Abstract
Background
We tested the hypothesis that testosterone depletion / blockade in male rats protects against trauma hemorrhagic shock-induced distant organ injury by limiting gut injury and subsequent production of biologically active mesenteric lymph.
Methods
Male, castrated male, or Flutamide-treated rats (25mg/kg sc following resuscitation) were subjected to a laparotomy (trauma), mesenteric lymph duct cannulation and 90 min of shock (35mmHg) or trauma sham-shock. Mesenteric lymph was collected pre, during and post shock. Gut injury was determined at 6 hours post shock using ex vivo ileal permeability with Fluorescein Dextran (FD4). Post-shock mesenteric lymph was assayed for biologic activity in vivo by injection into mice and measuring lung permeability, neutrophil activation and RBC deformability. In vitro neutrophil priming capacity of the lymph was also tested.
Results
Castrated and flutamide-treated male rats were significantly protected against trauma-hemorrhagic shock (T/HS)-induced gut injury as compared to hormonally-intact males. Post-shock mesenteric lymph from male rats had a higher capacity to induce lung injury, PMN activation and loss of RBC deformability when injected into naïve mice as compared to castrated and flutamide treated males. The increase in gut injury after T/HS in males directly correlated with the in vitro biologic activity of mesenteric lymph to prime neutrophils for an increased respiratory burst.
Conclusions
Following T/HS, gut protective effects can be observed in males after testosterone blockade / depletion. This reduced gut injury contributes to decreased biologic activity of mesenteric lymph leading to attenuated systemic inflammation and distant organ injury.
Keywords: Trauma, Shock, Sex Hormones, Mesenteric Lymph
Introduction
Since trauma is the leading cause of death in individuals under the age of 44 and more years of life are lost due to trauma than cancer or heart disease [1], extensive clinical and investigative efforts have been directed towards improving survival in this patient population. In this regard, one major area of investigation has focused on understanding the mechanisms by which trauma-hemorrhage leads to a systemic inflammatory state (SIRS), acute lung injury (ARDS) and the multiple organ dysfunction syndrome (MODS). One concept that has emerged from extensive preclinical animal studies is that sex hormonal status can modulate the early response to shock, trauma and sepsis [2, 3]. The mechanisms underlying these responses have been extensively studied and these studies have generally shown that estrogens exert a protective effect, while testosterone exerts either no or a deleterious effect on a number of systems, including vascular reactivity, the immune and inflammatory systems as well as several key organ systems including the gut, heart, liver and lung [2, 3]. While the preclinical animal studies were clear in showing estrogen is largely protective and testosterone deleterious, the human studies showed less consistency. Specifically, although most patient studies demonstrated a survival or clinical benefit for the female population [4, 5], some studies showed no difference between males and females [6], while a minority of the studies showed a benefit to being male [7]. Based on this body of clinical and preclinical data, recommendations for [8] and against early sex hormone manipulation in injured patients have emerged [9]. We believe that, in order to help resolve this controversy, more information is needed on the key physiologic effects of sex hormone manipulation. Since most trauma patients are male, in the current study we tested the hypothesis that testosterone is necessary for trauma-hemorrhagic shock-induced early gut injury and the production of biologically active mesenteric lymph and that the post trauma-shock administration of a testosterone antagonist (flutamide) would prevent gut injury and the production of pro-inflammatory and tissue injurious mesenteric lymph, thereby limiting the development of early SIRS and MODS.
Materials and Methods
Animals
Sprague-Dawley rats (Charles River, MA) weighing 350 g to 400 g and C57B wild type mice (B6 genetic background, Jackson Laboratories, Bar Harbor, ME) were used in the experiments. Animals were housed under barrier-sustained conditions kept at a temperature of 25°C with 12- hour light/dark cycle. The rats had free access to water and chow (Teklan 22/5 Rodent Diet W-8640; Harlan Tecklad, Madison, WI) for at least 1 week before the study. The castration procedures were done 2 weeks prior to the rats being subjected to sham or actual injury. All animals were maintained in accordance with the recommendation of the Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the New Jersey Medical School Animal Care and Use Committee.
