Abstract
We investigated the quantitative structure-activity relationships between hemolytic activity (log 1/H50) or in vivo mouse intraperitoneal (ip) LD50 using reported data for α,β-unsaturated carbonyl compounds such as (meth)acrylate monomers and their 13C-NMR β-carbon chemical shift (δ). The log 1/H50 value for methacrylates was linearly correlated with the δCβ value. That for (meth)acrylates was linearly correlated with log P, an index of lipophilicity. The ipLD50 for (meth)acrylates was linearly correlated with δCβ but not with log P. For (meth)acrylates, the δCβ value, which is dependent on the π-electron density on the β-carbon, was linearly correlated with PM3-based theoretical parameters (chemical hardness, η; electronegativity, χ; electrophilicity, ω), whereas log P was linearly correlated with heat of formation (HF). Also, the interaction between (meth)acrylates and DPPC liposomes in cell membrane molecular models was investigated using 1H-NMR spectroscopy and differential scanning calorimetry (DSC). The log 1/H50 value was related to the difference in chemical shift (ΔδHa) (Ha: H (trans) attached to the β-carbon) between the free monomer and the DPPC liposome-bound monomer. Monomer-induced DSC phase transition properties were related to HF for monomers. NMR chemical shifts may represent a valuable parameter for investigating the biological mechanisms of action of (meth)acrylates.
Keywords: (meth)acrylates, QSA(P)R, biological activity, NMR, chemical shift, β-carbon, theoretical parameters, DSC, liposomes
1. Introduction
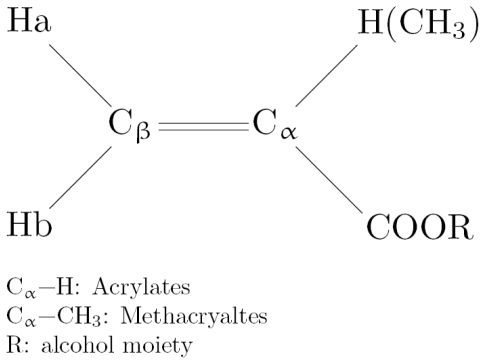
Acrylates and methacrylates, (meth)acrylates (Figure 1), are widely used in the formulation of polymeric materials for medical, dental and industrial applications.
Figure 1.
The structure of acrylates and methacrylates.
There have been many reports on the local and systemic toxicities of these monomers [1–5], whose volatility makes them a potential hazard in working environments [6]. In particular, methacrylates are widely used in resinous dental materials for dentures and cements, and also for restorations [7]. Methyl methacrylate (MMA) has been used as a bone cement for the fixation of prosthetic joints to adjacent bone [8]. Acrylates and methacrylates generally do not polymerize completely in air because oxygen acts as a biradical and suppresses polymerization. Residual unpolymerized free monomers on resin surfaces are released into bio-systems through direct contact or inhalation. Therefore, the potential toxicity of these monomers is a primary concern when developing new medical and dental materials. Dillingham et al. [3] and Tanii and Hashimoto [2] previously reported quantitative structure-activity relationships (QSARs) between the in vivo lethal dose (LD50) and the lipophilicity factor (log P) for acrylates and methacrylates based on the Hansch model [9]. The former researchers reported a good QSAR in terms of both log P and the molar refraction (MR), whereas the latter reported a good QSAR in terms of both log P and glutathione (GSH) reactivity. Dillingham et al. [3] also investigated the relationships between the hemolytic activity and ipLD50 of (meth)acrylates and found that the mechanism responsible for the toxicity of acrylates and methacrylates was membrane-mediated and relatively non-specific, and that in vivo biotransformation was not a significant factor for acute toxicity. However, the biotransformation of active acrylates has been reported to be causally linked to toxicity in vivo [10]. Freidig et al. [11] previously investigated the alkaline-catalyzed hydrolysis rate constants and GSH reactivity for (meth)acrylates and found that the hydrolysis rate constants and GSH reactivity of monomers are involved in their toxicity. Chan and O’Brien [5] investigated QSARs in terms of GSH reactivity, lowest unoccupied molecular orbital (LUMO), or the partial charge on the Cβ, Cα and carbonyl carbons for the experimentally determined hepatocyte IC50, and reported rat LD50 data for (meth)acrylates, demonstrating a correlation between in vitro or in vivo toxicity and physico-chemical parameters for separation of acrylates and methacrylates.
According to QSAR models used by the U.S. Environmental Protection Agency (EPA) for screening information data set (SIDS) endpoints, the chemical and physical properties of methacrylates, including their melting point, boiling point, vapor pressure, log P (Kow) and water solubility, are strongly independent variables, and Log P in the Hansch model has been employed successfully as an independent variable in QSAR equations [12]. Eroglu et al. have employed quantum-mechanical-based descriptors in quantitative structure-toxicity relationship (QSTR) equations for organic compounds using the AM1, PM3, and DFT levels of the theory [13].
To interpret the molecular mechanism responsible for the hemolytic activity of methacrylates, we previously investigated QSARs using the PM3-based theoretical parameters for monomers and found that the theoretical data were useful for estimating the mechanisms of hemolytic activity and toxicity [14,15]. Putz et al. have recently investigated QSARs for organic toxicants using chemical hardness (η) and electronegativity (χ) principles in addition to Hansch indices, and found that the mechanism responsible for the genotoxic carcinogenesis of toxicants can be interpreted using computational chemistry [16]. The highest occupied molecular orbital (HOMO) and LUMO for (meth)acrylates are evident at their α,β-unsaturated carbons [17]. The 13C-NMR chemical shift of the β-carbon (δCβ) of monomers is also quantitatively related to the π-electron density. The higher the π-electron density on the β-carbon, the higher the magnetic field where the NMR peak is observed; that is, as the π-electron density increases, the chemical shift value (δ) becomes smaller. Hence, it would be reasonable to correlate the magnitude of the chemical shift with the reactivity of acrylates and methacrylates [18]. Apart from log P, we have previously reported that the NMR chemical shifts of the α,β-unsaturated carbons for (meth)acrylates may be responsible for the toxicity resulting from reaction with tissue nucleophiles via Michael addition based on QSAR studies of published LD50 data in mice; there was a good correlation between the NMR chemical shifts of the β-carbon and the reactivity of reduced glutathione (GSH), and also between the GSH reactivity and LD50 [15,19]. However, in vitro-in vivo correlation studies have indicated that the 50% cytotoxic concentration (IC50) values for (meth)acrylates cannot be used reliably to predict LD50 values with a reasonable degree of precision [15].
