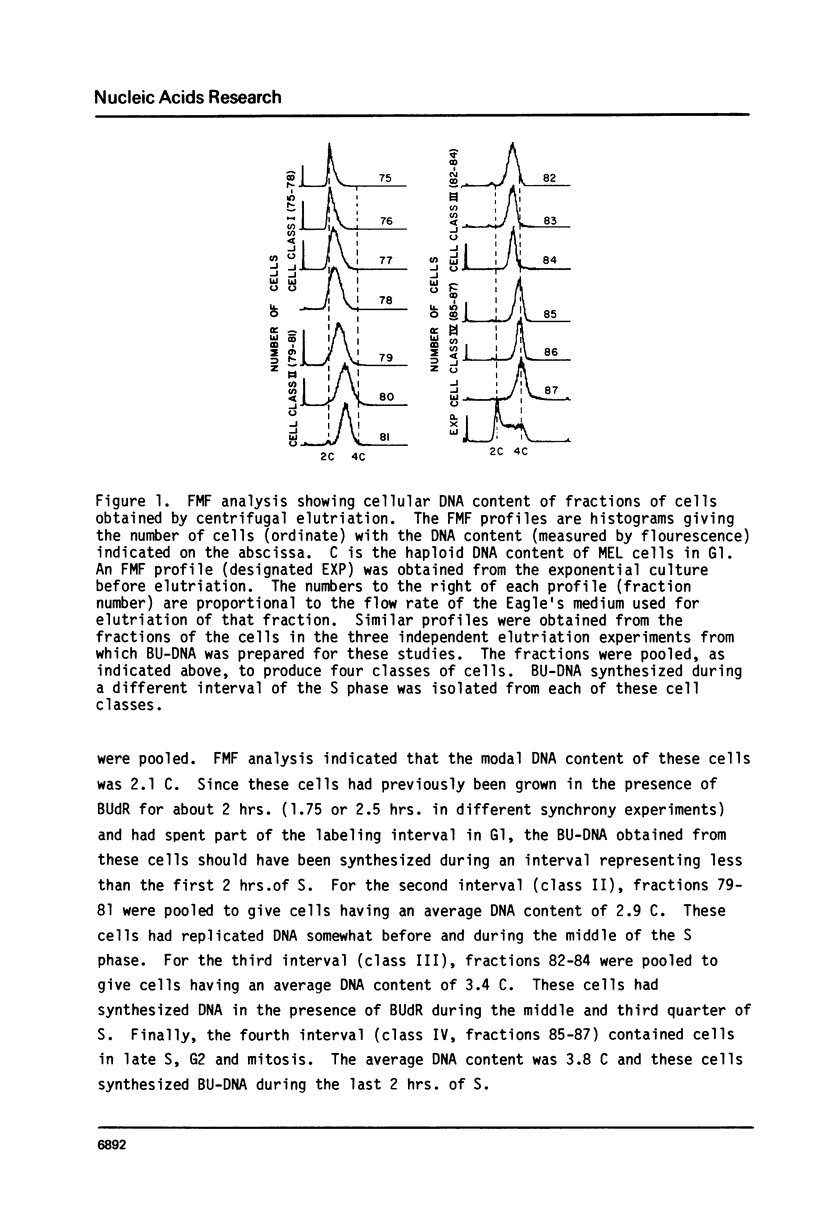
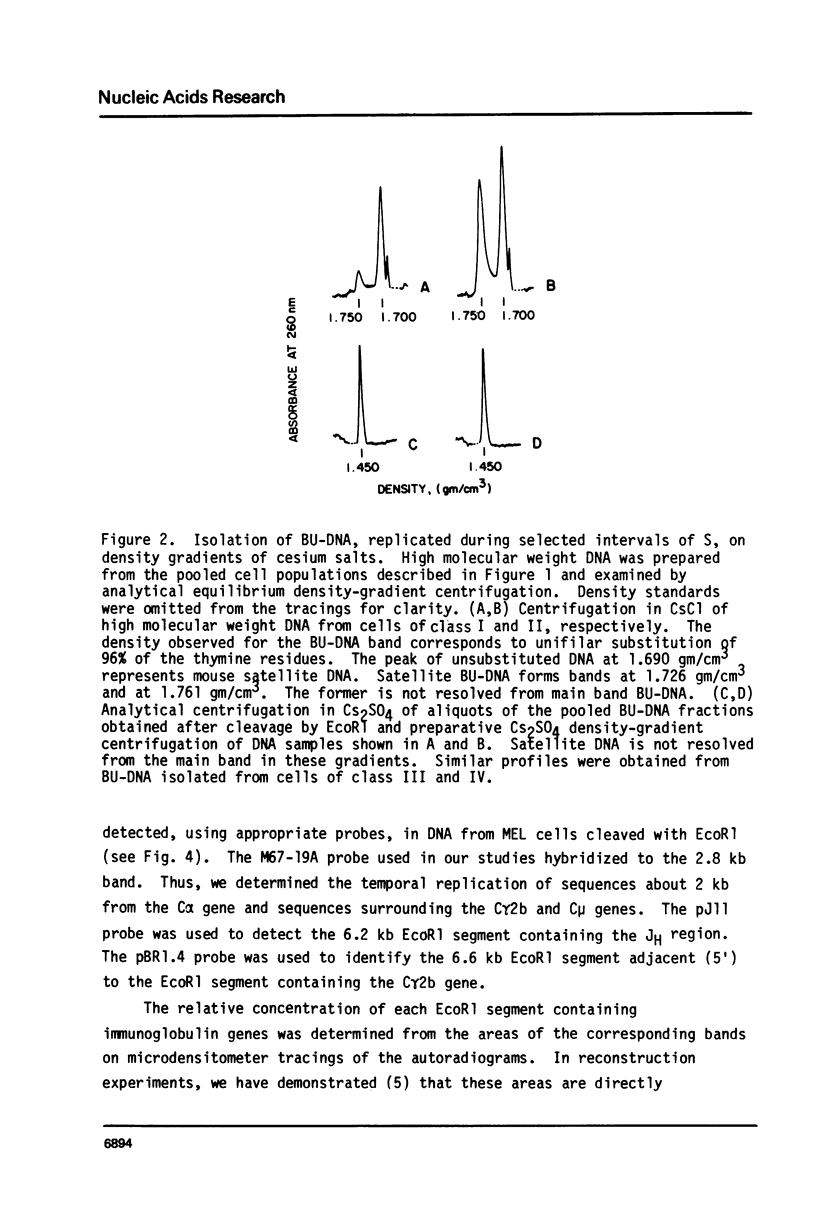
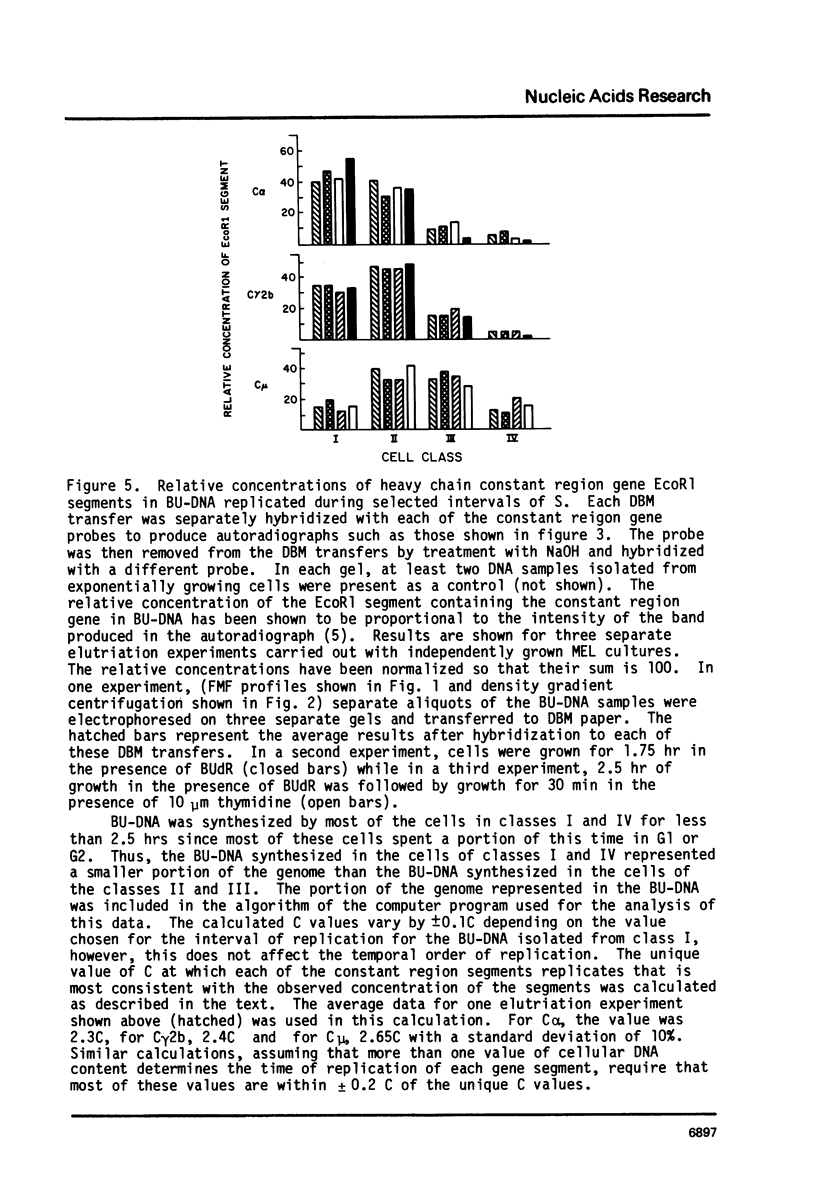
Abstract
The time of replication during the S phase in a murine erythroleukemia (MEL) cell line was determined for immunoglobulin heavy chain constant region C alpha, C gamma 2b and C mu sequences whose boundaries are defined by EcoR1 restriction endonuclease sites (EcoR1 segments). Logarithmically growing cultures of MEL cells with an S phase of about 7.5 hours were pulse labelled with 20 micrograms/ml of 5-bromodeoxyuridine (BUdR). The cells were then fractionated by centrifugal elutriation into 10-12 distinct populations containing cells in different stages of the cell cycle. Flow microfluorimetric (FMF) analysis of DNA content, measurements of cell volume and autoradiography after 3H-thymidine pulse labelling were used to determine position in the cell cycle. Fractions were pooled to represent four selected intervals of S in which BU-DNA was synthesized for 2.5 hrs or less. Newly replicated DNA which had incorporated BUdR into one strand was isolated, cleaved with EcoR1, and separated on neutral Cs2S04 gradients. Equal amounts of BU-DNA replicated during these four intervals of S were electrophoresed in 0.8% agarose gels, transferred to diazotized aminobenzyloxymethyl paper and hybridized with 32p probes containing the C alpha, C gamma 2b and C mu genes and flanking sequences. The relative amounts of segments replicated were assessed by quantitation of the appropriate bands on the autoradiograms by microdensitometry. The results indicate that the 2.8 kb C alpha, 6.6 kb