Abstract
The nucleotide sequence of the ompF gene coding for a major outer membrane protein of Escherichia coli K-12 has been determined and the amino acid sequence of the OmpF protein was deduced from it. The OmpF protein contains 340 amino acid residues, and is produced from a precursor having 22 extra amino acid residues, the signal peptide, at the amino terminus. The expected secondary structure of the OmpF protein had a high beta-sheet content with a low alpha-helix content. The promoter region and the transcription termination region of the ompF gene had a significantly high AT content, while the AT content of the coding region was about the same as the average AT content of the E. coli chromosome. Following the termination codon, a typical rho-independent transcription termination signal was observed. The codon usage in the ompF gene was highly nonrandom; the codons preferably utilized are those recognized by the most abundant species of isoaccepting tRNAs or those, among synonymous codons recognized by the same tRNA, that can interact more properly with the anticodon.
Full text
PDF


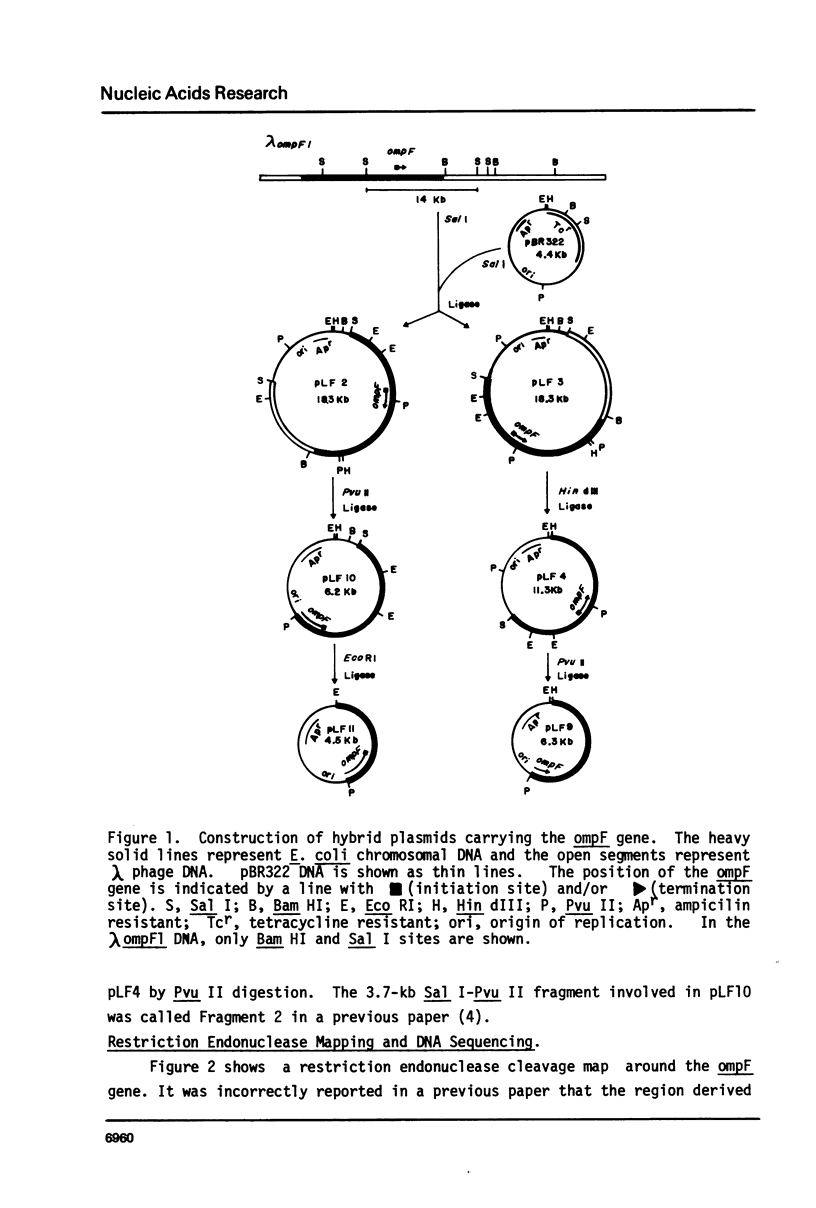



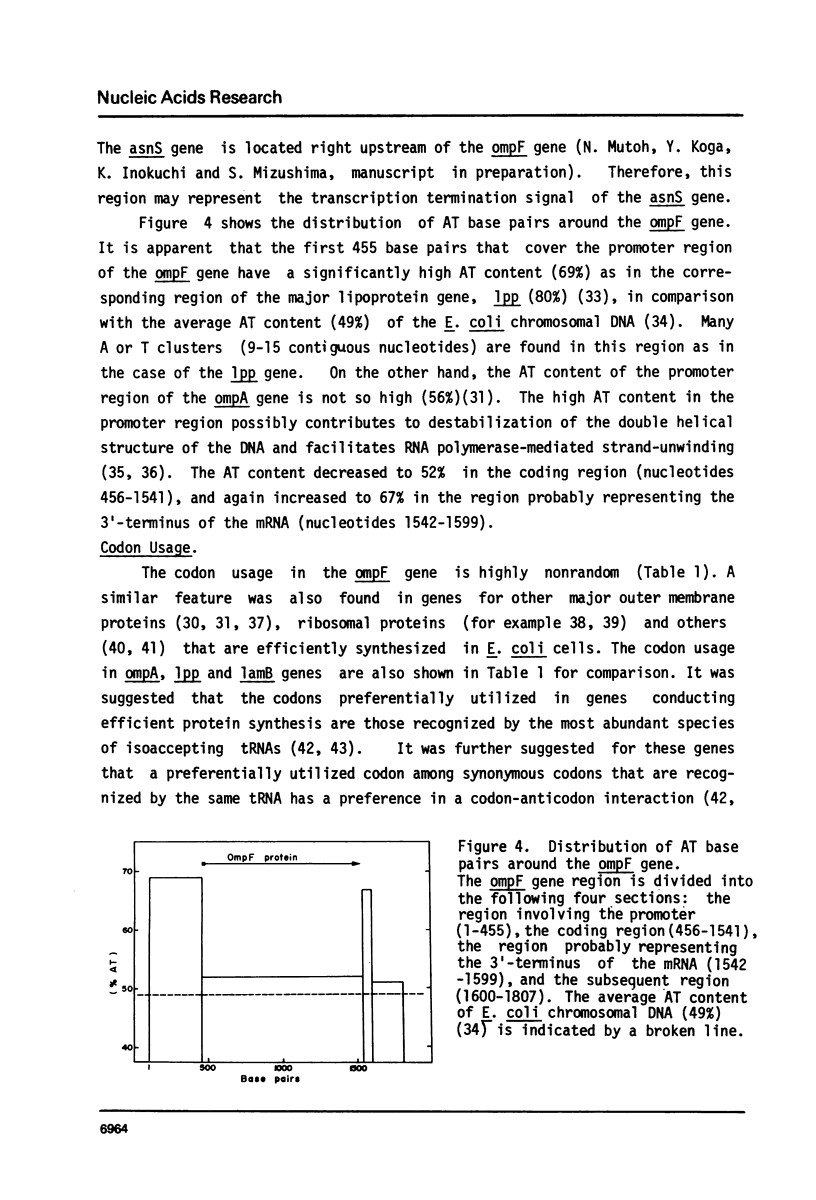
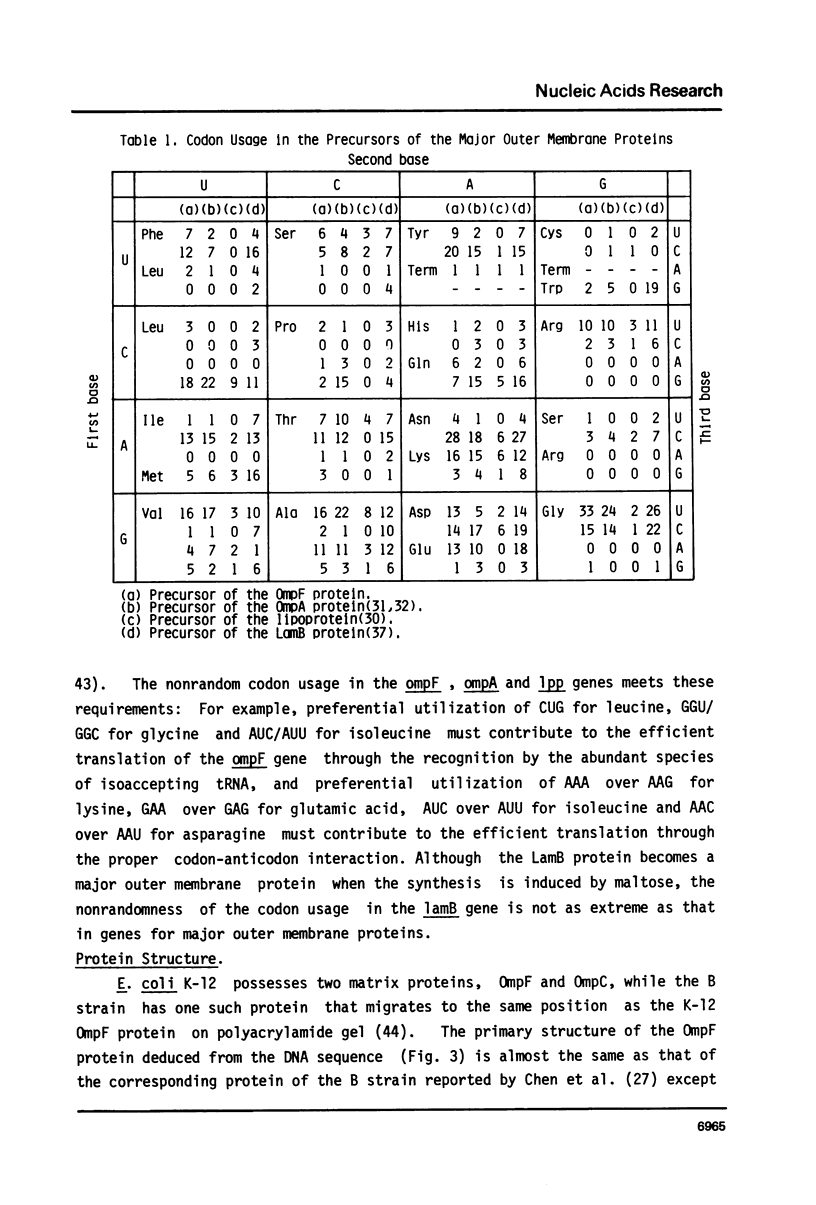
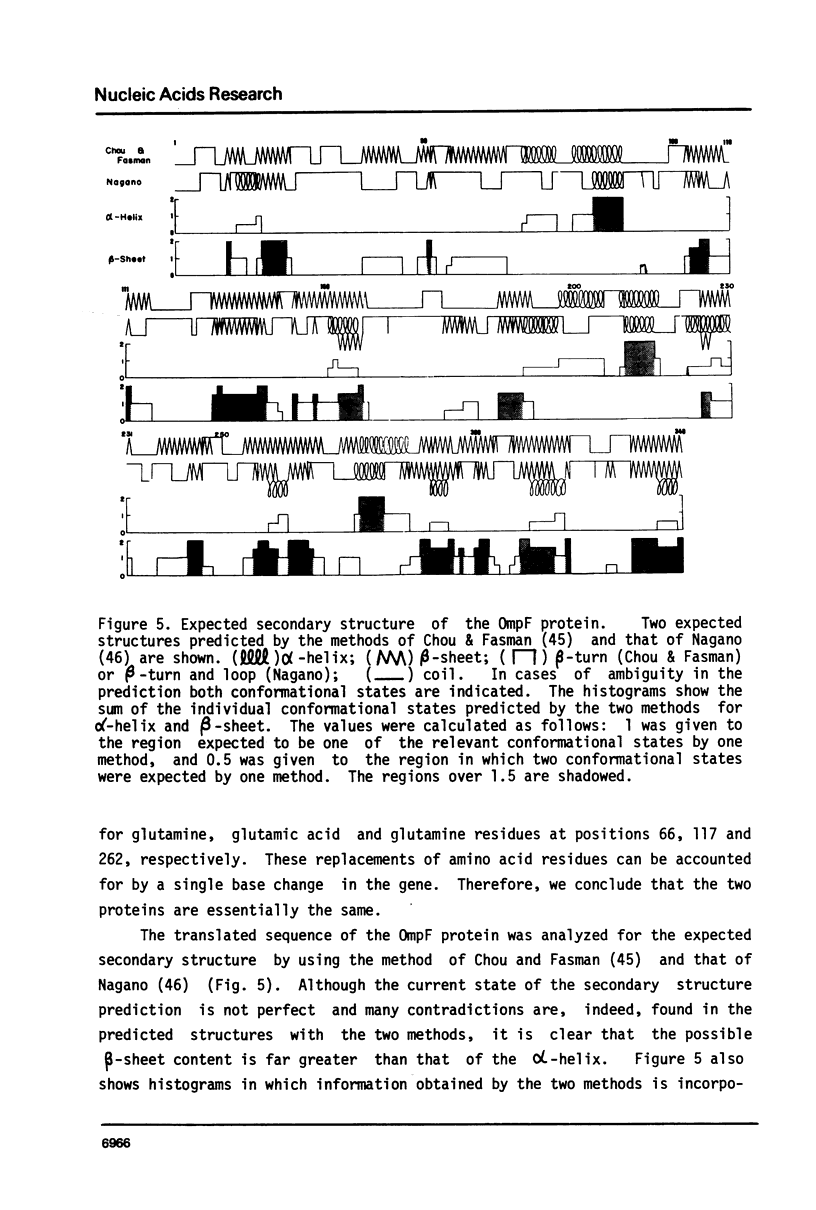


