Abstract
T cells are required for continuous PTH (cPTH) treatment to induce bone loss as they sensitize SCs to PTH through CD40 Ligand (CD40L), a surface molecule of activated T cells. Since CD40L expression is a feature of activated T cells, we investigated whether antigen (Ag) mediated T cell activation is required for PTH to exert its catabolic activity. We report that inhibition of Ag presentation through silencing of either class I or class II MHC-T cell receptor (TCR) interaction prevents the cortical bone loss induced by in vivo cPTH treatment. We also show that the bone loss and the stimulation of bone resorption induced by cPTH treatment are prevented by CTLA4-Ig, an inhibitor of T cell costimulation approved for the treatment of Rheumatoid Arthritis. Since inhibition of antigen driven T cell activation by blockade of either TCR signaling or T cell costimulation is sufficient to silence the catabolic activity of cPTH, antigen presenting cells and T lymphocyte interactions therefore play a critical role in the mechanism of action of PTH.
Keywords: parathyroid hormone, bone loss, T cell, lymphocyte, bone resorption
INTRODUCTION
Parathyroid hormone (PTH) is a major regulator of calcium metabolism, which defends against hypocalcemia, in part by stimulating bone resorption and thereby the release of calcium from the skeleton. Chronic excessive PTH production is a cause of skeletal and extraskeletal disease. Secondary hyperparathyroidism has been implicated in the pathogenesis of senile osteoporosis (1), while primary hyperparathyroidism (PHP), is associated with accelerated bone loss (2), osteopenia (3), and increased bone turnover (4), an independent risk factor for fractures. Furthermore, PHP is a cause of extra-skeletal manifestations stemming from increased bone resorption such as hypercalcemia, recurrent nephrolithiasis, renal failure, peptic ulcers and mental changes (3).
PHP is modeled by continuous PTH (cPTH) treatment, which leads to cortical bone loss due to an excess of bone resorption over formation (4). The main effect of PHP and cPTH treatment on cortical bone is that of stimulating resorption through an increase in osteoclast (OC) formation and activity (5). The effects on trabecular bone are more complex, as PHP and cPTH administration stimulate both trabecular bone resorption and formation. Depending on PTH levels, the net effect is a modest anabolic effect, or a catabolic activity (6–10).
Both the catabolic and the anabolic activities of PTH result from its binding to the PTH/PTH-related protein (PTHrP) receptor (PPR or PTH-1R), expressed on bone marrow (BM) SCs and their osteoblastic progeny (11,12). The catabolic effect of PTH has been shown to be mediated, in part, by enhanced production of RANKL and decreased production of OPG by SCs and osteoblasts (OBs) (13–15). Others have suggested a role for SC/OB produced IL-6 (16,17), although more recent investigations concluded that IL-6 is not required for the OC formation and bone loss that accompanies continuous elevation of PTH (18). PTH has also been shown to stimulate the production of TNFα by cells of the osteoblastic lineage (19), but the role of this cytokine as mediator of the catabolic effect of PTH in vivo remains to be determined.
The notion that PTH stimulates OC formation and bone resorption by modulating the osteoblastic production of pro- and anti-osteoclastogenic factors derives primarily from studies showing that PTH increases bone resorption when isolated osteoblasts are placed in close proximity to purified OC precursors or mature OCs (17,20–22). However, additional cell lineages contribute to the catabolic activity of PTH in vivo. Among them are T lymphocytes, a lineage now known to regulate bone homeostasis through secretion of pro- and anti-osteoclastogenic factors (23–25). T cells also express ligands for costimulatory molecules found on the surface of cells of the osteoblastic lineage (26). Furthermore, T cells express PPR (27,28,29), and PTH is known to activate T cell intracellular signaling (30) and T cell proliferation (31).
We have recently reported that cPTH treatment at doses which elevate serum PTH to levels typical of patients with PHP, stimulates OC formation and induces cortical bone loss only in the presence of T cells (32). We have also show that T cells increase the capacity of SCs to support PTH induced osteoclastogenesis through the CD40L/CD40 signaling system (32).