Experimental Design
The first goal of this study was to determine the role of testosterone depletion / blockade in protecting against T/HS-induced gut injury in male rats. The second goal was to determine whether this gut protection contributes to a reduction in distant organ injury by limiting the biological activity of post-shock mesenteric lymph.
To test this, we used three groups of male animals: hormonally unmanipulated males, castrated males (testosterone depleted) and male rats treated with flutamide (a potent non-steroidal testosterone receptor blocker). These animals underwent T/HS or trauma sham shock (T/SS) with mesenteric lymph duct cannulation. In the T/HS group, animals underwent a laparotomy and mesenteric lymph duct cannulation followed by 90 minutes of hemorrhagic shock. After shock, animals were resuscitated with their own shed blood. At 6 hours after the shock or sham period animals were sacrificed. Gut injury was measured using histologic evaluation of the percentage of injured villi. Gut permeability was determined in a fixed ileal segment using an ex-vivo method. Biologic activity of post-shock mesenteric lymph was assessed in vitro by its capacity to prime isolated naïve rat neutrophils for an increased respiratory burst. Lung injury, neutrophil activation and changes in RBC deformability following lymph infusion in naive mice were used to assess ability of testosterone depletion / blockade to abrogate the in vivo activity of post-shock mesenteric lymph.
Trauma–Hemorrhagic Shock and Lymph Cannulation Models
After a 7-day acclimatization period, rats underwent a laparotomy and mesenteric lymph duct cannulation, followed by T/HS or T/SS. In animals that were subjected to hemorrhagic shock, blood was withdrawn via the jugular vein catheter until the mean arterial pressure was reduced to ~35mm Hg and was maintained at this pressure for 90 minutes by reinfusing or withdrawing blood as needed. The shed blood was kept at 37°C. At the end of the shock period, the rats were resuscitated with their own shed blood. T/SS rats underwent the same procedure, but no blood was withdrawn or infused. The animal’s temperature was maintained above 36.3°Cwith the use of heating pads or lamps as necessary. Mesenteric lymph was collected continuously on ice. The lymph samples were centrifuged for 15 minutes at 400g to remove all cellular components and stored at -80°C until tested.
Flutamide (25mg/kg body weight subcutaneously, Sigma, St. Louis, MO) was dissolved in vehicle (propanediol, Sigma) and administered subcutaneously during T/HS resuscitation period as described previously [10].
Histological analysis of Ileal Villous Injury
After sacrifice, a segment of the terminal ileum was excised and fixed in 10% buffered formalin. After processing, semi-thin (2–4 mm) sections were cut, stained with Hematoxylin and Eosin, then examined using light microscopy at 100x magnification. In order to deem a villus injured, there must be microscopic evidence of injury. Injury ranged from submucosal edema at the villous tip to frank necrosis of the villi. A total of five random fields from each animal were examined, and the percentage of villous damage for each animal was determined. All results are expressed as the percentage of villi injured per total number of villi examined. All histologic evaluations were performed in a blinded fashion.
Measurement of Ex Vivo Intestinal Permeability
Intestinal permeability was determined using the everted gut sac method with fluorescein isothiocyanate dextran (molecular weight 4,000 Da; FD4; Sigma) tracer as described previously [11].
Lymph infusion protocol
Mice underwent laparotomy as well as internal jugular vein cannulation. Laparotomy was closed after 15 minutes using two layers of 3-0 silk suture. Next, pooled post-shock (1-3 hours) T/HS or T/SS rat lymph from various hormonally modulated groups was infused at a rate of 3.3 μL/g body weight per hour for 3 hours. The rationale for the lymph volumes infused was based on the actual amount of lymph produced by the rats (ml/kg/hr) subjected to T/HS or T/SS [12].