Here, in the light of currently available data, we investigated whether the NMR chemical shifts (δHa and δCβ) for (meth)acrylates are useful as independent variables for QSAR studies of reported data for in vitro hemolytic activity (50% hemolytic concentration) and in vivo lethal toxicity (LD50 in mice) [3]. Also, to clarify the mechanism responsible for the hemolytic activity of (meth)acrylates, we used DPPC liposomes as cell membrane molecular models and investigated the interaction between DPPC liposomes and (meth)acrylates using 1H-NMR spectroscopy and DSC.
2. Results and Discussion
2.1. Hemolytic Activity
1H-NMR chemical shifts are influenced by the π-electron density of the attached carbon. Ha represents the proton trans to the substituent, and Hb the proton cis to that (Figure 1). There was a good correlation between δHa and δCβ for monomers [18]. The 1H- and 13C-NMR chemical shift data for nine (meth)acrylates taken from the literature [18] and their biological activity (hemolytic activity, in vivo toxicity), physicochemical parameters and theoretical parameters, also taken from the literature [1,3,15], are shown in Tables 1, 2 and 3, respectively. We investigated QSARs between 1/H50 and log P or NMR chemical shifts (δHa, δCβ). The QSAR for 9 monomers (MA, EA, nPA, nBA, IBA, MMA, EMA, nPMA, and nBMA) yielded good results for log P (r2 = 0.95) (QSAR 1), whereas there was no QSAR for δHa. Similarly, a good linear QSAR between log 1/H50 and heat of formation (HF) for the same data set was obtained (r2 = 0.86) (QSAR 2). Since there was a good QSPR between log P and HF for monomers (r2 = 0.86), the entropic and enthalpic factors could affect the overall log P value. Dillingham et al. [3] previously reported a good QSAR between the hemolytic activity and log P for (meth)acrylates, and also a relationship between log P and molar refraction (MR) (r2 = 0.70) or molecular volume (Vm) (r2 = 0.92). We investigated a relation between Vm and HF (Table 3) for 9 (meth)acrylates and it was found that the good linear relationship was obtained at r2 = 0.97; as HF increased, Vm declined. Putz et al. [16] described Hansch physico-chemical parameters as follows: (1) hydrophobicity (log P), corresponding to trans-cellular membrane diffusion and with translation motion of the molecules; (2) polarizability, accounts for the dipole perturbation and ionic interaction; and (3) optimal total energy (Etot), which contains steric information about the molecule’s 3D structure since it is given by the equilibrium conformation. We previously investigated a relationship between log P and van der Waals (VDW) area, dipole moment (μ) or HOMO energy for seven methacrylates monomers that were calculated using the PM3 method and it was found that two molecular parameters, VDW area and HOMO energy, particularly the former, contributed significantly to the variation of log P values [15]. Thus, log 1/H50 could be related to HF (QSAR 2).
Table 1.
Hemolytic activity, in vivo toxicity and NMR chemical shifts for (meth)acrylates.
| Compound | log 1/H50 (mole/L) a | 7-days ipLD50 (mole/106 g) a | δHa (ppm) b | δCβ (ppm) b |
|---|---|---|---|---|
| Methyl acrylate (MA) | 0.63 | 2.95 | 5.82 | 130.56 |
| Ethyl acrylate (EA) | 0.95 | 5.98 | 5.807 | 130.24 |
| n-Propyl acrylate (nPA) | 1.59 | 5.80 | 5.809 | 130.22 |
| n-Butyl acrylate (nBA) | 2.61 | 6.64 | 5.805 | 130.21 |
| Isobutyl acrylate (IBA) | 2.84 | 5.92 | 5.813 | 130.23 |
| Methyl methacrylate (MMA) | 1.05 | 10.88 | 5.555 | 125.23 |
| Ethyl methacrylate (EMA) | 1.44 | 7.89 | 5.541 | 124.97 |
| n-Propyl methacrylate (nPMA) | 2.17 | 11.63 | 5.54 | 124.95 |
| n-Butyl methacrylate (nBMA) | 3.42 | 10.47 | 5.532 | 124.70 |
Table 2.
Physico-chemical parameters.
| Com. a | Log Pb | MR b | Vm (cm2/mole) b | Qσ (C) c | Rate constant (kapp) (liter mol−1min−1) d |
|---|---|---|---|---|---|
| MA | 0.625 | 21.85 | 49.02 | 0.1666 | 52.0 |
| EA | 1.165 | 26.03 | 59.25 | 0.1662 | 26.6 |
| nPA | 1.705 | 26.5 | 69.47 | -- | -- |
| nBA | 2.245 | 31.15 | 79.70 | 0.1662 | 38.7 |
| IBA | 2.245 | 31.15 | 79.70 | 0.1662 | -- |
| MMA | 0.945 | 27.5 | 59.25 | 0.1638 | 0.325 |
| EMA | 1.485 | 31.68 | 69.48 | 0.1634 | 0.139 |
| nPMA | 2.025 | 32.15 | 79.70 | -- | -- |
| nBMA | 2.565 | 36.8 | 89.94 | 0.1634 | No appreciable rate |
Table 3.
Theoretical parameters.
| Comp. | Heat of formation (HF) kcal/mol | EHOMO eV | η eV | χ eV | ω eV |
|---|---|---|---|---|---|
| MA | −67.387 | −11.066 | 5.492 | 5.574 | 2.829 |
| EA | −72.173 | −11.040 | 5.495 | 5.546 | 2.799 |
| nPA | −77.404 | −11.044 | 5.495 | 5.550 | 2.803 |
| nBA | −82.791 | −11.045 | 5.495 | 5.550 | 2.803 |
| IBA | −82.435 | −11.042 | 5.495 | 5.548 | 2.801 |
| MMA | −74.768 | −10.548 | 5.245 | 5.303 | 2.681 |
| EMA | −79.542 | −10.524 | 5.249 | 5.278 | 2.654 |
| nPMA | −84.767 | −10.529 | 5.248 | 5.281 | 2.657 |
| nBMA | −90.156 | −10.530 | 5.248 | 5.282 | 2.658 |
On the other hand, a linear relationship between log 1/H50 and δCβ was obtained for separations of acrylates and methacrylates, particularly the latter (r2 = 0.87). For acrylates, there was a good linear QSAR between log 1/H50 and HF (r2 = 0.96) or log P (r2 = 0.95), but not δCβ (r2 = 0.46). Thus, the hemolytic mechanism may differ between acrylates and methacrylates.
