Abstract
Objective:
Huperzine A is a natural cholinesterase inhibitor derived from the Chinese herb Huperzia serrata that may compare favorably in symptomatic efficacy to cholinesterase inhibitors currently in use for Alzheimer disease (AD).
Methods:
We assessed the safety, tolerability, and efficacy of huperzine A in mild to moderate AD in a multicenter trial in which 210 individuals were randomized to receive placebo (n = 70) or huperzine A (200 μg BID [n = 70] or 400 μg BID [n = 70]), for at least 16 weeks, with 177 subjects completing the treatment phase. The primary analysis assessed the cognitive effects of huperzine A 200 μg BID (change in Alzheimer's Disease Assessment Scale–cognitive subscale [ADAS-Cog] at week 16 at 200 μg BID compared to placebo). Secondary analyses assessed the effect of huperzine A 400 μg BID, as well as effect on other outcomes including Mini-Mental State Examination, Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change scale, Alzheimer's Disease Cooperative Study Activities of Daily Living scale, and Neuropsychiatric Inventory (NPI).
Results:
Huperzine A 200 μg BID did not influence change in ADAS-Cog at 16 weeks. In secondary analyses, huperzine A 400 μg BID showed a 2.27-point improvement in ADAS-Cog at 11 weeks vs 0.29-point decline in the placebo group (p = 0.001), and a 1.92-point improvement vs 0.34-point improvement in the placebo arm (p = 0.07) at week 16. Changes in clinical global impression of change, NPI, and activities of daily living were not significant at either dose.
Conclusion:
The primary efficacy analysis did not show cognitive benefit with huperzine A 200 μg BID.
Classification of evidence:
This study provides Class III evidence that huperzine A 200 μg BID has no demonstrable cognitive effect in patients with mild to moderate AD.
Huperzine A is an alkaloid extract of the plant Huperzia serrata that may be useful as a treatment for Alzheimer disease (AD). During the 1980s, investigators in China determined that huperzine A is a potent inhibitor of acetylcholinesterase (AChE),1 a finding that has been confirmed repeatedly.2,3 It is highly selective for AChE in vitro, it has good brain penetration, and it is relatively free of cholinergic toxicity.4–6 In addition, some studies have shown that huperzine A may shift amyloid precursor protein (APP) metabolism toward the nonamyloidogenic α-secretase pathway.7 Based on the 50% inhibitory concentration (IC50), huperzine A is more potent than tacrine, physostigmine, and galantamine with respect to inhibition of AChE activity. Hence, it is presumed that huperzine A, if efficacious, would exert its clinical efficacy in AD via this mechanism. Certainly, it should be noted that huperzine has been shown to exhibit other effects that may be beneficial AD: huperzine has been demonstrated to reduce glutamate-induced cytotoxicity by antagonizing cerebral NMDA receptors.8 Importantly, this trial was designed to assess short-term symptomatic effects more likely attributable to AChEI or memantine-like effects than to neuroprotective mechanisms.
Phase I studies in the United States explored escalating doses up to 400 μg twice daily in healthy older individuals.9 Most adverse effects (AEs) were rated as mild; there were no serious AEs (SAE).
In this phase II study, we aimed to demonstrate the safety, tolerability, and efficacy of huperzine A over 16 weeks in mild to moderate AD.
METHODS
Standard protocol approvals, registrations, and patient consents.
A total of 210 patients were enrolled in this study. The protocol was reviewed and approved by the institutional review board at each participating site. All research participants and caregivers gave written informed consent. Surrogate consent was used if criteria were met for its use. The Alzheimer's Disease Cooperative Study's Data and Safety Monitoring Board, which is advisory to the National Institute of Aging, provided safety oversight on an ongoing basis. The clinical trial identifier number for this study was NCT00083590. Blinded phase enrollment began June 2004 and last week 24 visit December 2007.
Subjects.