Evans blue dye lung permeability assay
After a 3-hour infusion period, all mice were injected with 1 mg of Evans blue dye through the internal jugular catheter. Twenty minutes after injection of the dye, all mice were sacrificed. Whole blood was collected via cardiac puncture and centrifuged at 1500 rpm for 20 minutes and the resultant plasma was serially diluted to obtain a standard curve. Bronchoalveolar lavage was performed on the excised lungs using phosphate buffered saline (PBS). 1ml of PBS was instilled, rinsed three times, and collected. The bronchoalveolar lavage fluid (BALF) was then centrifuged at 1500 rpm at 4°C for 20 minutes to remove any cells, and the supernatant fluid was assayed spectrophotometrically at 620 nm for dye concentration. The concentration of Evans blue dye in the BALF was then plotted on a standard curve, and the percentage relative to that present in the plasma was determined.
In vivo and in vitro determination of Neutrophil respiratory burst
In order to determine the in vivo effect of T/HS lymph samples on neutrophil activation, a blood sample was obtained in a heparinized syringe from each mouse at 3 hours, after the end of lymph injection. Respiratory burst activity was measured as previously described [13]. Briefly, the samples were treated with RBC lysis buffer (1% PharM Lyse; BD Pharmingen, San Diego, CA) and incubated for 15 minutes at room temperature to remove the RBCs. They were then centrifuged at 25°C and washed twice with Hank’s balanced salt solution , and the supernatant was then discarded. The resulting pellet was suspended in Hank’s balanced salt solution at a concentration of 4 × 106 cells. Dihydrorhodamine (DHR, 15 ng/mL) was added to 100μL of the PMN sample and warmed to 37°C. Phorbol myristic acid (PMA, 0.4 μmol/L, 15 minutes, 37°C) was then added to stimulate the cells. The PMN respiratory burst was measured by flow cytometry, where the neutrophils were identified by forward and side scatter analysis. The data are expressed as the mean fluorescence index.
The biologic activity of post-shock mesenteric lymph to activate PMNs in vitro was measured as follows. Whole blood was obtained via cardiac puncture from naïve male rats. PMNs were isolated as described above. Medium or the lymph samples (5% v/v) were then added and the tubes were placed into the incubator for 5 mins, following which DHR (15 ng/mL) was added to the tubes. Five minutes after the DHR was added, the PMNs were stimulated with PMA. After 15 mins at 37°C, the PMN respiratory burst was measured by flow cytometry as described previously.
In vivo RBC deformability
In order to determine the in vivo effect of lymph on RBC deformability, a blood sample was obtained in a heparinized syringe from each mouse at 3 hours, after the end of lymph injection. RBC deformability was determined with a laser-assisted ektacytometer (LORCA, RR Mechatronics, The Netherlands), as previously described [13]. Briefly, an aliquot of RBCs (15 μL) containing approximately 30 million cells was suspended in 1 mL of 5% polyvinylpyrrolidone (molecular weight 360,000; Sigma, St. Louis, MO) in phosphate-buffered saline at a final viscosity of 30 mPa. After gently mixing for 15 minutes at room temperature to assure complete oxygenation of the hemoglobin, cell deformability was determined at 37°C. Cell deformability was assessed by calculating the elongation index (EI) at shear stresses ranging from 0.3 Pa to 30 Pa, as described previously [14] . RBC deformability alterations were assessed by either the elongation index (EI) measured at low shear stress similar to that occurring during low flow conditions in the microcirculation (0.3 Pa) as well as by calculating KEI, which is an indicator of the overall status of RBC deformability. Since EI is a direct measure of RBC deformability, the smaller the number the less deformable are the cells. In contrast, since the KEI is the amount of stress needed to deform the RBC to half-maximal deformation, an increase in KEI indicates a decrease in RBC deformability
Statistical Analysis
Results were expressed as mean ± standard deviation (SD). Continuous data were analyzed by one-way analysis of variance using the post hoc Tukey’s multiple comparison tests. Correlation was analyzed using Pearson correlation. Statistical significance was considered to be a p value < 0.05.