Acrylates and methacrylates can be classified together as a single group of unspecifically reactive chemicals [21], or as two groups: the acrylates as electrophiles and the methacrylates as ester narcotics [22]. In general, QSARs for acrylates and methacrylates are based on the assumption that their monomers with the same functional group (e.g., R: the alcohol moiety in Figure 1) have the same mode of action. However, the classes of these chemicals may not be identical to each other because the metabolic activity or conjugation with GSH differs between acrylates and methacrylates; a difference in GSH reactivity (kGSH) has been observed between readily reactive acrylates and more slowly reactive methacrylates [5,11,20]. We previously investigated the relationship between kGSH values using reported data and δCβ values for 12 acrylates and methacrylates and it was found that a good relationship was obtained [19]. The unsaturated β-carbon atom in (meth)acrylate molecules is the most probably site of attack in the Michael addition [11]. As shown in QSPR 1, in this work there was a significant relationship between GSH rate constants and δCβ for (meth)acrylates. McCarthy et al. [20] investigated depression of the erythrocyte GSH by acrylates and methacrylates in vitro, and found that alkylation of the erythrocyte membrane may be result from interaction between erythrocytes and the reactive acrylates, MA and EA, and also that there may be a process that can reduce the effective intracellular acrylate concentration, consequently leading to a decrease of cellular GSH depression in the erythrocyte system. Koleva et al. [23] reported that α,β-unsaturated carbonyl compounds such as acrylates are common environmental pollutants that are able to interact with proteins, enzymes, and DNA through various mechanisms. A common mechanism of action (Michael-type addition) may not be responsible for the hemolytic activity of acrylates because there was no QSAR between log 1/H50 and δCβ for these monomers. Therefore we investigate the hemolytic mechanism of acrylates and methacrylates using DPPC liposomes as a model of erythrocyte membranes. The results are described in Section 2.3.
2.2. In Vivo Toxicity
Next, we investigated the QSARs between ipLD50 and δHa or δCβ for (meth)acrylates, and the results are shown in Table 4. A significant linear QSAR was obtained for δHa or δCβ (QSARs 3 and 4; in both cases, r2 = 0.78). An increase of δHa or δCβ enhanced the in vivo toxicity. We found good QSPRs between δCβ and the χ-, η- or ω-term (in three cases, r2 = 0.99). As expected, there were also good QSARs between ipLD50 and the χ-, η- or ω- term for methacrylates (QSARs 7, 8 and 9; r2 = 0.7 − 0.8). Furthermore, there was a parabolic, not linear, QSAR (QSAR 6) for log P for (meth)acrylates. This finding was similar to that reported previously [1]. The QSAR in terms of both δCβ and log P yielded a better result (QSAR 5, r2 = 0.94).
Table 4.
Quantitative structure-property relationship (QSPR) (A) and quantitative structure-activity relationship (QSAR) (B) for (meth)acrylates.
| (A) | |
| Equation (1) | |
| Equation (2) | |
| Equation (3) | |
| Equation (4) | |
| For (meth)acrylates: | |
| kapp = −941.33 (±9.43) + 7.53 (±1.64) δCβ (n = 5, r2 = 0.88, p < 0.05) | QSPR 1 |
| For MA, EA, MMA and EMA at 40 mM DPPC: | |
| ΔδHa = −0.320 (±0.012) − 0.005 (±0.001) HF (n = 4, r2 = 0.92, p < 0.05) | QSPR 2 |
| (B) | |
| For (meth)acrylates: | |
| Log 1/H50 = −0.44 (±0.24) − 0.36 (±0.12) log P (n = 9, r2 = 0.95, p < 0.001) | QSAR 1 |
| Log 1/H50 = −5.55 (±0.27) − 0.09 (±0.14) HF (n = 9, r2 = 0.86, p < 0.001) | QSAR 2 |
| ipLD50 = 123.0 (±1.5) − 0.9 (±0.2) δCβ (n = 9, r2 = 0.78, p < 0.01) | QSAR 3 |
| ipLD50 = 109.0 (±1.5) − 17.8 (±3.6) δHa (n = 9, r2 = 0.78, p < 0.01) | QSAR 4 |
| ipLD50 = 1.02 (±0.26) − 0.01 (±0.03) δCβ + 1.40(±0.14) log P (n = 9, r2 = 0.94, p < 0.001) | QSAR 5 |
| ipLD50 = −1.1 + 8.8 (±2.0) log P − 2.1 (±0.6) log P2 (n = 9, r2 = 0.78, p < 0.01) | QSAR 6 |
| ipLD50 = 270.2 (±16) − 1592.8 (±429.9) Qσ(C) (n = 7, r2 = 0.73, p < 0.05) | QSAR 7 |
| ipLD50 = 111.2 (±1.5) − 19.3 (±4.2) η (n = 9, r2 = 0.75, p < 0.01) | QSAR 8 |
| ipLD50 = 105.1 (±1.5) − 17.5 (±3.7) χ (n = 9, r2 = 0.75, p < 0.01) | QSAR 9 |
| ipLD50 = 98.5 (±1.4) − 33.1 (±6.6) ω (n = 9, r2 = 0.78, p < 0.01) | QSAR 10 |
| For MA, EA, MMA and EMA: | |
| Log 1/H50 = 0.57 (±0.13) + 117.55 (±27.48) ΔδHa (n = 4, r2 = 0.90, p < 0.05) | QSAR 11 |
Lawrence et al. [1] also previously reported a good QSAR between ipLD50 (mice) and the σ charge on the carbonyl carbon, Qσ(C) for (meth)acrylates using the Hansch model; Qσ(C) was associated with high toxicity [9]. They obtained Qσ(C), using the method of del Re [24] employing parameters that reproduce dipole moments. We also investigated the QSPR between δCβ and Qσ(C) for selected (meth)acrylates and obtained a good linear QSPR (r2 = 0.99). As shown for QSAR 7, the σ charge of monomer molecules was considered to play a role in the toxicity, since ester hydrolysis and nucleophilic attack are affected by the σ charge [1,11].