Participants were 50 years or older and diagnosed with probable AD, as defined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria. Subjects had Mini-Mental State Examination (MMSE) scores between 10 and 24, inclusive, and were in stable medical condition for 3 months prior to screening. Individuals receiving stable doses of memantine were included. Exclusion criteria included use of cholinesterase inhibitors within 2 months of screening; regular use of narcotic analgesics (>2 doses per week) within 4 weeks of screening; use of medications with significant CNS anticholinergic activity within 2 months of screening (e.g., tricyclic antidepressants, diphenhydramine); and use of anti-Parkinson medications (including Sinemet, amantadine, bromocriptine, pergolide, and selegiline) within 2 months of screening.
Study design.
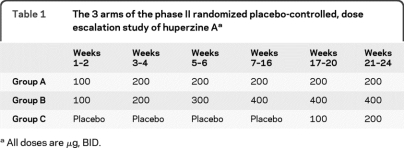
This was a multicenter, 3-arm, randomized, double-blind, placebo-controlled, dose-escalation trial. Participants were randomly assigned with equal probability to 3 groups: group A received huperzine A 100 μg BID for 2 weeks, then 200 μg BID for 22 weeks. Group B received huperzine A 100 μg BID for 2 weeks, then 200 μg BID for 2 weeks, then 300 μg BID for 2 weeks, then 400 μg BID for 18 weeks. Group C received placebo for 16 weeks then huperzine A 100 μg BID for 4 weeks, then 200 μg BID for 4 weeks (table 1).
Table 1.
The 3 arms of the phase II randomized placebo-controlled, dose escalation study of huperzine Aa
All doses are μg, BID.
Safety assessments and laboratory measures.
All AEs occurring during the study were characterized. Safety data, such as vital signs, ECG data, and laboratory test results, were collected throughout the trial. Pill counting was used as a measure for medication compliance. Vital signs were recorded at each study visit, with ECGs performed at baseline and at weeks 2, 4, 8, 11, 16, 20, and 24. DNA testing for APOE genotype was performed.
Clinical measures.
At baseline and at weeks 8, 11, 16, 20, and 24, cognitive performance was assessed using the Alzheimer's Disease Assessment Scale cognitive subscale (ADAS-Cog)10 and the MMSE,11 daily function was assessed by the Alzheimer's Disease Cooperative Study Activities of Daily Living Scale (ADCS-ADL),12 behavior was assessed by the Neuropsychiatric Inventory (NPI),13 and global status was rated using the Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change (CGIC).14
Efficacy analysis.
Our intention was to evaluate 2 doses of huperzine A, 200 μg BID and 400 μg BID, for safety and efficacy. The primary efficacy analysis was change from baseline to 16 weeks in the ADAS-Cog score using analysis of covariance (ANCOVA), with baseline score as a covariate. Sixteen weeks was chosen to balance concerns about prolonged placebo exposure with interest in longer comparison to placebo. Studies of cholinesterase inhibitors generally show efficacy at 12 weeks. The available funds allowed us to study a total of 210 subjects, or 70 subjects per arm. A power calculation indicated that this group size would provide 80% power to demonstrate a 3-point difference in ADAS-Cog change at 16 weeks (assuming a SD of 5 points for the change in ADAS-Cog score, an estimate based on the observed SD of 4.5 for the ADAS-Cog change score in the placebo arm at 6 months in the ADCS high-dose B vitamin trial15), for a single comparison between 2 groups, with an α level of 0.05; for comparison, a meta-analysis of published studies has indicated that the mean effect on this measure of currently available cholinesterase inhibitors in comparison to placebo at 12–24 weeks is 2.7 points.16 With this marginal statistical power, we selected a single comparison for the primary analysis: 200 μg BID (the dose shown to be effective in China) vs placebo at 16 weeks.
For the primary analysis, missing values were imputed using last observation carried forward (LOCF). Prespecified secondary analyses examined the high-dose group, other cognitive and clinical assessments, an alternative method for imputing missing values by applying to the last observation a slope derived from individuals in the same arm with observed data over the missing interval,17 and mixed-effects modeling, with time treated as a categorical variable, and unspecified correlation structure. The interaction between treatment and time was included in the mixed-effects model.
AE data are reported using intention-to-treat analyses (n = 210) across 16 weeks. To maximize the sensitivity of the safety measures comparisons, no adjustments were made for multiple comparisons.