Results
There was no significant difference observed in any of the parameters between the hormonally-intact, the castrated or the flutamide-treated T/SS groups and hence they have been represented as a single group.
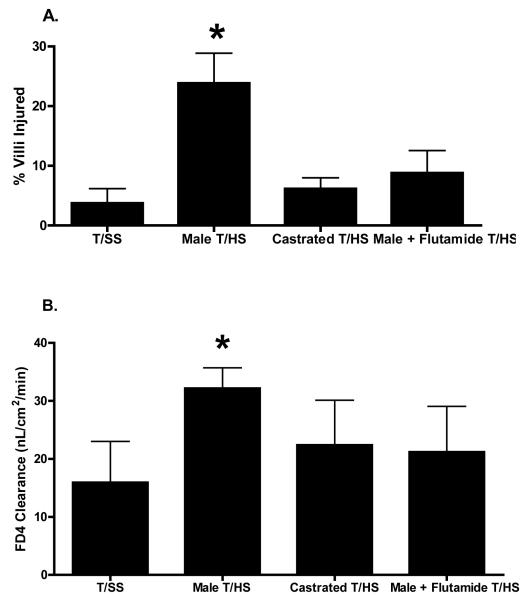
As compared to T/SS rats, T/HS resulted in gut injury as measured by morphologic villous injury as well as increased gut permeability to the permeability probe FD 4 (Figure 1). Testosterone appeared to be necessary for T/HS-induced gut injury, since the castrated and flutamide-treated rats were protected and both gut morphology and gut permeability of these two groups of T/HS rats were similar to that observed after T/SS (Figure 1).
Figure 1.
Castrated males and Flutamide-treated males subjected to T/HS were more resistant to loss of gut barrier function and morphologic changes of villous damage as compared to the Male T/HS group. *p < 0.05 vs. all other groups. Data expressed as Mean ± SD; N = 5-8 for each group.
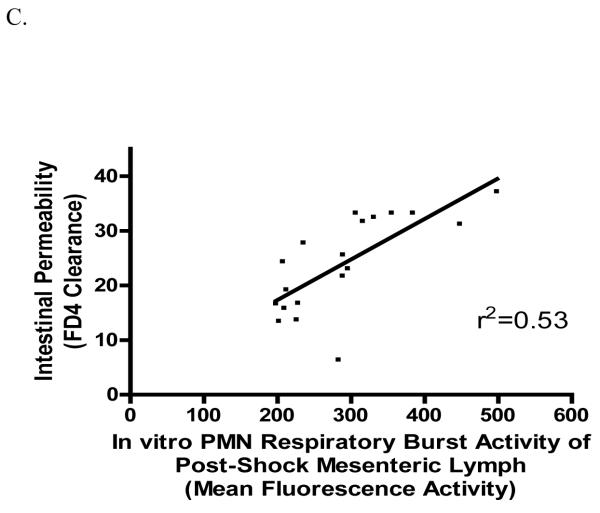
As previously reported [15], lymph from male rats subjected to T/HS primed naïve rat neutrophils for an augmented in vitro respiratory burst (Figure 2A). Similar to what was observed in terms of gut injury, both castration and flutamide treatment abrogated this T/HS lymph-induced neutrophil response (Figure 2A). Furthermore, a direct correlation was observed between the magnitude of gut injury, whether measured as morphologic injury (Figure 2B) or increased permeability (Figure 2C), and the magnitude of in vitro lymph-induced neutrophil priming. This data indicated that the degree of neutrophil priming by the T/HS lymph samples was at least partly related to the magnitude of gut injury and thereby supports the concept that T/HS-induced gut injury results in the production of biologically-active mesenteric lymph.
Figure 2.
