Beaton A. R., Bogorad L., Rich A. Location of the single gene for the large subunit of ribulosebisphosphate carboxylase on the maize chloroplast chromosome. J Biol Chem. 1979 Feb 10;254(3):905–910. [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad L. Chloroplasts. J Cell Biol. 1981 Dec;91(3 Pt 2):256s–270s. doi: 10.1083/jcb.91.3.256s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Driesel A. J., Speirs J., Bohnert H. J. Spinach chloroplast mRNA for a 32 000 dalton polypeptide: size and localization on the physical map of the chloroplast DNA. Biochim Biophys Acta. 1980 Dec 11;610(2):297–310. doi: 10.1016/0005-2787(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Lemeshko V. V., Kaliman P. A., Nikitchenko Iu V. Perekisnoe okislenie lipidov v post "iadernoĩ i mikrosomal'noĩ fraktsiiakh gomogenatov pecheni krys pri starenii organizma. Biokhimiia. 1981 Apr;46(4):620–627. [PubMed] [Google Scholar]
- Link G. Cloning and mapping of the chloroplast DNA sequences for two messenger RNAs from mustard (Sinapis alba L.). Nucleic Acids Res. 1981 Aug 11;9(15):3681–3694. doi: 10.1093/nar/9.15.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Palmer J. D. Physical and gene mapping of chloroplast DNA from Atriplex triangularis and Cucumis sativa. Nucleic Acids Res. 1982 Mar 11;10(5):1593–1605. doi: 10.1093/nar/10.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Clone banks of the mung bean, pea and spinach chloroplast genomes. Gene. 1981 Oct;15(1):21–26. doi: 10.1016/0378-1119(81)90100-1. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Rearrangements in the chloroplast genomes of mung bean and pea. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5533–5537. doi: 10.1073/pnas.78.9.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozier C., Rocipon M., Mache R. Post-maturation of the plastid ribosomal RNA in the plant kingdom. J Mol Evol. 1979 Nov;13(4):271–279. doi: 10.1007/BF01731367. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G. L., Matthews H. M., Bingham S. E., Hallick R. B. The gene for the large subunit of ribulose-1,5-bisphosphate carboxylase in Euglena gracilis chloroplast DNA: location, polarity, cloning, and evidence for an intervening sequence. Nucleic Acids Res. 1982 Jun 11;10(11):3427–3444. doi: 10.1093/nar/10.11.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]