C gamma 2b and 12 kb C mu EcoR1 segments in these MEL cells replicated during defined intervals of the first half of the S phase. The order of replication of these EcoR1 segments as the cells proceeded through S was C alpha, C gamma 2b, C mu, corresponding to the linear order of the genes determined by restriction endonuclease mapping.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balazs I., Brown E. H., Schildkraut C. L. The temporal order of replication of some DNA cistrons. Cold Spring Harb Symp Quant Biol. 1974;38:239–245. doi: 10.1101/sqb.1974.038.01.027. [DOI] [PubMed] [Google Scholar]
- Braun R., Wili H. Time sequence of DNA replication in Physarum. Biochim Biophys Acta. 1969 Jan 21;174(1):246–252. doi: 10.1016/0005-2787(69)90248-2. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Schildkraut C. L. Perturbation of growth and differentiation of Friend murine erythroleukemia cells by 5-bromodeoxyuridine incorporation in early S phase. J Cell Physiol. 1979 May;99(2):261–278. doi: 10.1002/jcp.1040990213. [DOI] [PubMed] [Google Scholar]
- Cory S., Jackson J., Adams J. M. Deletions in the constant region locus can account for switches in immunoglobulin heavy chain expression. Nature. 1980 Jun 12;285(5765):450–456. doi: 10.1038/285450a0. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Epner E., Rifkind R. A., Marks P. A. Replication of alpha and beta globin DNA sequences occurs during early S phase in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3058–3062. doi: 10.1073/pnas.78.5.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L. Separation of very large DNA molecules by gel electrophoresis. Nucleic Acids Res. 1978 Mar;5(3):653–665. doi: 10.1093/nar/5.