Post-shock mesenteric lymph from castrated and flutamide-treated males had a significant reduction in its capacity of induce PMN respiratory burst in vitro (A). *p < 0.05 vs. all other groups. Data expressed as Mean ± SD; N = 5-8 for each group. The increase in gut permeability (B) and intestinal villous injury (C) after T/HS is affected by hormonal modulation and directly correlates (p<0.001) with the biologic activity of post-shock mesenteric lymph to prime neutrophils for an increased respiratory burst. All shock and sham animals were used.
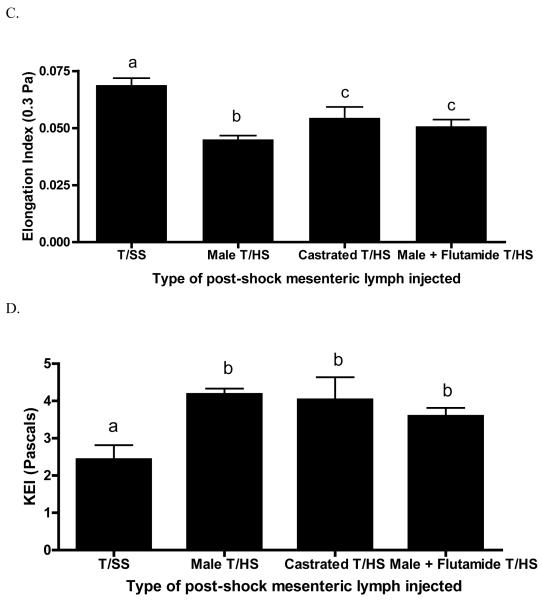
The injection of T/HS but not T/SS lymph into naïve mice resulted in a significant increase in lung permeability, which was totally abrogated in the castrated and flutamide-treated male T/HS rats (Figure 3A). The injection of T/HS lymph also primed the murine neutrophils for an increased respiratory burst and this T/HS lymph-induced neutrophil priming was not observed in the mice injected with T/HS lymph from the castrated males (Figure 3B). However, in contrast to what was observed in the mice injected with lymph from the castrated rats, flutamide-treatment did not abrogate the ability of T/HS lymph to prime neutrophils (Figure 3B).
Figure 3.
Post-shock mesenteric lymph from males but not castrated or flutamide treated males induced in vivo lung injury (A) when injected into mice. In vivo effects of post-shock mesenteric lymph on PMN activation (B) and RBC deformability (C,D) when injected in naive mice. n=4-6 mice per group. Data expressed as mean ± SD, with means being significantly different (p<0.05) if they do not share the same letter
The ability of castration or testosterone blockade to limit T/HS lymph-induced RBC dysfunction was observed when the RBC from the lymph injected mice were tested at low shear stresses (0.3 Pa) such as occurs in the microcirculation (Figure 3C). However, neither was effective, when the RBCs were subjected to higher shear stresses, such as those observed in larger blood vessels (Figure 3D).
Discussion
Emerging data describes a sexual dimorphism in the response to major injury or sepsis with differences demonstrated in immune function as well as susceptibility to organ injury and infection [2, 3]. Although somewhat controversial, the majority of the emerging clinical [2, 16,17], and experimental [2, 18] evidence suggest that males are more susceptible to the adverse consequences of shock, trauma and sepsis than females. This has led to a paradigm where sex hormones are important modulators of the response to major injury, shock or sepsis. In its most basic form, this sex hormone paradigm predicts that the patient’s sex hormonal status at the time of injury modulates the host’s response with testosterone contributing to and estradiol limiting the development of adverse clinical consequences. However, recent clinical data evaluating post-insult sex hormonal levels as predictors of morbidity and mortality [19] has challenged the notion that estradiol is good and testosterone is bad, since, in these studies, it was increased post-injury estradiol levels that were associated with adverse clinical outcomes. The reason why a sex hormone pattern of high estradiol and low testosterone would be protective if present at the time of the insult, yet be associated with an adverse outcome when present several days post-injury is not clear or fully understood. Resolution of this paradox will require studies where clear causality, rather than just associations, between sex hormonal status and outcome can be assessed. Likewise, these studies will need to focus either on the immediate post-injury period or on the several day later post-injury period. In this context, we tested the hypothesis that testosterone is involved in potentiating the immediate and early systemic adverse effects of T/HS and that these effects are related to testosterone’s permissive effect on T/HS-induced gut injury and the production of biologically active mesenteric lymph. The results of these studies provide support for these hypotheses. First, both castration and flutamide administration prevented T/HS-induced gut injury and abrogated the ability of mesenteric lymph from these animals to prime naïve neutrophils for an augmented in vitro respiratory burst. Furthermore, the fact that a direct correlation was found between the magnitude of T/HS-induced gut injury and the neutrophil priming effect of the T/HS lymph specimens supports a direct relationship between gut injury and the production of biologically-active mesenteric lymph.