All statistical tests were 2-sided. Analyses were performed using the R statistical package, version 2.3.1.11.18 Baseline values of outcome measures were entered as covariates into each model to account for any baseline group differences. In addition, age and APOE genotype were prespecified as potential confounders; if either was found to have between-group differences at baseline and to correlate with the outcome measure being tested, it would be entered as a confounder into the ANCOVA model. Neither covariate met both criteria for inclusion in any analysis.
RESULTS
Participants.
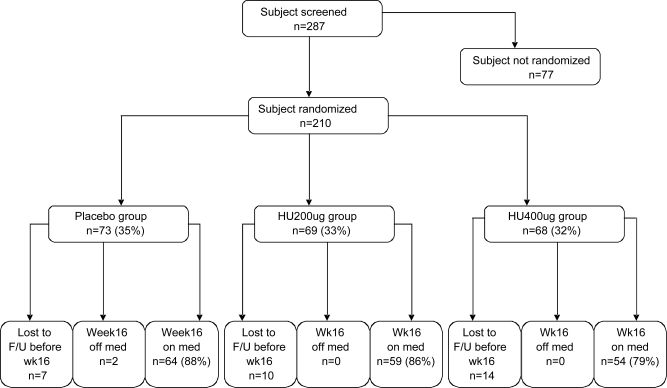
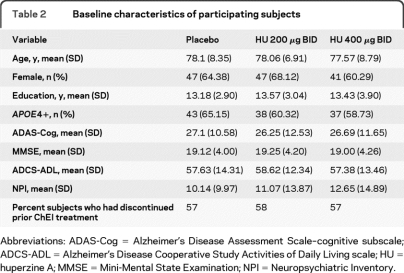
Flow of subjects through the study is shown in figure 1. Of 287 participants who were screened for the trial, 210 were eligible and randomized. The screen failure rate was 27%. The most common reason for screen failure was MMSE score being too high (39%). No subjects discontinued the medication because of SAEs attributed to the treatment, and there were no differences in treatment discontinuation rates (for placebo, dropout = 9 subjects; for 200 μg BID arm, dropout = 10 subjects; for 400 μg BID arm, dropout = 14 subjects) or in medication compliance (96%–99%) among the 3 groups. Of the 33 subjects who withdrew, 17 did so due to AEs. The 3 groups were balanced at baseline regarding all demographics, clinical measures (MMSE scores), and APOE4 distribution (table 2). The percentage of subjects in the trial who had discontinued prior cholinesterase inhibitor treatment (a baseline characteristic) is also reported, by treatment group (table 2). Peripheral AChE inhibition was measured in all 3 groups. The data are reported in table e-1 on the Neurology® Web site at www.neurology.org.
Figure 1. Participant flow through the phase II huperzine A trial.
A total of 287 subjects were screened for the trial, and 210 were eligible and randomized. The most common reason for screen failure was Mini-Mental State Examination score being too high.
Table 2.
Baseline characteristics of participating subjects
Abbreviations: ADAS-Cog = Alzheimer's Disease Assessment Scale–cognitive subscale; ADCS-ADL = Alzheimer's Disease Cooperative Study Activities of Daily Living scale; HU = huperzine A; MMSE = Mini-Mental State Examination; NPI = Neuropsychiatric Inventory.
Safety and tolerability.
Huperzine A was generally well-tolerated at doses of up to 400 μg BID for 24 weeks, even in subjects unable to take other cholinesterase inhibitors. Fifty-seven percent of subjects had been on ChEI treatment prior to enrolling in this study. Sixty-six percent of them had discontinued due the experience of an AE (most commonly nausea). The remainder discontinued due to other reasons, including lack of perceived benefit (22%) and expense (12%). Vital signs were recorded at each study visit, with ECGs performed at baseline and at weeks 2, 4, 8, 11, 16, 20, and 24. There were no statistically significant differences between the placebo, 200 μg, or 400 μg groups. All AEs that occurred in at least 4% of participants (treatment or placebo) are listed in table e-2. SAEs occurred in 6/73 (8.22%) in placebo and 9/69 (13.0%) in 200 μg BID arm group (p = 0.42) and 11/68 (16.2%) in 400 μg BID arm (p = 0.20). No SAE was considered possibly related to study medication. There was one death in the placebo group, 3 in the 200 μg BID arm, and none in the 400 μg BID arm. Rates of withdrawal due to AEs, by treatment group, separately for subjects who did and did not discontinue cholinesterase inhibitors due to AEs prior to entering the trial by arm are presented in table e-3.
