Abstract
Background:
Epidemiologic studies have suggested different prevalence of neuromyelitis optica (NMO) in different ethnic groups. However, data on the incidence and prevalence of NMO in Caucasians are scarce.
Objective:
To estimate the incidence and prevalence of NMO in a predominantly Caucasian population based on the Wingerchuk 2006 criteria.
Methods:
The study was a population-based retrospective case series with longitudinal follow-up. Patients with multiple sclerosis (MS), optic neuritis (ON), acute transverse myelitis (TM), and NMO from the 4 neurology and 3 ophthalmology departments in the Region of Southern Denmark having been diagnosed between 1998 and 2008 were investigated. Patients were included based on 1) episodes of ON or TM and 2) an initial brain MRI not diagnostic for MS. An immunofluorescence assay was used to determine aquaporin-4 (AQP-4) antibodies.
Results:
A total of 477 patients with MS, TM, or ON were evaluated: 163 fulfilled the inclusion criteria, 42 (26%) qualified for the diagnosis of NMO, 26 (62.0%) of these were AQP4 antibody positive. All except one were Caucasian, the female:male ratio was 2.8:1, and mean age at onset was 35.6 years (range 15–64 years). The clinical presentation was heterogeneous including TM, longitudinally extensive TM, ON, and brainstem syndromes. The yearly incidence rate of NMO in the population was estimated to be 0.4 per 105 person-years (95% confidence interval [CI] 0.30–0.54) and the prevalence was 4.4 per 105 (95% CI 3.1–5.7).
Conclusions:
Despite being a rare disease, NMO is more common in a Caucasian population than earlier believed.
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease (IDD) of the CNS and probably the most common IDD apart from multiple sclerosis (MS).1–3 NMO is considered to be a rare disorder in Caucasians, but this view is based on few studies with small patient populations from tertiary hospitals.4–7 No population-based studies have been carried out so far in Caucasians.
The main clinical features of NMO consist of optic neuritis (ON) and acute transverse myelitis (TM). Longitudinally extensive TM (LETM) or more limited TM from the cervical spine reaching into the brainstem is regarded as typical for NMO.2,8–11 NMO follows a relapsing course in 80%–90% of cases, is more common in females, and is associated with older age. Serum immunoglobulin G (IgG) aquaporin 4 (AQP4) antibodies have been shown to be a highly specific (85–99%) but less sensitive (58–76%) serum biomarker for NMO.12–14 Based on AQP4 antibody determinations, NMO has recently been recognized to have a more heterogeneous clinical presentation, including clinical signs or lesions in the CNS outside of the optic nerve and spinal cord.11,15,16
NMO has a poor prognosis so early diagnosis based on robust criteria is critical. Several diagnostic criteria have been suggested, notably the Wingerchuk criteria,5,11 and the US National Multiple Sclerosis Society (NMSS) criteria.17
The aim of the present study was to estimate the incidence and prevalence of NMO in the Region of Southern Denmark based on the Wingerchuk 2006 criteria.11
METHODS
Setting.
The Region of Southern Denmark is one of 5 administrative units in Denmark established January 1, 2007, with a 12,191 km2 area and a geographically well-defined population. The adult population (≥15 years of age) of the Region in 2006–2008 was 952,000. Of the total population, 94.1% were ethnic Danes and 5.9% were immigrants.
The Region has 4 hospital units with neurology departments including MS clinics to which all patients with demyelinating disorders of the CNS are referred from private practice (neurologists and general practitioners). Treatment is free of charge for the patient. The Region had 4 ophthalmology departments at the time, but information could only be obtained from 3 as one department closed and their patients were referred to the other departments in the Region.
Data sources.
As the primary data source the neurology and ophthalmology departments of the Region were asked to report patients who obtained a diagnosis of MS, NMO, TM, or ON during the time period January 1, 1998–December 31, 2008. A separate registration for patients with MS treated with biological therapy (natalizumab) was available from the neurologic departments because this treatment is centralized to university hospitals. These data were used as a supplementary source of information.