According to the QSARs 3 and 4, (meth)acrylates with a large δHa (δCβ) value should have potent toxicity. Talalay et al. [25] previously reported that MA, acrylonitrile and acrolein were more highly active inducers of QR (NAD(P)H: (quinone-acceptor) oxidoreductase) in Hepa 1clc7 cells in comparison to MMA. MMA and acrylamide are inactive intracellular inducers of QR. The relationship between QR reactivity, GSH reactivity or in vivo toxicity and the NMR chemical shifts of the β-carbon for these active acrylates are summarized in Table 5.
Table 5.
Concentration of double quinone reductase (QR) in Hepa 1clc7 cells, glutathione reactivity (kapp), in vivo oral or ipLD50 (mouse) and NMR chemical shifts for reactive acrylates.
| Name | Acrylate | Concentration of QR | kapp | Reported oral-LD50, (mg kg−1) | NMR chemical shift h |
|---|---|---|---|---|---|
| Structure | (mM) a | (M−1min−1) b | (ipLD50, mol kg−1) c | δHa(δCβ), ppm | |
| MA | CH2=CHCOOCH3 | 20 | 41.8 | 857 (5.5) d | 5.825(130.56) |
| MMA | CH2=C(CH3)COOCH3 | I | 16.8 | 5,197 (10.3) d | 5.555(125.23) |
| Acrolein | CH2=CHCHO | 130 | 94.6 | 40 (0.5) e | 6.495(137.57) |
| Acrylonitrile | CH2=CHC≡N | 50 | 91.4 | 27 (0.8) f | 6.083(137.14) |
| Acrylamide | CH2=CHCONH2 | I | 17.9 | 107 (8.4) g | 5.700(127.38) |
The δHa (δCβ) (ppm) declined in the order acrolein > acrylonitrile > MA > acrylamide > MMA. MA, acrylamide and acrolein, which are potent QR inducers, showed a large NMR chemical shift value of the β-carbon, compared to that of acrylamide; these compounds are known to be major intracellular reducing agents, and scavengers of reactive oxygen species (ROS) generated in various cellular processes [29]. Electrophilic xenobiotics such as vinyl monomers become conjugated to GSH and decrease its level within the cell [5,30]. When cellular GSH is exhausted, unscavenged ROS accumulate in cells, thus exerting toxic effects [31]. Ishikawa et al. [32] previously showed that MMA upregulates the expression of genes encoding phase II enzymes such as glutathione S-transferase and quinone oxidoreductase (NAD(P)H) in L929 cells. However, from the present result based on the NMR chemical shifts, it was assumed that such reactivity of MMA would be markedly lower than that of active acrylates such as acrolein, acrylonitrile and MA. Acrolein [33] and acrylonitrile [34] are well-known carcinogens. McCarthy et al. [20] reported that the carcinogenetic mechanism of acrylates may be related to alkylation of protein thiols involved in tumor promotion. Oxidative stress caused by these compounds, and the resulting oxidative damage, induce apoptosis and are involved in carcinogenesis. We predicted the GSH reactivity (kapp) under cell-free conditions, and found that kapp declined in the order acrolein > acrylonitrile > MA > acrylamide > MMA. This strong decrease of GSH in hepatocytes for acrolein and acrylonitrile has been reported previously [35], and is supported by the predicted kapp data for these monomers. Also, the predicted ipLD50 value declined in the order acrolein > acrylonitrile > MA > acrylamide > MMA. Both acrolein and acrylonitrile were most toxic, as supported by reported oral-LD50 data. The induction of QR activity and GSH reactivity for active acrylates was dependent on the NMR chemical shifts of the β-carbon. On the other hand, although acrylamide was an inactive QR inducer and its predicted toxicity was relatively low, it showed toxicity under experimental conditions (Table 5). Biotransformation of acrylamide is thought to occur through glutathione conjugation and decarboxylation, with the formation of toxic glycinamide [36].
Induction of phase II enzymes and elevation of the glutathione level are well known to be involved in the toxic and carcinogenic effects of electrophiles and reactive forms of oxygen.
2.3. Interaction between DPPC Liposomes and (Meth)Acrylates
2.3.1. NMR Chemical Shifts of Ha
Liposomes have been employed in model systems to study the interaction of lipid-soluble drugs and monomers with biological membranes [17,37–40]. Liposomes consist of lipid bilayers, and closely resemble the structure of biological membranes. Depending on their hydrophobicity, an exogenous hydrophobic compound will reside predominantly in liposomes and a hydrophilic one will be located in aqueous medium. NMR is one of the most powerful methods for studying not only the characterization of small unilamellar, large unilamellar and multilamellar liposomes [41,42] but also the interaction between (meth)acrylate monomers and liposomes as a model of cell membranes and transport phenomena across membranes [43,44]. We previously investigated the changes in 1H and 13C-NMR chemical shifts of methacrylates in DPPC liposomes. The chemical shift of Ha and β-carbon for methacrylates was shifted markedly to a higher field by their interaction with liposomes [38–40]. These findings may allow interpretation of the mechanism responsible for the hemolytic activity and cytotoxicity of monomers in vitro [39,40].
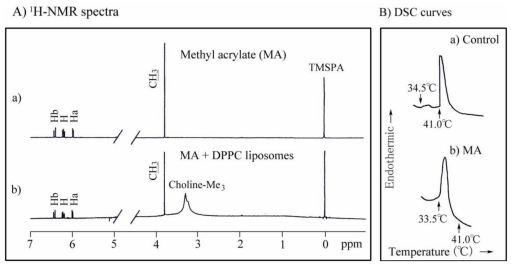
We investigated the interaction between unilamellar DPPC liposomes and MMA, EMA, EA or MA using 1H-NMR spectroscopy. As an example, 1H-NMR spectra of MA and DPPC liposome-bound MA are shown in Figure 2.
Figure 2.