Since in vitro and in vivo results may not always correlate, we also tested the biologic activity of the various lymph samples in vivo. These studies were based on our earlier work showing that the intravenous injection of T/HS, but not T/SS, lymph from male animals into naïve male rats and mice was sufficient to recreate the lung [12] and RBC injuries observed after actual T/HS [20]. In the current study, we found that the injection of T/HS lymph from the hormonally intact rats led to acute lung injury, while neither T/HS lymph from the castrated or the flutamide-treated rats cause lung injury. These lymph injection study results are consistent with our earlier studies showing that castration abrogates acute lung injury after actual T/HS [21] and studies showing that castration or testosterone blockade limits T/HS-induced liver and cardiac injury [22-24]. The observation that testosterone was necessary for the production of lung-injurious mesenteric lymph is of potential clinical importance since the lung is generally the commonest organ to failure after shock-trauma and its failure is a sentinel event in the cascade of MODS [25].
In these in vivo lymph injection studies, we also measured neutrophil priming and RBC injury, in addition to acute lung injury, for the following reasons. Neutrophil activation was measured because trauma-shock-induced changes in neutrophil activation have been implicated in the pathogenesis of ARDS and as a contributor to the development of MODS [25]. Likewise, recent work suggests that impaired RBC deformability, which occurs in sepsis, after trauma and during shock states [26-28], contributes to the development of MODS by impairing tissue microvascular blood flow [29]. The mechanism by which the failure of 7 micron RBCs to deform when passing through 4 micron capillaries leads to MODS is thought to be related to the rigidified RBCs blocking the microcirculation with resultant decreases in cellular oxygen delivery [30, 31]. This phenomenon is recognized in primary RBC disease states, such as sickle cell anemia, where stress-induced changes in RBC deformability and endothelial sticking leads to clinically significant vasoactive crises [30, 31]. In contrast to the observation that the T/HS lymph samples from the castrated and flutamide-reated rats did not cause lung injury, the in vivo effects of the injection of these T/HS lymph samples on neutrophil priming and RBC deformability were more variable. For example, T/HS lymph from the flutamide-treated, but not the castrated rats, retained the ability to prime neutrophils in vivo. Additionally, castration and flutamide treatment, protected against T/HS lymph-induced RBC rigidification at low shear stresses (0.3 Pa), as observed in the microcirculation. However, RBC deformability was impaired at higher shear stresses.