All Danish citizens are identified by a unique personal identification number facilitating a cross-check of data with information from The Danish National Patient Registry (DNPR) where all hospital visits including outpatient contacts are registered. Residents in the Region of Southern Denmark who during the time period 1998–2008 appeared in DNPR with a diagnosis of MS, ON, TM, or NMO (WHO ICD-10 codes: G 37.3, G35, G360, H46.9) were identified.
Study population.
The study population was established based on the following inclusion criteria: 1) episodes of ON or TM and 2) an initial brain MRI (obtained within the first year of the onset of symptoms) that did not meet diagnostic criteria for MS at disease onset (McDonald dissemination in space criteria).18,19 A total of 99.2% of the patient population were Caucasian.
Study design.
The study was a population-based retrospective consecutive case series with longitudinal follow-up. Information was provided from a questionnaire, a clinical examination, reevaluation of all MRIs, supplementary MRIs, visual evoked potentials (VEP), and serum AQP4 antibody determination.
Diagnosis.
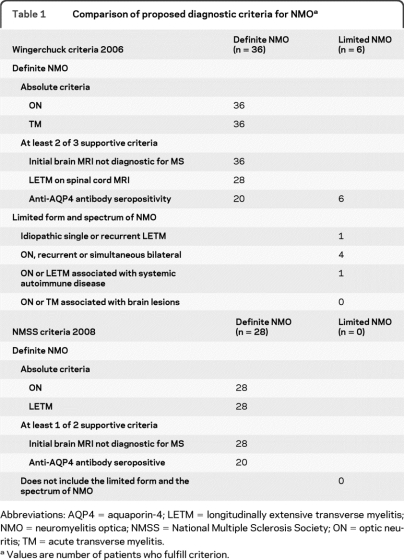
The diagnosis of NMO was based on the criteria of Wingerchuk et al.11 For comparison, the diagnostic criteria for NMO from the NMSS17 were used (table 1). The diagnostic process was performed in 3 independent parts.
Table 1.
Comparison of proposed diagnostic criteria for NMOa
Abbreviations: AQP4 = aquaporin-4; LETM = longitudinally extensive transverse myelitis; NMO = neuromyelitis optica; NMSS = National Multiple Sclerosis Society; ON = optic neuritis; TM = acute transverse myelitis.
Values are number of patients who fulfill criterion.
The clinical files were collected and analyzed; the patients completed a questionnaire and underwent a clinical examination by a neurologist. The neurologist was blinded to anti-AQP4 results and the reevaluated and supplementary MRIs of the CNS at this stage.
Prestudy MRIs at disease onset and subsequent MRIs were reevaluated by the neuroradiologist. Supplementary MRI was performed if unavailable or if a relapse was suspected since the last MRI. If brain MRI at disease onset was unavailable the supplementary MRI was used provided it was negative for MS-like changes. Brain MRIs were reported as normal, as nonspecific, or as MS-like, i.e., meeting the Barkhof criteria18 for dissemination in space used in the McDonald criteria.19 Spinal cord MRI were reported as normal, as LETM (cord lesion extending 3 or more vertebral segments) on T2-weighted MRI and hypointensities on T1-weighted images when obtained during acute episodes of myelitis, or abnormal with a smaller lesion not suggestive of NMO.11,20 The neuroradiologist had no prior knowledge of clinical history or results of other investigations. MRI data were reported in a written form by the neuroradiologist. Integration of clinical data and imaging was made by the neurologist and established the clinical NMO diagnosis.
Anti-AQP4 antibodies were determined. The laboratory staff was blinded to the clinical status of patients when performing the assay.
The final diagnosis of NMO was based on the clinical data and anti-AQP-4 antibody positivity.
Laboratory methods.