1H-NMR spectra (A) of: (a) MA and (b) DPPC liposome-bound MA (molar ratio: DPPC:MA = 10:1) in D2O (pD 7.0 phosphate buffer) at 25 °C, and DSC curves (B) of: (a) DPPC liposomes (control) and b) MA-treated DPPC liposomes (molar ratio: MA:DPPC = 1:1). The NMR chemical shift of Ha, Hb, H (α-CH) and CH3 was derived from MA molecule. By contrast, that of choline-Me3, N-(CH3)3 was derived from DPPC molecule [39,40].
The difference in chemical shift (ΔδHa) between monomers and liposomal membrane-bound monomers was calculated. As a typical example, the shift for each proton of the MMA molecule at 25 and 50 °C is shown in Table 6.
Table 6.
The chemical shift difference (ΔδHa, ppm) between free MMA and DPPC liposome-bound MMA at 25 and 50 °C.
| MMA, structure and numbering | H attached to the carbon | ΔδHa, ppm | |
|---|---|---|---|
| 25 °C | 50 °C | ||
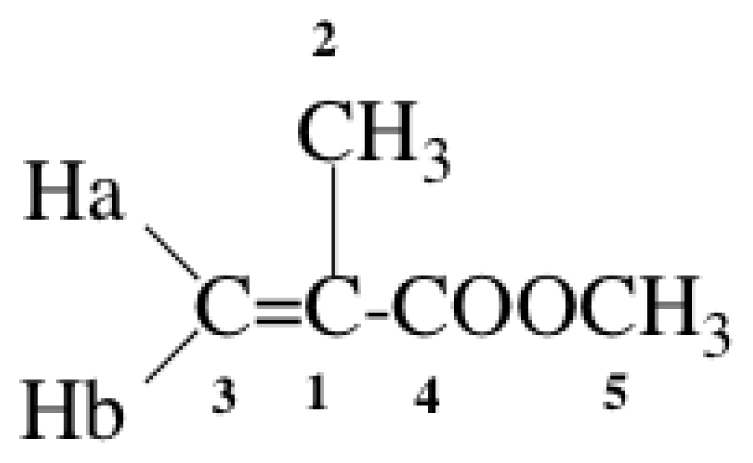 |
Ha | −0.01 | −0.05 |
| Hb | −0.005 | −0.01 | |
| 5H | −0.004 | 0.00 | |
| 2H | −0.001 | 0.03 | |
MMA, 4 mM; DPPC:MMA = 10:1 (molar ratio). The negative value for each proton in the MMA molecule exhibited a shift to a higher field, whereas the corresponding positive value exhibited a shift to a lower field.
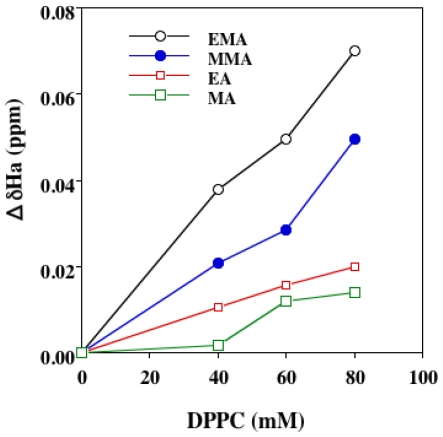
The 1H-NMR-chemical shift (ppm) of MMA in D2O (free monomer) for Ha, Hb, 2H and 5H was 5.72, 6.13, 1.94 and 3.79, respectively. That for Ha, Hb, 2H and 5H derived from membrane-bound monomers was determined by varying the DPPC liposomal concentrations. The ΔδHa for each proton in the MMA molecule at a DPPC:MMA molar ratio of 10:1 was investigated, and this revealed that the ΔδHa for Ha at 25 °C was about 10 times greater than that for the corresponding protons in the MMA molecule, the value being even higher at 50 °C. Since the DPPC concentration exceeds that of MMA monomers, Langmuir’s adsorption isotherm probably holds well. It was clear that the Ha attached to the β-carbon of monomers was highly impregnated into liposomes in a DPPC concentration-dependent manner. The relationship between ΔδHa for the monomers EMA, MMA, EA and MA and increasing DPPC concentration is shown in Figure 3. The decline in the ΔδHa value was dependent on DPPC concentration in the order EMA > MMA > EA > MA. The phase transition temperature (T) of DPPC liposomes was approximately 41 °C, and therefore the DPPC liposomes exist in a gel phase at 25 °C. We also examined the ΔδHa at 50 °C, at which DPPC liposomes exist in a liquid-crystalline phase. The ΔδHa at 50 °C declined in the order EMA > MMA > EA > MA, which was identical to that at 25 °C (data not shown). We also examined the QSAR between the ΔδHa at 40 mM DPPC and log 1/H50 for each monomer, and this revealed that the log 1/H50 value for monomers was linearly correlated with their ΔδHa (QSAR 11). This suggested that the hemolytic activity of monomers may be related to their membrane permeation activity.
Figure 3.
The chemical shift difference (ΔδHa, ppm) between free monomers and DPPC liposome-bound monomers as a function of DPPC concentration. The concentration of each monomer was 4 mM. The δ (ppm) values for Ha were determined with external TMSPA at 25 °C using 1H-NMR spectroscopy. The values represent the means of two or three separate experiments.
2.3.2. DSC Phase Transition Property
In earlier experiments, we examined changes in the phase transition properties of methacrylate-induced DPPC liposomes using DSC [38–40]. In this series, we investigated DSC changes in the phase transition temperature (T) (main transition peak), enthalpy (ΔH) and entropy (ΔS) of DPPC liposomes induced by (meth)acrylates. The results are shown in Table 7. The ΔS value was calculated according to ΔS = ΔH/T (Equation 4) and that has been is also given in Table 7. As an example of DSC scans, DSC curves of control and MA are also shown in Figure 2B. The T, ΔH and ΔS values for DPPC liposomes without any additives, as a control, were approximately 41.0 °C, 8.8 kcal/mol and 28.0 cal mol−1 K−1, respectively. Control showed two peaks of pre-transition at 34.5 °C with a very small ΔH and main transition at 41.0 °C with a large ΔH, 8.8 kcal/mol. Acrylates, MA, EA and nBA, showed a shift of T to a much lower temperature range of 32–34 °C, whereas methacrylates, MMA and EMA showed a shift of T to a slightly lower temperature range of 39.5–40.5 °C. The decrease in ΔH for nBA and EMA was greater than that for MA, EA and MMA. Shifts of the T and ΔS values for acrylates were greater than those for methacrylates, possibly due to differences between the α-CH3 and α-H substituents in the monomer molecule (Figure 1). The ΔS values for acrylates, MA and EA were greater than those for the corresponding methacrylates, MMA and EMA, possibly as a result of the effect of the steric factor of the α-substituent in the monomer molecule. The ΔH value for nBA was the lowest, probably in view of the hydrophobicity of its butyl substituent (Table 2).