Our previous studies have disclosed that PTH does not increase antigen (Ag) presentation and T cell activation. However, since the expression of CD40L is a feature of activated T cells, it is likely that baseline Ag presentation, which leads to spontaneous activation of T cells in the bone marrow (BM), may be required for PTH to induce its catabolic effect. Indeed the BM contains a relative large number of memory T cells which have increased reactivity to self peptides and foreign Ag (33). T cell activation takes place in the presence of several signals. The first is the presentation to the T cell receptor (TCR) of Ag derived peptides bound to MHC molecules which are expressed on the surface of Ag presenting cells (APCs). A second set of signals is provided by the interaction of the costimulatory molecules on APCs with the T cell expressed counter receptors such as CD28 and CD40L.
In the present study we investigated the role of Ag presentation in cPTH induced bone loss. We report that cPTH failed to induce bone loss in mice lacking Ag presentation due to impaired MHC - TCR interaction, and in mice treated with the inhibitor of co-stimulation Abatacept (CTLA4-Ig).
RESULTS
1. Silencing of antigen presentation prevents PTH induced bone loss
We have found (data not shown) that cPTH does not increase Ag presentation, but we hypothesize that activated T cells which physiologically reside in the BM are required for PTH to exert its catabolic activity. Thus, neutralization of baseline antigen dependent T cell activation should prevent PTH induced bone loss.
To determine whether PTH requires APC-T cell interactions in order to cause bone loss, cPTH was infused for 2 weeks as previously described (32) into OT-I/RAG1, OT-II/RAG1 and WT mice. At sacrifice femurs were harvested and analyzed by μCT. OT-I/RAG1 and OT-II/RAG1 mice are characterized by impaired by MHC class I - TCR interaction and MHC class II-TCR interaction, respectively. We found that infusion of cPTH for 2 weeks induced significant loss of femoral cortical thickness and cortical volume in WT control mice. In contrast, both OT-I/RAG1, OT-II/RAG1 mice were completely protected against PTH induced bone loss (fig 1). These findings indicate that silencing of Ag presentation to either CD4+ cells or CD8+ cells is sufficient to completely block the capacity of cPTH to induce cortical bone loss.
Figure 1.
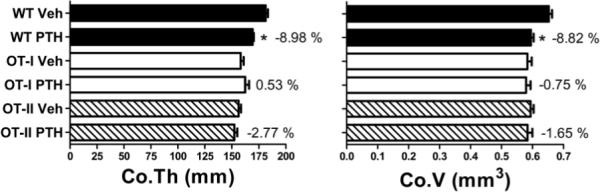
Effect (Mean ± SEM) of in vivo treatment with cPTH infusion (80 μg/kg/day) for 2 weeks in WT, OT-I/RAG1 and OT-II/RAG1 mice. Figure shows femoral cortical thickness and cortical volume. (n = 10 mice per group). * = p <0.05 compared to the corresponding vehicle treated group.
2. Treatment with CTLA-4 Ig prevents the bone loss and the increase in bone resorption induced by PTH
In the context of Ag recognition, the interaction of the surface costimulatory molecules CD80 and CD86 on Ag presenting cells with the costimulatory receptor CD28 expressed by T cells is required for T cell activation. Abatacept (CTLA-4 Ig) is a human fusion protein combining the extracellular portion of cytotoxic T lymphocyte Ag 4 (CTLA-4) with the constant-region of human IgG1 (34). CTLA-4 Ig binds to human and murine CD80 and CD86 blocking their interaction with CD28, promoting anergy and T cell apoptosis (35). Abatacept also blunts the expression of CD40L in T cells. Therefore, CTLA-4 Ig is a potent suppressor of T cell activation in vivo (34). WT mice were injected with either CTLA-4 Ig (100 μg IP 3 times a week) or irrelevant Ig for one week. Mice were also treated for 2 weeks with either PTH (80μg/kg/day) or vehicle, staring 3 days after the first CTLA-4 Ig injection. Femurs were harvested at sacrifice and analyzed by μCT. These studies showed that CTLA-4 Ig treatment prevented the decrease in femur cortical bone thickness and volume (fig 2) induced by PTH infusion. Furthermore, whereas in vivo CTLA-4 Ig treatment prevented PTH induced increase in serum levels of CTX, a marker of bone resorption, it did not inhibit PTH induced increase in serum levels of osteocalcin, a marker of bone formation (fig 2). These findings indicate that the presence of activated T cells is required for cPTH to stimulate bone resorption and cause cortical bone loss.