IgG AQP4 antibodies were measured with a recombinant immunofluorescence assay using HEK293 cells transfected with recombinant human full-length AQP4 gene (Euroimmun, Lubeck, Germany). Patient sera were screened at a 1:10 dilution. Analyses were done in an accredited laboratory at the Department of Clinical Immunology, Odense University Hospital.
Statistical methods.
Incidence rates were calculated as the number of patients with NMO during the follow-up period divided by the total numbers of person-years at risk and reported per 105 person-years. Prevalence was estimated as a percentage in the IDD groups and as number of cases per 105 persons in the total population. Means, medians, and ranges were calculated and 95% confidence intervals (CI) were estimated. All data analyses were performed in STATA version 11.
Standard protocol approvals, registrations, and patient consents.
The study was approved by The Committee on Biomedical Research Ethics for the Region of Southern Denmark (ref. no. S-20080142) and The Danish Data Protection Agency (ref. no. 2008–41-2826). All patients provided written informed consent.
RESULTS
Patient ascertainment.
A total of 477 cases (277 MS, 8 NMO, 128 ON, 64 TM) were ascertained including 66 patients with MS treated with natalizumab, who were not listed in the primary reports. All these diagnoses were confirmed in the data list extracted from the DNPR. Research protection was registered for 42 patients (9 MS, 22 ON, 11 TM) who were not approached. The rest of the patients were contacted by means of questionnaire (response rate 70%). A total of 42 patients did not want to participate in the study (16 MS, 19 ON, 7 TM). The inclusion criteria were not met in 166 patients with MS (including 91 patients who had been diagnosed before the inclusion period), 3 patients with NMO, 43 patients with ON, and 18 patients from the TM group, leaving 163 patients (86 MS, 5 NMO, 44 ON, 28 TM) who fulfilled the inclusion criteria. These 163 patients all participated fully in the study except one patient with NMO who died before clinical examination and blood sampling for the study were performed. Two patients with NMO died after completion of the examinations.
Diagnosis.
Flow chart of the diagnosis of NMO in the study is depicted in the figure.
Figure. Flow chart of the diagnosis of neuromyelitis optica (NMO) in the study.
The figure shows the diagnostic process of the selected study population. The clinical data and the imaging data were prepared to establish the clinical NMO diagnosis without knowledge of the aquaporin-4 (AQP4) antibody serologic status. The AQP4 antibodies were measured without knowledge of the clinical status. As an endpoint, the final diagnosis of NMO was made. *Number of patients where the diagnosis depended on AQP4 antibody positivity. MS = multiple sclerosis; ON = optic neuritis; TM = transverse myelitis.
MS.
Of the 86 patients from the at-risk MS group, 28 (33%) were classified as NMO, 19 on purely clinical grounds based on the association of ON, LETM, and absence of brain changes suggestive of MS,5 and 9 additionally with positive anti-AQP4 antibodies. A total of 54% of patients with NMO from the MS group were positive for serum anti-AQP4 antibodies.
Previously diagnosed NMO.
The 5 patients with NMO diagnosed during 1998–2008 all had definite NMO. Three were positive for anti-AQP4 antibodies. Two patients were previously found positive for anti-AQP4 antibodies as measured by another assay (Seelig Labs, Karlsruhe, Germany). One died before blood sampling and one, who received azathioprine therapy, was negative for anti-AQP4 antibodies when analyzed in the present study.
TM.
The TM group consisted of 28 patients. Of those, 13 had single TM, 7 had LETM, and 9 had relapsing TM. Four were classified as having NMO based on positive anti-AQP4 antibodies, 3 LETM, and one recurrent TM. Two of the patients with LETM developed ON later during the study period and were reclassified as definite NMO. The patient with recurrent TM developed bilateral ON and later a cervical LETM, which led to respiratory failure and death.
ON.