The fact that T/HS lymph from the flutamide-treated, but not the castrated rats, retained the ability to prime neutrophils in vivo, while neither of these two groups of T/HS lymph samples had in vitro neutrophil priming capability, raises several potentially important points. First, it stresses the key concept that in vitro results are not always replicated in vivo as was observed in the discordant in vitro and in vivo neutrophil priming results observed with the flutamide-treated T/HS lymph samples. Secondly, since, in contrast to the flutamide T/HS lymph samples, the T/HS lymph samples from the castrated rats did not manifest in vivo neutrophil priming activity, it appears that the biologic activity and hence the molecular composition of these lymph samples may differ. These results also suggest that different factors in T/HS lymph may have different in vivo physiologic effects. One possible explanation for castration being more effective than flutamide in abrogating the in vivo neutrophil priming activity of T/HS lymph is that castration is a pretreatment modality, while the administration of flutamide is a post-treatment regimen. That is, in the castrated animals, no testosterone was present during the shock period itself, while in the flutamide-treated animals, testosterone blockade was only initiated during the resuscitation period. Consequently, in the flutamide-treated rats, there was a period of time when the action of testosterone was unopposed and hence it could have resulted in T/HS lymph that differs from that observed in the castrated rats. Since the flutamide-treated T/HS lymph did not directly prime neutrophils in vitro but did induce neutrophil priming in vivo, it is possible that its in vivo neutrophil priming activity was indirect and related to secondary systemic mediators induced by the T/HS lymph. Nonetheless, although T/HS lymph from the flutamide-treated rats caused PMN priming, it did not cause acute lung injury. This observation suggests that lymph-induced PMN priming may not be sufficient by itself to cause T/HS-lymph-induced acute lung injury. Clearly, more study into the biology of these observations is needed, but these observations do indicate that post-shock treatment with a testosterone receptor blocker is sufficient to prevent gut injury and the production of lung injurious T/HS lymph.
The results from this work showing that testosterone is involved in gut injury and the production of mesenteric lymph with pro-inflammatory and tissue injurious properties, provides insight into how sex hormones modulate the initial and early response to T/HS. The potential clinical relevance of these rodent studies to early post T/HS-induced SIRS and MODS are supported by porcine T/HS studies showing that castration reduced the magnitude of gut injury and abrogated the biologic activity of intestinal lymph [32, 33]. Since androgen receptor blockade with flutamide is clinically safe [34], this approach could be utilized in the early post-trauma period and flutamide administration would be a potential alternative to estrogen administration. This option is particularly relevant in light of recent clinical studies in ICU patients showing that the development of elevated estradiol levels is associated with a poorer prognosis [9, 19].
Acknowledgments
Supported by NIH grants GM 59841 and T32 069330
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan P, Deitch EA. Gut origin sepsis and MODS: the role of sex hormones in modulating intestinal and distant organ injury: a review. XX vs XY. 2003;1:108–117. [Google Scholar]
- 3.Angele MK, Wichman M, Eisenmenger S, et al. Immunologic effects of sex hormones following hemorrhagic shock: potential therapeutic applications: a review. XX vs XY. 2003;1:39–45. [Google Scholar]
- 4.Schroder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 5.Wohltmann CD, Franklin GA, Boaz PW, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 6.Gannon CJ, Napolitano LM, Pasquale M, et al. A statewide population-based study of gender differences in trauma: validation of a prior single-institution study. J Am Coll Surg. 2002;195:11–18. doi: 10.1016/s1072-7515(02)01187-0. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree TD, Pelletier SJ, Gleason TG, et al. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 8.Wigginton JG, Pepe PE, Idris AH. Rationale for routine and immediate administration of intravenous estrogen for all critically ill and injured patients. Crit Care Med. 2010;38:S620–629. doi: 10.1097/CCM.0b013e3181f243a9. [DOI] [PubMed] [Google Scholar]