Figure 2.
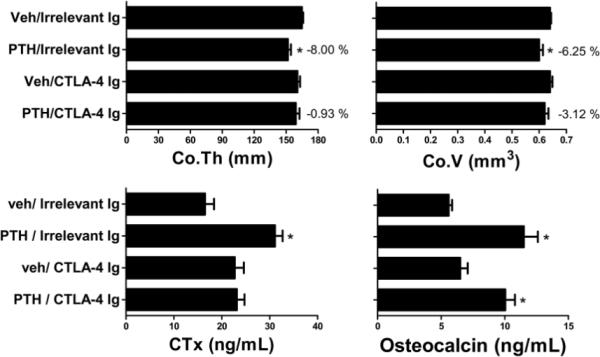
Effect (Mean ± SEM) of in vivo treatment with cPTH infusion (80 μg/kg/day) for 2 weeks in WT mice treated with CTLA-4 Ig or irrelevant Ig for 1 week. Figure shows femoral cortical thickness, cortical volume, serum CTX and serum osteocalcin. (n = 10 mice per group). * = p <0.05 as compared to vehicle treated groups and to PTH/CTLA-4 Ig group
DISCUSSION
This study was designed to determine whether Ag driven T cell activation is required for cPTH treatment to induce cortical bone loss. The results demonstrate that arrest of Ag presentation through silencing of class I and class II MHC-TCR interactions, or blockade of costimulation, prevents the capacity of cPTH to induce cortical bone loss. These findings provide further evidence of a novel regulatory link between the immune system and the mechanism of action of PTH.
The presentation of antigenic peptides to the TCR was prevented using a genetic approach. The T cells of both the OT-I/RAG1 mouse and the OT-II/RAG1 mouse express exclusively a TCR specific for ovalbumin, an antigen not present in mammals. In fact, the OT-I/RAG1 mouse is homozygous for a transgene that encodes a T-cell receptor specific for chicken ovalbumin 257–264 presented by the MHC class I molecule H-2Kb. It is also deficient in the RAG1 gene and therefore does not develop mature T or B cells expressing endogenous receptors. OT-I/RAG1 mice lack CD4+ cells and virtually all peripheral CD8+ cells exhibit a single TCR specificity toward the foreign Ag chicken albumin (ovalbumin) (36). The OT-II2/RAG1 line is homozygous for a transgene that encodes a T-cell receptor specific for chicken ovalbumin 323–339 presented by the MHC class II molecule I-Ab. It is also deficient in the Rag1 gene and therefore does not develop endogenous mature T or B cells. Importantly, OT-II/RAG1 mice lack CD8+ cells and all peripheral CD4+ cells exhibit a single TCR specificity toward ovalbumin (37).
T cell costimulation was instead blocked using a pharmacological approach. Abatacept is an agent approved for the treatment of Rheumatoid Arthritis which has also been shown to block the bone loss induced by ovariectomy (38). The finding that PTH induced bone loss may be prevented using an inhibitor of costimulation may lay the foundation for a new pharmacological approach to the management of hyperparathyroidism.
Our investigation was limited to an analysis of cortical bone because continuous delivery of PTH into young mice does not cause trabecular bone loss. However, cortical bone represents about 80 % of the entire skeletal mass (39) and cortical volume and thickness are major predictors of bone strength and fracture risk (40). Thus, the T cell dependent bone effects of PTH are relevant for the risk of long bone fractures associated with primary and secondary hyperparathyroidism (1–4)
Previous studies have shown that T cells play a pivotal role in stimulating OC formation and inducing bone loss in autoimmune diseases (41), periodontal bone disease (42) estrogen deficiency (23) and cPTH treatment (32). The current investigation demonstrates the key role of Ag presentation in a model of hyperparathyroidism, reinforcing the evidence that that T cells may be central for stimulating OC formation above baseline both in inflammatory and hormone mediated bone loss. However, T cells mediate PTH induced bone loss by regulating SC function through a membrane bound signaling molecule, while they cause other forms of bone loss by secreting osteoclastogenic cytokines.