Of the 44 patients with ON, 28 had single ON, 12 recurrent ON, and 4 bilateral ON. Five cases—3 from the recurrent ON group, one from the bilateral ON group, and one from the single ON group—were classified as having NMO limited form based on AQP4 antibody positivity. The case with single ON was associated with rheumatoid arthritis.
Final NMO diagnosis.
A total of 42 patients were classified as having NMO, 36 with definite and 6 with limited form of NMO according to the Wingerchuk 2006 criteria (table 1).5 Demographic features of patients with NMO are illustrated in table 2. All were Caucasians except for one patient of African descent. The group consisted of 31 females and 11 males (ratio 2.8:1). Mean age at onset was 35.6 years (range 15–64 years). When the NMSS diagnostic criteria for NMO15 were applied, 28 of the patients also fulfilled these criteria. The 14 patients who did not fulfill the NMSS criteria were all AQP4 antibody–positive including 8 patients from the MS group who did not have LETM and 6 from the limited NMO group.
Table 2.
Demographic features of patients with NMO
Abbreviations: BON = bilateral optic neuritis; LETM = longitudinally extensive TM; MS = multiple sclerosis; NMO = neuromyelitis optica; PNMO = previously diagnosed neuromyelitis optica; RON = recurrent optic neuritis; RTM = recurrent TM; TM = acute transverse myelitis.
Incidence and prevalence.
The prevalence of NMO in the at-risk MS group was 33%. In the total MS group (including patients with MS who did not fulfill the inclusion criteria), the prevalence was 17%. In the total number of patients with MS, TM, and ON, the prevalence was 26%. The yearly incidence rate of NMO in the population was estimated at 0.4 per 105 person-years (95% CI 0.30–0.54) and the prevalence was 4.4 per 105 (95% CI 3.1–5.7).
Clinical manifestations.
Clinical characteristics are depicted in table 3. At disease onset, 24 of the 36 patients with definite NMO had TM, 15 of whom had evaluable MRI at spinal cord; 6 (40%) of these had LETM. At follow-up, 28 of the 36 (78%) had had events of LETM. Five patients had cervical TM that led to respiratory failure and intractable hiccups and nausea; 3 died during the study period. Brainstem symptoms occurred in 2 at disease onset, increasing to 26 (72%) at follow-up.
Table 3.
Clinical characteristics of patients with NMOa
Abbreviations: BON = bilateral optic neuritis; BS = brainstem symptoms; EDSS = Expanded Disability Status Scale; LETM = longitudinally extensive transverse myelitis; MS = multiple sclerosis; NMO = neuromyelitis optica; ON = optic neuritis; TM = acute transverse myelitis; VEP = visual evoked potentials.
Values are n (%) of patients.
Three asymptomatic cases had VEP abnormalities.
All patients with definite NMO followed a relapsing course. A total of 34 (81%) patients were in remission at the time of investigation. Patients with NMO were assigned Expanded Disability Status Scale (EDSS) score (table 3). Three patients died, at 63, 56, and 47 years of age, during the study period.
CSF.
Complete CSF analysis was available at disease onset for 32 of the 42 patients. CSF abnormalities were detected in 27 (table 3).
MRI-CNS.
The Regional Health Service was not obligated by law to keep radiologic investigations for more than 5 years and reports of radiologic examinations for more than 10 years, so a significant proportion of MRIs at disease onset were unavailable. Supplementary MRI of brain was performed in 58 and supplementary MRI of spinal cord in 108 patients. All patients who were diagnosed with definite or limited NMO in the study had evaluable MRI of brain at disease onset. Characteristics of the brain MRI in patients with NMO are described in table 3. Spinal cord MRI demonstrated LETM in 29 (69%) of the patients in the NMO group. Cervical LETM was present in 20 (69%), of those 7 (35%) reached into the brainstem. Thoracic TM occurred in 9 (31%) cases.
Treatment of NMO group.