- 9.Kauffmann RM, Norris PR, Jenkins JM, et al. Trends in estradiol during critical illness are associated with mortality independent of admission estradiol. J Am Coll Surg. 2011;212:703–712. doi: 10.1016/j.jamcollsurg.2010.12.017. discussion 712-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu HP, Choudhry MA, Shimizu T, et al. Mechanism of the salutary effects of flutamide on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of estrogen receptor-{beta}-dependent HO-1. J Leukoc Biol. 2006;79:277–284. doi: 10.1189/jlb.0705363. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe SM, Qin X, Lu Q, et al. Loss of the Intestinal Mucus Layer in the Normal Rat Causes Gut Injury, but Not Toxic Mesenteric Lymph nor Lung Injury. Shock. 2010 doi: 10.1097/SHK.0b013e3181dc3ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senthil M, Watkins A, Barlos D, et al. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246:822–830. doi: 10.1097/SLA.0b013e3180caa3af. [DOI] [PubMed] [Google Scholar]
- 13.Doucet DR, Bonitz RP, Feinman R, et al. Estrogenic hormone modulation abrogates changes in red blood cell deformability and neutrophil activation in trauma hemorrhagic shock. J Trauma. 2010;68:35–41. doi: 10.1097/TA.0b013e3181bbbddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condon MR, Kim JE, Deitch EA, et al. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol. 2003;284:H2177–2184. doi: 10.1152/ajpheart.01069.2002. [DOI] [PubMed] [Google Scholar]
- 15.Adams JM, Hauser CJ, Adams CA, Jr., et al. Entry of gut lymph into the circulation primes rat neutrophil respiratory burst in hemorrhagic shock. Crit Care Med. 2001;29:2194–2198. doi: 10.1097/00003246-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Magnotti LJ, Fischer PE, Zarzaur BL, et al. Impact of gender on outcomes after blunt injury: a definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. 2008;206:984–991. doi: 10.1016/j.jamcollsurg.2007.12.038. discussion 991-982. [DOI] [PubMed] [Google Scholar]
- 17.Deitch EA, Livingston DH, Lavery RF, et al. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. discussion 453-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 19.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36:62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Condon M, Senthil M, Xu DZ, et al. Intravenous injection of mesenteric lymph produced during hemorrhagic shock decreases RBC deformability in the rat. J Trauma. 2011;70:489–495. doi: 10.1097/TA.0b013e31820329d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananthakrishnan P, Cohen DB, Xu DZ, et al. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Remmers DE, Cioffi WG, Bland KI, et al. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Ann Surg. 1998;227:790–799. doi: 10.1097/00000658-199806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarrar D, Wang P, Knoferl MW, et al. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery. 2000;128:246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 24.Remmers DE, Wang P, Cioffi WG, et al. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–2925. doi: 10.1152/ajpheart.1997.273.6.H2919. [DOI] [PubMed] [Google Scholar]
- 25.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurd TC, Dasmahapatra KS, Rush BF, Jr., Machiedo GW. Red blood cell deformability in human and experimental sepsis. Arch Surg. 1988;123:217–220. doi: 10.1001/archsurg.1988.01400260101012. [DOI] [PubMed] [Google Scholar]
- 27.Baskurt OK, Temiz A, Meiselman HJ. Red blood cell aggregation in experimental sepsis. J Lab Clin Med. 1997;130:183–190. doi: 10.1016/s0022-2143(97)90094-9. [DOI] [PubMed] [Google Scholar]
- 28.Machiedo GW, Powell RJ, Rush BF, Jr., et al. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 29.Machiedo GW, Zaets SB, Berezina TL, et al. Trauma-hemorrhagic shock-induced red blood cell damage leads to decreased microcirculatory blood flow. Crit Care Med. 2009;37:1000–1010. doi: 10.1097/CCM.0b013e3181962d39. [DOI] [PubMed] [Google Scholar]
- 30.Wick TM, Eckman JR. Molecular basis of sickle cell-endothelial cell interactions. Curr Opin Hematol. 1996;3:118–124. doi: 10.1097/00062752-199603020-00003. [DOI] [PubMed] [Google Scholar]
- 31.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302:992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 32.Deitch EA, Senthil M, Brown M, et al. Trauma-shock-induced gut injury and the production of biologically active intestinal lymph is abrogated by castration in a large animal porcine model. Shock. 2008;30:135–141. doi: 10.1097/shk.0b013e318161724f. [DOI] [PubMed] [Google Scholar]
- 33.Senthil M, Brown M, Xu DZ, et al. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958–965. doi: 10.1097/01.ta.0000215500.00018.47. discussion 965-957. [DOI] [PubMed] [Google Scholar]
- 34.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]