The BM has long been recognized as a primary lymphoid organ, but it is now clear that the BM is also a secondary lymphoid organ that plays a key role in the immune response by hosting and regulating adaptive immunity. Many T cell clones present in lymphatic organs including the BM are reactive to self Ag. These clones survive negative selection in the thymus because the self Ag to which they respond is not expressed in the thymus (43–46). In the BM and other lymphoid organs self Ag can be presented to T cells, and induce low grade T cell activation, typically without causing any autoimmune pathology (47). Thus, a low number of activated T cells is physiologically present in healthy humans and rodents due to presentation by MHCII and MHCI molecules of both self and foreign peptides to CD4+ and CD8+ cells (48). This autoreactive response may be beneficial, and could promote immune cell survival and renewal (49) as well as enhance the sensitivity of mature T cells to foreign Ag (50,51).
The demonstration that the process of co-stimulation is required for the catabolic activity of PTH extends our previous finding regarding the relevance of the CD40L/CD40, which is another pair of costimulatory molecules involved in the mechanism of action of PTH. Indeed one feature of activated T cells is the expression of CD40L, a ligand for the costimulatory molecule CD40, which is expressed on APCs and cells of the osteoblastic lineage (26). CD40L provides proliferative and survival cues to stromal cells (SCs) and sensitize SCs to PTH.
In summary, our findings demonstrate that Ag presentation is required for cPTH to induce bone loss. Inhibition of Ag presentation by blockade of either MHC/TCR interactions or costimulatory molecules may thus represent novel therapeutic targets for hyperparathyroidism.
METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. Female WT mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained under sterile conditions. OT-I/RAG1 (C57BL/6-Tg(OT-I)-RAG1tm1Mom) mice and OT-II/RAG1 (C57BL/6-TgN(OT-II.2a)-RAG1tm1Mom) were purchased from Taconic Farms.
In vivo PTH infusion
ALZET osmotic pump model-1002 (DURECT Corporation, Cupertino, CA) were implanted subcutaneously in female mice of 10 weeks of age. The pumps contained either hPTH1–34 (Bachem California Inc., Torrance, CA) or vehicle. PTH was delivered at a dose of 80 μg/kg/day for 2 weeks by implanting a single pump with a delivery rate of 0.25 μl/hr, containing 16.2 pM of hPTH1–34.
CTLA4-Ig treatment
WT mice were injected IP with CTLA4-Ig (kindly provided by Dr. Robert Mittler, Emory University) or irrelevant Ig, at a dose of 100 μg per mouse, three times a week for one week, starting 3 days before PTH or vehicle infusion.
μCT measurements of cortical bone
μCT scanning and analysis was performed as reported previously (52) by a technician blind to grouping of animals, using a Scanco μCT-40 scanner (Scanco Medical, Bassersdorf, Switzerland). Bones were scanned at a resolution of 12 μm, tomographic images were obtained at conditions of 70 KV and 114 μA by collecting 500 projections. Cortical bone volume and cortical thickness were determined by analyzing 80 slices at the mid –diaphysis of the femurs.
Measurement of serum markers of bone turnover
Serum C-terminal telopeptide of collagen (CTX), a marker of bone resorption, was measured by a rodent specific ELISA assay (Immunodiagnostic Systems Inc. Fountain Hills AZ). Serum osteocalcin, a specific marker for bone formation, was measured using Rat-MID™ Osteocalcin ELISA kit ((Immunodiagnostic Systems Inc. Fountain Hills AZ).