At the time of diagnosis, all patients with NMO from the MS group received immunosuppressive and immunomodulatory reagents: natalizumab 15 (36%), interferon beta 6 (14%), azathioprine 5 (12%), and rituximab 1.
Anti-AQP4 antibodies.
Anti-AQP4 antibodies were positive in 26/42 (62%) patients. Antibody positivity was necessary to confirm the diagnosis in 15 cases (36%), whereas 27 (64%) were diagnosed solely on clinical criteria. Fifty patients with MS were examined clinically and radiologically verifying the MS diagnosis and were used as disease controls together with 50 healthy controls. None were positive for anti-AQP4 antibodies.
DISCUSSION
In this study including longitudinal follow-up for a decade we found a yearly incidence rate of NMO in the Region of Southern Denmark of 0.4 per 105 person-years (95% CI 0.30–0.54) and a prevalence of 4.4 per 105 (95% CI 3.1–5.7). The study estimated the incidence and prevalence of NMO in a Caucasian population.
Among the patients with the most common IDD, the prevalence of NMO was 26% (95% CI 19%–32%). In the total MS group, the prevalence was 17%, and in the at-risk MS group 33%. These data are at variance with a multicenter study from Italy21 of NMO in patients with IDD, reporting a low prevalence of NMO of 1.5% (95% CI 0.7–2.4) with an MS: NMO ratio of 42.7. The study was not population-based and it is not clear to what extent NMO antibodies (NMO-IgG) was used in the initial diagnosis.
In our study, the diagnosis of NMO by the Wingerchuk 2006 criteria11 could be made purely on clinical grounds in a high proportion of cases. Similarly, a study from France22 using the same criteria found that clinical and MRI-based diagnosis of definite NMO was sufficient in 90% of 125 patients with NMO. Furthermore, the clinical phenotype in our study was similar to the results from these 2 studies21,22 including the high female:male ratio and the clinical heterogeneity of NMO with TM, LETM, ON, and brainstem syndromes. Danish NMO cases are thus comparable to other Caucasian patients with NMO with regard to the gender distribution and clinical manifestations.
Low sensitivity of AQP4 antibody determination has been observed previously.12,14,21,22 Antibody titers are probably influenced both by the clinical status and immunosuppressive treatment, and most of the patients in the present study were in remission or on immunomodulatory or immunosuppressive treatment. All 8 patients who had a clinical relapse during the study period were seropositive for anti-AQP4 antibodies. The MS controls and the healthy controls were uniformly negative for AQP4 antibodies. Thus, the present study confirms a high diagnostic specificity and moderate to low sensitivity of anti-AQP4 antibody determination.
A possible limitation of this study was the fact that a lower than expected number of MS cases were reported from the clinical departments. The low number could be due to misclassification of MS as a clinically isolated syndrome, as inflammatory demyelinating disease, or as other disorders. Secondly, identification of newly diagnosed patients in the study period presented difficulties both for the clinical departments and the DNPR, possibly leading to unequal sampling. It has previously been described for rheumatologic diseases that up to 22% of the diagnoses were not correct in the DNPR.23 Finally, susceptible patients with ON may be seen by ophthalmologists in private practice and with regard to TM by rheumatology departments in the Region.
Another possible limitation of the study was the selection of an at-risk MS group who fulfilled the inclusion criteria. Of those who fulfilled the inclusion criteria, 35 were on natalizumab treatment, which according to general treatment guidelines in the region was given to patients with high disease activity. This strategy may hinder the diagnosis of NMO, but evidently increases the prevalence of NMO in the MS group. Furthermore, it was not possible to retrieve the initial MRI for evaluation in a number of patients. A LETM may appear in multiple shorter plaques (≤3 vertebral segments) during remission or following treatment with high-dose steroids, and normal appearance or shorter lesions can be found early during relapse or in the residual atrophic stage.11,24,25 This natural development of the disease may limit the number of patients with NMO in the study, as 34 of the total group of patients with NMO were in remission at the time of investigation. Furthermore, the majority of patients received immunosuppressive or immunomodulatory treatment, which further lowered the probability of diagnosing NMO. It may be concluded that the number of patients with NMO found in the study provides a conservative estimate of the incidence and prevalence.