Table 7.
Changes in DSC phase transition properties of multilamellar DPPC liposomes induced by (meth)acrylates.
| Compound | Phase transition temperature (T) °C | Enthalpy (ΔH) kcal/mol | Entropy (ΔE) cal mo1−1K−1 |
|---|---|---|---|
| Control | 41.0 | 8.8 | 28.03 |
| MA a | 33.5 | 7.9 | 25.77 |
| MMA a | 39.5 | 6.7 | 21.44 |
| Control | 41.5 | 8.9 | 28.30 |
| EA b | 32.5 | 7.7 | 25.20 |
| nBA b | 31.5 | 4.0 | 13.13 |
| EMA b | 40.5 | 5.7 | 18.18 |
Values are the means for two or three separate experiments. T: S.E. < 0.01%; ΔH: S.E. < 10%.; 75 mM DPPC.
75 mM;
25 mM.
MA and EA, although showing great in vivo toxicity, possessed less hemolytic activity (Table 1). Jain et al. [43] previously reported that antihemolytic small molecules have the ability to expand the lipid bilayer of biomembranes and cause large changes in the phase transition properties of the DPPC bilayer. Marique-Moreno et al. [44] investigated the effects of non-steroidal anti-inflammatory drug on human erythrocytes, and on liposomes as a cell membrane molecular model, and found that these drugs interacted strongly with dimyristoylphosphatidylcholine (DMPC) multilayers; DSC data also indicated a decrease in the melting (phase transition) temperature (T) of DMPC liposomes, which was attributed to destabilization of the gel phase. Taken together, it was concluded from these findings that the low hemolytic activity of MA, despite its high toxicity, may be attributable to its ability to expand the lipid bilayer of erythrocytes. The hemolytic activity of (meth)acrylates may be controlled by HF and hydrophobicity of the monomers, resulting from a good QSAR in the HF term and log P, whereas the in vivo toxicity of (meth)acrylates may be controlled by their π-electron density, σ-charge or η and χ reactivity principles, resulting from a good QSAR for these descriptors (Table 4). In vivo toxicity may be controlled by the Michael-type reactivity of the monomers [11]. As in vivo experiments are too complex to allow simple interpretation, liposome studies may help to clarify the mechanisms of in vitro and in vivo toxicity.
3. Experimental Section
3.1. Chemicals
The following chemicals and reagents were obtained from the indicated sources. Methyl acrylate (MA), ethyl acrylate (EA), methyl methacrylate (MMA), ethyl methacrylate (EMA) and n-butyl acrylate (nBA)(Tokyo Chemical Industry Co., Ltd., Tokyo, Japan); l-α-dipalmitoylphosphatidylcholine (DPPC)(Sigma Chemical Co., USA); deuterium oxide, 3-(trimethylsilyl)propionic acid sodium salt (TMSPA)(Merck, Darmstadt, Germany).
3.2. NMR Spectra
The 1H- and 13C-NMR chemical shift data for various monomers in chloroform-d (CDCl3) were taken from the literature [18,19]. Briefly, the chemical shifts of the indicated monomers were measured in CDCl3 at 35 °C at 125 and/or 500 MHz, respectively, using tetramethylsilane (TMS) as an internal standard.
3.3. NMR Study
Preparation of liposomes: Briefly, the method of DPPC liposome preparation was as follows: DPPC was accurately weighed and dissolved in chloroform. The solution was evaporated to a dry thin film on the bottom of a round-bottom test tube and left under vacuum for 30 min. Deuterium oxide (D2O, Merck, Darmstadt, Germany) in 0.01 M phosphate buffer at pD 7.0 was then added, and sonication was performed under a nitrogen atmosphere for 10 min at 60 °C. After incubation for 20 min at room temperature, unilamelar DPPC liposomes were prepared by centrifugation at 20,000× g for 15 min. 1H-NMR spectra were measured at 25 °C on a JEOL (Tokyo, Japan) JNM-GX 270 or ALPHA 500 instrument at a resolution of 0.01 ppm and 0.0013 ppm, respectively. An NMR sample tube with a coaxial capillary was used. The coaxial capillary with monomers was inserted into an NMR sample tube with the liposomes. Then, NMR spectra were measured at 25 °C and 50 °C, respectively. The external standard was TMSPA [17,38].
3.4. DSC Study
An aliquot sample (20 μL) of DPPC and the indicated concentration of monomer in 0.01 M phosphate buffer solution at pH 7.0 was placed into a DSC specimen container. The specimen was allowed to equilibrate for 14 h at 5 °C, then the specimen was scanned in a sealed calorimetric container on a DSC-Rigaku calorimeter (Rigaku Denki Co. Ltd., Tokyo, Japan) at a heating rate of 5 °C min−1 with a range setting of 0.5 mcal s−1. The instrument was calibrated with indium as a standard. The enthalpy (ΔH) was calculated from the area under the DSC curve [17,38,39].
3.5. Hemolytic Activity
The concentration eliciting 50% (H50) hemolysis of rabbit erythrocytes for (meth)acrylates was taken from the literature [3].
3.6. In Vivo Toxicity
LD50 (50% lethal dose) data for intraperitoneal injection of mice with acrylate and methacrylate monomers were taken from the literature [3]. Briefly, male albino ICR mice weighing 25 ± 5 g were used as the test animals, and the LD50 dose for each compound was calculated in terms of 7-day mortality.