Clinical measures.
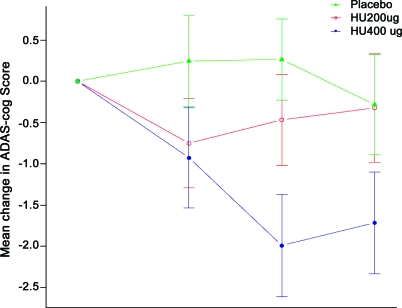
The primary analysis demonstrated that huperzine A 200 μg BID had no effect on ADAS-Cog change at week 16 (−0.32 ± 15.37 vs −0.34 ± 5.17, p = 0.98, figure 2). The slope imputation ANCOVA and mixed effects model analysis of ADAS-Cog change were similarly negative. Change in MMSE, the secondary cognitive measure, suggested improved cognition with treatment compared to placebo at weeks 6 and 16 (0.66 ± 2.85 vs −0.33 ± 3.31, p = 0.06, LOCF ANCOVA), though not at week 11 (table 3 and figure e-1). There was no effect of this dose on the ADL or NPI assessments (figure e-1). Changes from baseline in cognitive and functional measures in each treatment arm, at each timepoint, using LOCF are shown in table 3.
Figure 2. Intention-to-treat last observation carried forward analyses of Alzheimer's Disease Assessment Scale–cognitive subscale (ADAS-Cog) change score.
Placebo n = 69, 200 μg n = 68, 400 μg n = 73.
Table 3.
Changes from baseline in cognitive and functional measures by treatment arm, at each timepoint, using last observation carried forward
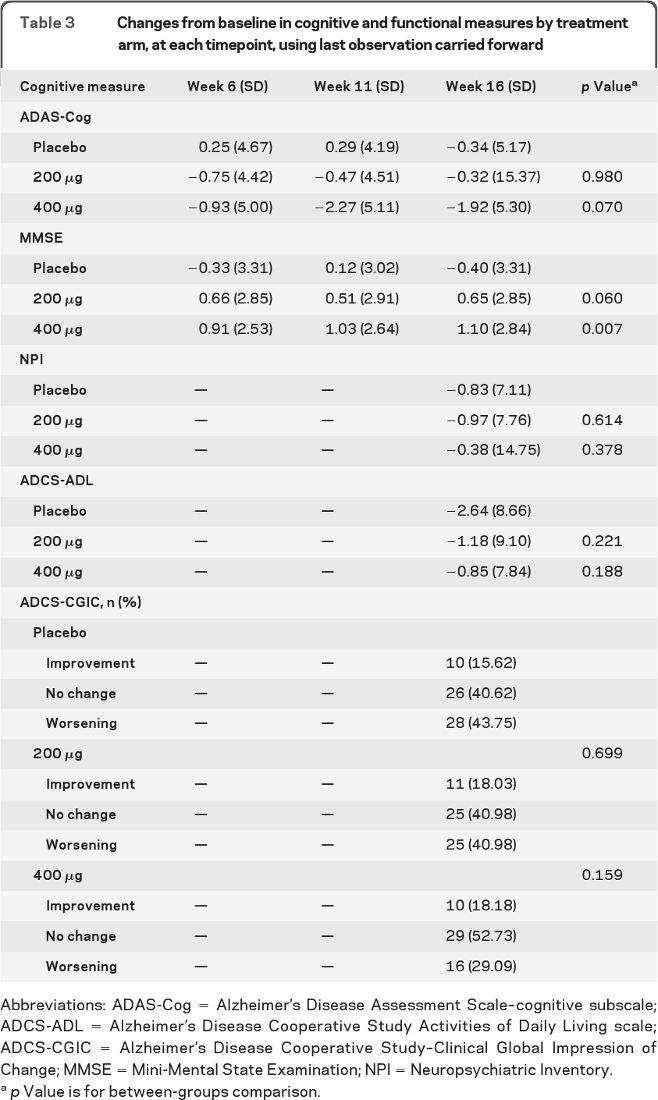
Abbreviations: ADAS-Cog = Alzheimer's Disease Assessment Scale–cognitive subscale; ADCS-ADL = Alzheimer's Disease Cooperative Study Activities of Daily Living scale; ADCS-CGIC = Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change; MMSE = Mini-Mental State Examination; NPI = Neuropsychiatric Inventory.
p Value is for between-groups comparison.