A primary strength of this study was the design of the diagnostic algorithm for NMO, as the clinical diagnosis was established without knowledge of AQP4 antibody results and vice versa, diminishing the risk of bias. All MRIs were reevaluated and supplementary MRI was taken if missing or if a relapse was suspected since the last MRI, giving a high degree of diagnostic completeness. VEP was used as an objective indication of visual pathophysiology in the diagnosis of patients with NMO in the study. VEP abnormalities were observed in 86% of the patients with NMO, suggesting that VEP analysis may support the diagnosis of NMO.6
We discovered a number of patients with NMO in a predominantly Caucasian population. A consequence of this finding is that NMO may be considered a more obvious differential diagnosis than previously thought in diagnostic algorithms for MS as well as for ON and TM. We observed heterogeneity of clinical disease manifestations similar to the findings of previous studies.21,22 Future studies may relate the diverse clinical findings in NMO to the immunogenetic background, e.g., the human leukocyte antigen distribution. Furthermore, other forms of autoimmunity seem to be prevalent in patients with NMO and could be more closely delineated.
ACKNOWLEDGMENT
The authors thank Professor Trevor Owens for suggestions and advice for the study design; Mads Henrik Ravnborg, MD, Kevin K. Mortensen, MD, and Carsten Bisgaard, MD, for giving access to their patients and for creating facilities for patient investigation; student scientist Peter Haagerup for IT management; The Clinics of Neurophysiology at Sygehus Lillebælt, Vejle, and at Odense University Hospital for VEP analysis; the 4 MS clinics in the Region of Southern Denmark for support, in particular secretarial assistance from Anette Ravn Jacobsen, Elisabeth Dal, Mette Thingholm, Edel Eriksen, and Alice Hoyer Nissen; and Technician Mette Hviid and The Autoimmunity Lab at Department of Clinical Immunology, Odense University Hospital, in particular Lis Rasmussen, for technical assistance.
Footnotes
- AQP4
- aquaporin-4
- CI
- confidence interval
- DNPR
- Danish National Patient Registry
- EDSS
- Expanded Disability Status Scale
- IDD
- inflammatory demyelinating disease
- IgG
- immunoglobulin G
- LETM
- longitudinally extensive transverse myelitis
- MS
- multiple sclerosis
- NMO
- neuromyelitis optica
- NMSS
- National Multiple Sclerosis Society
- ON
- optic neuritis
- TM
- acute transverse myelitis
- VEP
- visual evoked potential
AUTHOR CONTRIBUTIONS
N. Asgari: study concept and design, acquisition of data, statistical analysis and interpretation of results, writing of manuscript. S.T. Lillevang: concept of study, laboratory determination of aquaporin-4 antibodies, revising manuscript, and approving final version. H.P.B. Skejoe: MRI reevaluation and analysis of follow-up MRI investigations, revising manuscript, and approving final version. M. Fallah: acquisition of data, revising manuscript, and approving final version. E. Stenager: study concept and design, interpretation of statistical result, revising manuscript, and approving final version, clinical co-supervisor. K.O. Kyvik: study concept and design, interpretation of statistical result, revising manuscript and approving final version, study supervisor, and epidemiologic contribution.
DISCLOSURE
Dr. Asgari has received funding for travel from Biogen Idec and Novartis and receives research support from the Sonderborg Hospital Research Fund, the Vejle Hospital Research Fund, the Esbjerg, Sønderborg, and Vejle Hospitals of the Region of Southern Denmark, the Danish Foundation for Neurological Research, and the Ole Jacobsen Commemoration Foundation. Dr. Lillevang, Dr. Skejoe, and Dr. Falah report no disclosures. Dr. Stenager has received funding for travel from Biogen Idec, Merck Serono, Bayer Schering Pharma, sanofi-aventis, and Novartis. Dr. Kyvik receives research support from the Danish Medical Research Council, the NIH/NIA, and Interreg Iva.