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Chen-Schmeisser U., Henning U. Primary structure of major outer-membrane protein I (ompF protein, porin) of Escherichia coli B/r. Biochem J. 1982 Apr 1;203(1):33–43. doi: 10.1042/bj2030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Danna K. J. Determination of fragment order through partial digests and multiple enzyme digests. Methods Enzymol. 1980;65(1):449–467. doi: 10.1016/s0076-6879(80)65055-1. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Horn G. T., Wells R. D. The leftward promoter of bacteriophage lambda. Structure, biological activity, and influence by adjacent regions. J Biol Chem. 1981 Feb 25;256(4):2003–2009. [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Arrangement of proteins O-8 and O-9 in outer membrane of Escherichia coli K-12. Existence of homotrimers and heterotrimers. Eur J Biochem. 1979 Oct 15;100(2):321–328. doi: 10.1111/j.1432-1033.1979.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Characterization of major outer membrane proteins O-8 and O-9 of Escherichia coli K-12. Evidence that structural genes for the two proteins are different. J Biochem. 1978 Apr;83(4):1095–1100. doi: 10.1093/oxfordjournals.jbchem.a131998. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Strain specificity of outer membrane proteins in Escherichia coli. J Biochem. 1977 May;81(5):1525–1530. [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Amino acid sequence of the signal peptide of ompA protein, a major outer membrane protein of Escherichia coli. J Biol Chem. 1980 Jan 10;255(1):27–29. [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980 Nov 5;143(3):317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Inokuchi K., Mizushima S. Amino acid sequence of the signal peptide of OmpF, a major outer membrane protein of Escherichia coli. FEBS Lett. 1982 Jan 25;137(2):171–174. doi: 10.1016/0014-5793(82)80341-4. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Nagasawa T., Mizushima S. Specialized transducing bacteriophage lambda carrying the structural gene for a major outer membrane matrix protein of Escherichia coli K-12. J Bacteriol. 1981 Feb;145(2):1085–1090. doi: 10.1128/jb.145.2.1085-1090.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K. Triplet information in helix prediction applied to the analysis of super-secondary structures. J Mol Biol. 1977 Jan 15;109(2):251–274. doi: 10.1016/s0022-2836(77)80033-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Pirtle I. L., Takeishi K., Inouye M. Messenger ribonucleic acid of the lipoprotein of the Escherichia coli outer membrane. II. The complete nucleotide sequence. J Biol Chem. 1980 Jan 10;255(1):210–216. [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sato T., Yura T. Chromosomal location and expression of the structural gene for major outer membrane protein Ia of Escherichia coli K-12 and of the homologous gene of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):468–477. doi: 10.1128/jb.139.2.468-477.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Yura T. Regulatory mutations conferring constitutive synthesis of major outer membrane proteins (OmpC and OmpF) in Escherichia coli. J Bacteriol. 1981 Jan;145(1):88–96. doi: 10.1128/jb.145.1.88-96.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk W. J., Weisberg R. A. A simple method for making new transducing lines of coliphage lambda. Mol Gen Genet. 1975;137(2):101–107. doi: 10.1007/BF00341676. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Fiandt M., Szybalski W. A relationship between DNA helix stability and recognition sites for RNA polymerase. Science. 1979 Aug 3;205(4405):508–511. doi: 10.1126/science.377494. [DOI] [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Lugtenberg B., van Boxtel R., Hack A. M., Verhoef C., Havekes L. meoA is the structural gene for outer membrane protein c of Escherichia coli K12. Mol Gen Genet. 1979 Jan 31;169(2):147–155. doi: 10.1007/BF00271665. [DOI] [PubMed] [Google Scholar]