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigwood N. L., Jahn C. L., Hutchison C. A., 3rd, Edgell M. H. Locations of three repetitive sequence families found in BALB/c adult beta-globin clones. Nucleic Acids Res. 1981 Mar 11;9(5):1133–1150. doi: 10.1093/nar/9.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kataoka T., Kawakami T., Takahashi N., Honjo T. Rearrangement of immunoglobulin gamma 1-chain gene and mechanism for heavy-chain class switch. Proc Natl Acad Sci U S A. 1980 Feb;77(2):919–923. doi: 10.1073/pnas.77.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki R., Traunecker A., Sakano H., Roeder W., Tonegawa S. Exon shuffling generates an immunoglobulin heavy chain gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2138–2142. doi: 10.1073/pnas.77.4.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M. A., Roufa D. J. Replication of viral DNA sequences integrated within the chromatin of SV40-transformed Chinese hamster lung cells. Cell. 1981 Oct;26(2 Pt 2):245–258. doi: 10.1016/0092-8674(81)90307-x. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Mueller G. C., Kajiwara K. Early- and late-replicating deoxyribonucleic acid complexes in HeLa nuclei. Biochim Biophys Acta. 1966 Jan 18;114(1):108–115. doi: 10.1016/0005-2787(66)90258-9. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- WALKER P. M., MCLAREN A. SPECIFIC DUPLEX FORMATION IN VITRO OF MAMMALIAN DNA. J Mol Biol. 1965 Jun;12:394–409. doi: 10.1016/s0022-2836(65)80263-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]