REFERENCES
- 1. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 3. Jacob A, Matiello M, Wingerchuk DM, Lucchinetti CF, Pittock SJ, Weinshenker BG. Neuromyelitis optica: changing concepts. J Neuroimmunol 2007;187:126–138 [DOI] [PubMed] [Google Scholar]
- 4. Asgari N, Owens T, Frokiaer J, Stenager E, Lillevang ST, Kyvik KO. Neuromyelitis optica (NMO): an autoimmune disease of the central nervous system (CNS). Acta Neurol Scand Epub 2010 Sep 29. [DOI] [PubMed] [Google Scholar]
- 5. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107–1114 [DOI] [PubMed] [Google Scholar]
- 6. Wu JS, Zhang MN, Carroll WM, Kermode AG. Characterisation of the spectrum of demyelinating disease in Western Australia. J Neurol Neurosurg Psychiatry 2008;79:1022–1026 [DOI] [PubMed] [Google Scholar]
- 7. Cabre P, Heinzlef O, Merle H, et al. MS and neuromyelitis optica in Martinique (French West Indies). Neurology 2001;56:507–514 [DOI] [PubMed] [Google Scholar]
- 8. Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol 2006;59:566–569 [DOI] [PubMed] [Google Scholar]
- 9. Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 2010;17:1019–1032 [DOI] [PubMed] [Google Scholar]
- 10. de Seze J, Stojkovic T, Ferriby D, et al. Devic's neuromyelitis optica: clinical, laboratory, MRI and outcome profile. J Neurol Sci 2002;197:57–61 [DOI] [PubMed] [Google Scholar]
- 11. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 12. Fazio R, Malosio ML, Lampasona V, et al. Anti-aquaporin 4 antibodies detection by different techniques in neuromyelitis optica patients. Mult Scler 2009;15:1153–1163 [DOI] [PubMed] [Google Scholar]
- 13. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. Int MS J 2008;15:99–105 [PubMed] [Google Scholar]
- 15. Magana SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 2009;72:712–717 [DOI] [PubMed] [Google Scholar]
- 16. Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 2005;65:1479–1482 [DOI] [PubMed] [Google Scholar]
- 17. Miller DH, Weinshenker BG, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 2008;14:1157–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120:2059–2069 [DOI] [PubMed] [Google Scholar]
- 19. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 20. Sellner J, Luthi N, Buhler R, et al. Acute partial transverse myelitis: risk factors for conversion to multiple sclerosis. Eur J Neurol 2008;15:398–405 [DOI] [PubMed] [Google Scholar]
- 21. Bizzoco E, Lolli F, Repice AM, et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J Neurol 2009;256:1891–1898 [DOI] [PubMed] [Google Scholar]
- 22. Collongues N, Marignier R, Zephir H, et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 2010;74:736–742 [DOI] [PubMed] [Google Scholar]
- 23. Nickelsen TN. [Data validity and coverage in the Danish National Health Registry: a literature review. ] Ugeskr Laeger 2001;164:33–37 [PubMed] [Google Scholar]
- 24. Krampla W, Aboul-Enein F, Jecel J, et al. Spinal cord lesions in patients with neuromyelitis optica: a retrospective long-term MRI follow-up study. Eur Radiol 2009;19:2535–2543 [DOI] [PubMed] [Google Scholar]
- 25. Weinshenker BG, Wingerchuk DM. Neuromyelitis optica: clinical syndrome and the NMO-IgG autoantibody marker. Curr Top Microbiol Immunol 2008;318:343–356 [DOI] [PubMed] [Google Scholar]