3.7. Theoretical Parameters
Parameters η, χ and ω were calculated using Equations 1–3, respectively (Table 4). HOMO, LUMO, and heats of formation were taken from our reported studies [14,15]. Briefly, calculations of heats of formation were performed using the PM3/CONFLEX method. To obtain fine geometry details in the present study, initial geometry optimization was first performed using CONFLEX5 (Conflex, Tokyo, Japan). Thereafter, calculations using the PM3 method in the MOPAC 2000 program were carried out on a Tektronix CAChe workstation (Fujitsu Ltd., Japan).
4. Conclusions
QSARs between in vitro toxicity (1/H50) or in vivo toxicity (ipLD50) based on reported data and their NMR chemical shifts (δHa or δCβ) or PM3-based theoretical parameters (HF, η, χ, ω) were investigated. There was a good linear QSAR between log 1/H50 and log P or HF for (meth)acrylates. Also, a good QSAR between ipLD50 and the δCβ, η-, χ-, or ω-term for methacrylates was obtained, indicating that a common mechanism of action (Michael-type addition) of these monomers may be responsible for their in vivo toxicity. The interaction between DPPC liposomes and (meth)acrylates, investigated using NMR and DSC methods, indicated that the hemolytic activity of monomers may be controlled by their HF, which may be attributed to the monomer-induced phase transition properties of the erythrocyte lipid bilayer. NMR data may be an important tool for evaluating the biological activity of new vinyl monomers in medical and dental applications.
Acknowledgments
We thank M. Ishihara of Meikai University for semi-empirical calculations.
References
- 1.Lawrence W.H., Bass G.E., Purcell W.P., Autian J. Use of mathematical models in the study of structure-toxicity relationships of dental compounds: I. Esters of acrylic and methacrylic acids. J. Dent. Res. 1972;51:526–535. doi: 10.1177/00220345720510024701. [DOI] [PubMed] [Google Scholar]
- 2.Tanii H., Hashimoto K. Structure-toxicity relationship of acrylates and methacrylates. Toxicol. Lett. 1982;11:125–129. doi: 10.1016/0378-4274(82)90116-3. [DOI] [PubMed] [Google Scholar]
- 3.Dillingham E.O., Lawrence W.H., Autian J. Acrylate and methacrylate esters: Relationship of hemolytic activity and in vivo toxicity. J. Biomed. Mater. Res. 1983;17:945–957. doi: 10.1002/jbm.820170606. [DOI] [PubMed] [Google Scholar]
- 4.Yoshii E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997;37:517–524. doi: 10.1002/(sici)1097-4636(19971215)37:4<517::aid-jbm10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Chan K., O’Brien P.J. Structure-activity relationships for hepatocyte toxicity and electrophilic reactivity of α,β-unsaturated esters, acrylates and methacrylates. J. Appl. Toxicol. 2008;28:1004–1015. doi: 10.1002/jat.1366. [DOI] [PubMed] [Google Scholar]
- 6.Cosmetic Ingredient Review. Final report of the safety assessment of methacrylic acid. Int. J. Toxicol. 2005;24:33–51. doi: 10.1080/10915810500434191. [DOI] [PubMed] [Google Scholar]
- 7.Bakopoulou A., Papadopoulos T., Garefis P. Molecular toxicology of substances related from resin-based dental restorative materials. Int. J. Mol. Sci. 2009;10:3861–3899. doi: 10.3390/ijms10093861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukeoka T., Suzuki M., Ohtsuki C., Sugino A., Tsuneizumi Y., Miyagi J., Kuramoto K., Moriya H. Mechanical and histological evaluation of a PMMA-based bone cement modified with γ-methacryloxypropyltrimethoxysilane and calcium acetate. Biomaterials. 2006;27:3897–3903. doi: 10.1016/j.biomaterials.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Hansch C., Fujita T. p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964;86:1616–1626. [Google Scholar]
- 10.Linhart I., Vosmanská M., Smejkal J. Biotransformation of acrylates. Excretion of mercapturic acids and changes in urinary carboxylic acid profile in rat dosed with ethyl and 1-butyl acrylate. Xenobiotica. 1994;24:1043–1052. doi: 10.3109/00498259409043301. [DOI] [PubMed] [Google Scholar]
- 11.Freidig A.P., Verhaar H.J.M., Hermens J.L.M. Quantitative structure-property relationships for the chemical reactivity of acrylates and methacrylates. Environ. Toxicol. Chem. 1999;18:1133–1139. [Google Scholar]
- 12.Cronin M.T., Walker J.D., Jaworska J.S., Comber M.H., Watts C.D., Worth A.P. Use of QSARs in international decision-making frameworks to predict ecologic effects and environmental fate of chemical substances. Environ. Health Perspect. 2003;111:1376–1390. doi: 10.1289/ehp.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eroglu E., Palaz S., Oltulu O., Turkmen H., Ozaydın C. Comparative QSTR study using semi-empirical and first principle methods based descriptors for acute toxicity of diverse organic compounds to the fathead minnow. Int. J. Mol. Sci. 2007;8:1265–1283. [Google Scholar]
- 14.Ishihara M., Fujisawa S. Quantum-chemical descriptors for estimating hemolytic activity of aliphatic and aromatic methacrylates. Chemosphere. 2008;70:1898–1902. doi: 10.1016/j.chemosphere.2007.07.070. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara M., Fujisawa S. A structure-activity relationship study on the mechanisms of methacrylate-induced toxicity using NMR chemical shift of β-carbon, RP-HPLC log P and semiempirical molecular descriptor. Dent. Mater. J. 2009;28:113–120. doi: 10.4012/dmj.28.113. [DOI] [PubMed] [Google Scholar]
- 16.Putz M.V., Ionaşcu C., Putz A.M., Ostafe V. Alert-QSAR. Implications for electrophilic theory of chemical carcinogenesis. Int. J. Mol. Sci. 2011;12:5098–5134. doi: 10.3390/ijms12085098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujisawa S., Kadoma Y., Ishihara M., Atsumi T., Yokoe I. Dipalmitoylphosphatidylcholine (DPPC) and DPPC/cholesterol liposomes as predictors of the cytotoxicity of bis-GMA related compounds. J. Liposome Res. 2004;14:39–49. doi: 10.1081/lpr-120039662. [DOI] [PubMed] [Google Scholar]