Acknowledgement
This study was supported by a grant from the National Institutes of Health (AR54625)
REFERENCES
- 1.Riggs BL, Melton LJ. Medical progress: involutional osteoporosis. N. Eng. J. Med. 1986;314:1676–1684. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 2.Grey AB, Stapleton JP, Evans MC, Reid IR. Accelerated bone loss in post-menopausal women with mild primary hyperparathyroidism. Clin Endocrinol (Oxf) 1996;44(6):697–702. doi: 10.1046/j.1365-2265.1996.744565.x. [DOI] [PubMed] [Google Scholar]
- 3.Potts J. Primary hyperparathyroidism. In: LVAaS Krane., editor. Metabolic Bone Diseases. #rd ed. vol. 1. Academic Press; San Diego: 1998. pp. 411–442. [Google Scholar]
- 4.Parisien M, Dempster DW, Shane E, Bilezikian JP. Basic and clinical concepts. Academic Press; San Diego: 2001. Histomorphometric analysis of bone in primary hyperparathyroidism The parathyroids; pp. 423–436. [Google Scholar]
- 5.Guo CY, Thomas WE, al-Dehaimi AW, Assiri AM, Eastell R. Longitudinal changes in bone mineral density and bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81(10):3487–91. doi: 10.1210/jcem.81.10.8855790. [DOI] [PubMed] [Google Scholar]
- 6.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1–34) on rat bone. Bone. 1995;16(4):477–84. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 7.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7(1):65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Shen V, Dempster DW, Lindsay R. Continuous parathyroid hormone and estrogen administration increases vertebral cancellous bone volume and cortical width in the estrogen-deficient rat. J Bone Miner Res. 2001;16(7):1300–7. doi: 10.1359/jbmr.2001.16.7.1300. [DOI] [PubMed] [Google Scholar]
- 9.Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 1999;84(5):1562–6. doi: 10.1210/jcem.84.5.5652. [DOI] [PubMed] [Google Scholar]
- 10.Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, Dempster DW, Lindsay R. Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol. 2005;186(3):549–57. doi: 10.1677/joe.1.06270. [DOI] [PubMed] [Google Scholar]
- 11.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277–86. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15(2):60–5. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology. 1999;140(8):3552–61. doi: 10.1210/endo.140.8.6887. [DOI] [PubMed] [Google Scholar]
- 14.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–54. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 15.Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem. 2003;89(1):180–90. doi: 10.1002/jcb.10490. [DOI] [PubMed] [Google Scholar]
- 16.Grey A, Mitnick MA, Masiukiewicz U, Sun BH, Rudikoff S, Jilka RL, Manolagas SC, Insogna K. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology. 1999;140(10):4683–90. doi: 10.1210/endo.140.10.7036. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield EM, Shaw SM, Gornik SA, Banks MA. Adenyl cyclase and interleukin 6 are downstream effectors of parathyroid hormone resulting in stimulation of bone resorption. J Clin Invest. 1995;96(3):1238–44. doi: 10.1172/JCI118157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien CA, Jilka RL, Fu Q, Stewart S, Weinstein RS, Manolagas SC. IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice. Am J Physiol Endocrinol Metab. 2005;289(5):E784–93. doi: 10.1152/ajpendo.00029.2005. [DOI] [PubMed] [Google Scholar]
- 19.Grey A, Mitnick MA, Shapses S, Ellison A, Gundeberg C, Insogna K. Circulating levels of Intreleukin-6 and tumor necrosis factor α are elevated in primary hyperparathyroidism and correlate with markers of bone resorption. J.Clin.Endo.Metab. 1996;81:3450–3468. doi: 10.1210/jcem.81.10.8855783. [DOI] [PubMed] [Google Scholar]
- 20.McSheehy PM, Chambers TJ. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology. 1986;118(2):824–8. doi: 10.1210/endo-118-2-824. [DOI] [PubMed] [Google Scholar]
- 21.McSheehy PM, Chambers TJ. Osteoblast-like cells in the presence of parathyroid hormone release soluble factor that stimulates osteoclastic bone resorption. Endocrinology. 1986;119(4):1654–9. doi: 10.1210/endo-119-4-1654. [DOI] [PubMed] [Google Scholar]
- 22.Evely RS, Bonomo A, Schneider HG, Moseley JM, Gallagher J, Martin TJ. Structural requirements for the action of parathyroid hormone-related protein (PTHrP) on bone resorption by isolated osteoclasts. J Bone Miner Res. 1991;6(1):85–93. doi: 10.1002/jbmr.5650060114. [DOI] [PubMed] [Google Scholar]