- 18.Hatada K., Kitayama T., Nishiura T., Shibuya W. Relation between reactivities of vinyl monomers and their NMR spectra. Curr. Org. Chem. 2002;6:121–153. [Google Scholar]
- 19.Fujisawa S., Kadoma Y. Prediction of the reduced glutathione (GSH) reactivity of dental methacrylate monomers using NMR spectra—Relationship between toxicity and GSH reactivity. Dent. Mater. J. 2009;28:722–729. doi: 10.4012/dmj.28.722. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy T.J., Hayes E.P., Schwartz C.S., Witz G. The reactivity of selected acrylate esters toward glutathione and deoxyribonucleosides in vitro: Structure-activity relationships. Fundam. Appl. Toxicol. 1994;22:543–548. doi: 10.1006/faat.1994.1061. [DOI] [PubMed] [Google Scholar]
- 21.Verhaar H.J.M., van Leeuwen C.J., Hermens J.L.M. Classifying environmental pollutants. 1: Structure-activity relationships for prediction of aquatic toxicity. Chemosphere. 1992;25:471–491. [Google Scholar]
- 22.Russom C.L., Bradbury S.P., Broderius S.J., Hammermeister D.E., Drummond R.A. Predicting modes of toxic action from chemical structure: Acute toxicity in the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 1997;16:948–967. doi: 10.1002/etc.2249. [DOI] [PubMed] [Google Scholar]
- 23.Koleva Y.K., Madden J.C., Cronin M.T. Formation of categories from structure-activity relationships to allow read-across for risk assessment: Toxicity of α,β-unsaturated carbonyl compounds. Chem. Res. Toxicol. 2008;21:2300–2312. doi: 10.1021/tx8002438. [DOI] [PubMed] [Google Scholar]
- 24.del Re G. A simple MO-LCAO method for the calculation of charge distributions in saturated organic molecules. J. Chem. Soc. 1958:4031–4040. doi: 10.1039/JR9580004031. [DOI] [Google Scholar]
- 25.Talalay P., de Long M.J., Prochaska H.J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The University of Oxford. Department of Chemistry MSDS web resource. [accessed on 19 October 2011]. http://msds.chem.ox.uk/AC/acrolein.html.
- 27.The University of Oxford. Department of Chemistry MSDS web resource. [accessed on 15 November 2011]. http://msds.chem.ox.uk/AC/acrylonitrile.html.
- 28.The University of Oxford. Department of Chemistry MSDS web resource. [accessed on 7 February 2005]. http://msds.chem.ox.uk/AC/acrylamide.html.
- 29.Dickinson D.A., Forman H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 30.Geurtsen W., Leyhausen G. Chemical-biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA) J. Dent. Res. 2001;80:2046–2050. doi: 10.1177/00220345010800120401. [DOI] [PubMed] [Google Scholar]
- 31.Schweikl H., Spagnuolo G., Schmalz G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006;85:870–877. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa T., Li Z.S., Lu Y.P., Rea P.A. The GS-X pump in plant, yeast, and animal cells: Structure, function, and gene expression. Biosci. Rep. 1997;17:189–207. doi: 10.1023/a:1027385513483. [DOI] [PubMed] [Google Scholar]
- 33.Tanel A., Averill-Bates D.A. The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim. Biophys. Acta. 2005;1743:255–267. doi: 10.1016/j.bbamcr.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H., Kamendulis L.M., Jiang J., Xu Y., Klaunig J.E. Acrylonitrile-induced morphological transformation in Syrian hamster embryo cells. Carcinogenesis. 2000;21:727–733. doi: 10.1093/carcin/21.4.727. [DOI] [PubMed] [Google Scholar]
- 35.Zitting A., Heinonen T. Decrease of reduced glutathione in isolated rat hepatocytes caused by acrolein, acrylonitrile, and the thermal degradation products of styrene copolymers. Toxicology. 1980;17:333–341. doi: 10.1016/0300-483x(80)90014-1. [DOI] [PubMed] [Google Scholar]
- 36.Besaratinia A., Pfeifer G.P. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis. 2007;28:519–528. doi: 10.1093/carcin/bgm006. [DOI] [PubMed] [Google Scholar]
- 37.Papahadjopoulos D. Liposomes and their uses in biology and medicine. Ann. N. Y. Acad. Sci. 1978;308:1–462. [PubMed] [Google Scholar]
- 38.Fujisawa S., Kadoma Y., Masuhara E. A calorimetric study of the interaction of synthetic phospholipid liposomes with vinyl monomers, acrylates and methacrylates. J. Biomed. Mater. Res. 1984;18:1105–1114. doi: 10.1002/jbm.820180912. [DOI] [PubMed] [Google Scholar]
- 39.Fujisawa S., Atsumi T., Kadoma Y. Cytotoxicity of methyl methacrylate (MMA) and related compounds and their interaction with dipalmitoylphosphatidylcholine (DPPC) liposomes as a model for biomembranes. Oral Dis. 2000;6:215–221. doi: 10.1111/j.1601-0825.2000.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 40.Fujisawa S., Atsumi T., Kadoma Y. Cytotoxicity and phospholipid-liposome phase-transition properties of 2-hydroxyethyl methacrylate (HEMA) Artif. Cells Blood Substit. Immobil. Biotechnol. 2001;29:245–261. doi: 10.1081/bio-100103048. [DOI] [PubMed] [Google Scholar]
- 41.Lasic D.D. Magnetic resonance methods in the studies of liposomes. Bull. Magn. Reson. 1991;13:3–13. [Google Scholar]
- 42.Cruciani O., Mannina L., Sobolev A.P., Cametti C., Segre A. An improved NMR study of liposomes using 1-palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as model. Molecules. 2006;11:334–344. doi: 10.3390/11050334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain M.K., Wu N.Y., Wray L.V. Drug-induced phase change in bilayer as possible mode of action of membrane expanding drugs. Nature. 1975;255:494–496. doi: 10.1038/255494a0. [DOI] [PubMed] [Google Scholar]
- 44.Marique-Moreno M., Suwalsky M., Villena F., Garidel P. Effects of the nonsteroidal anti-inflammatory drug naproxen on human erythrocytes and on cell membrane molecular models. Biophys. Chem. 2010;147:53–58. doi: 10.1016/j.bpc.2009.12.010. [DOI] [PubMed] [Google Scholar]