- 23.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–68. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum SL. Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc Natl Acad Sci U S A. 2004;101(48):16711–2. doi: 10.1073/pnas.0407335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–27. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144(5):1761–9. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- 27.McCauley LK, Rosol TJ, Merryman JI, Capen CC. Parathyroid hormone-related protein binding to human T-cell lymphotropic virus type I-infected lymphocytes. Endocrinology. 1992;130(1):300–6. doi: 10.1210/endo.130.1.1309334. [DOI] [PubMed] [Google Scholar]
- 28.Shurtz-Swirski R, Shkolnik T, Shasha SM. Parathyroid hormone and the cellular immune system. Nephron. 1995;70(1):21–4. doi: 10.1159/000188538. [DOI] [PubMed] [Google Scholar]
- 29.Perry HM, 3rd, Chappel JC, Bellorin-Font E, Tamao J, Martin KJ, Teitelbaum SL. Parathyroid hormone receptors in circulating human mononuclear leukocytes. J Biol Chem. 1984;259(9):5531–5. [PubMed] [Google Scholar]
- 30.Stojceva-Taneva O, Fadda GZ, Smogorzewski M, Massry SG. Parathyroid hormone increases cytosolic calcium of thymocytes. Nephron. 1993;64(4):592–9. doi: 10.1159/000187406. [DOI] [PubMed] [Google Scholar]
- 31.Geffner ME, Bersch N, Cortez AB, Bailey RC, Golde DW. Growth-promoting actions of parathyroid hormone, adrenocorticotrophic hormone, and thyroid-stimulating hormone: in vitro studies in normal and pygmy T-lymphoblast cell lines. Pediatr Res. 1995;37(4 Pt 1):507–11. doi: 10.1203/00006450-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y, Wu X, Terauchi M, Li JY, Grassi F, Galley S, Yang X, Weitzmann MN, Pacifici R. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab. 2008;8(2):132–45. doi: 10.1016/j.cmet.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Rosa F, Santoni A. Memory T-cell competition for bone marrow seeding. Immunology. 2003;108(3):296–304. doi: 10.1046/j.1365-2567.2003.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257(5071):792–5. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 35.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338(25):1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 36.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 37.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 38.Grassi F, Tell G, Robbie-Ryan M, Gao Y, Terauchi M, Yang X, Romanello M, Jones DP, Weitzmann MN, Pacifici R. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci U S A. 2007;10438:15087–92. doi: 10.1073/pnas.0703610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 40.Cheng X, Li J, Lu Y, Keyak J, Lang T. Proximal femoral density and geometry measurements by quantitative computed tomography: Association with hip fracture. Bone. 2006 doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 42.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001;12(2):125–35. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 43.Robey EA, Ramsdell F, Gordon JW, Mamalaki C, Kioussis D, Youn HJ, Gottlieb PD, Axel R, Fowlkes BJ. A self-reactive T cell population that is not subject to negative selection. Int Immunol. 1992;4(9):969–74. doi: 10.1093/intimm/4.9.969. [DOI] [PubMed] [Google Scholar]
- 44.Tanchot C, Rocha B. Peripheral selection of T cell repertoires: the role of continuous thymus output. J Exp Med. 1997;186(7):1099–106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossman Z. Cellular tolerance as a dynamic state of the adaptable lymphocyte. Immunol Rev. 1993;133:45–73. doi: 10.1111/j.1600-065x.1993.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272(5258):54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 47.Rammensee HG, Falk K, Rotzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–44. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 48.Grossman Z, Paul WE. Self-tolerance: context dependent tuning of T cell antigen recognition. Semin Immunol. 2000;12(3):197–203. doi: 10.1006/smim.2000.0232. discussion 257–344. [DOI] [PubMed] [Google Scholar]
- 49.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276(5321):2057–62. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 50.Kondo T, Cortese I, Markovic-Plese S, Wandinger KP, Carter C, Brown M, Leitman S, Martin R. Dendritic cells signal T cells in the absence of exogenous antigen. Nat Immunol. 2001;2(10):932–8. doi: 10.1038/ni711. [DOI] [PubMed] [Google Scholar]
- 51.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420(6914):429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 52.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117(1):122–32. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]