In the 400 μg BID of huperzine A arm, the ADAS-Cog change at week 16 demonstrated a 1.92 ± 5.30–point improvement (p = 0.07 for the LOCF ANCOVA), and at week 11, 400 μg BID dose showed a 2.27-point improvement vs a 0.29-point decline (p = 0.001 for the LOCF ANCOVA). The mixed-effects model of ADAS-Cog change comparing the 400 μg BID dose to placebo over 16 weeks showed a significant treatment group by time effect (p = 0.03), with greater cognitive improvement in the active treatment arm. For MMSE, the results at week 16 for placebo group demonstrated a 0.40-point decline, while active treatment showed a 1.1-point improvement (p = 0.007). Placebo subjects switched to 400 μg BID at week 16 did not experience statistically significant benefits on the ADAS-Cog. There was no effect of this dose on the ADL, NPI, or CGIC assessments (table 3). All subjects were followed from week 16 through week 24 on active drug. These data were not included in the primary analysis as this phase was not placebo-controlled. ADAS-Cog data for this phase are presented in figure e-2.
Effectiveness of allocation concealment was assessed for this trial. Four groups were queried: clinician, psychometrist, study partner, and coordinator. The coordinators did better than chance in their assessment of active vs placebo (p < 0.001). The other groups' responses were no different from chance (see table e-4).
DISCUSSION
The present study represents an initial attempt to characterize the impact of short-term huperzine A treatment on individuals with mild to moderate AD. The primary endpoint for this trial, cognitive benefit as indicated by the change in ADAS-Cog score for the 200 μg BID dose at 16 weeks compared to placebo, did not indicate efficacy; we therefore cannot conclude that huperzine A provides cognitive enhancement in mild to moderate AD. However, the 200 μg BID did favorably influence MMSE scores at 16 weeks, and the 400 μg BID dose showed evidence of cognitive enhancement as measured by the ADAS-Cog and the MMSE. Thus, the results suggest a possible short-term symptomatic benefit of huperzine A, which requires confirmation in additional studies. Huperzine was generally well-tolerated at both doses tested.
It is notable that most subjects in this trial had discontinued prior treatment with cholinesterase inhibitors; there was no evidence of a different response in such individuals when compared to individuals who had never taken such drugs. Fifty-seven percent of subjects had been on ChEI in the past and 66% had discontinued because of AEs, most commonly nausea. However, 11.4% of subjects were unable to tolerate huperzine A due to AEs, with nausea being the most common reason. This comparison, however, is limited by the incompleteness (e.g., titration schedule, dosage, and duration of past ChEI treatment) and uncertain quality of the historical information. Although there is limited power, the concomitant treatment with memantine did not seem to influence response to huperzine A.
Analysis of effectiveness of allocation concealment showed that study coordinators did better than chance in guessing which subjects were in the treatment arm vs placebo. This is presumably as a result of AE reporting, which is performed by study coordinators. The clinicians, psychometrists, and study partners did no better than chance in guessing which subjects were in the treatment arm vs placebo. This suggests maintenance of blindness was adequate for the objective measurement of study endpoints.
Huperzine A at a dose of 200 μg BID is ineffective in the treatment of AD. Some secondary analyses in this trial suggest that a higher dose, 400 μg BID, may improve cognition; further studies to identify the maximal tolerated dose and evaluate long-term treatment effects may warrant consideration.
Supplementary Material
- AChE
- acetylcholinesterase
- AD
- Alzheimer disease
- ADAS-Cog
- Alzheimer's Disease Assessment Scale–cognitive subscale
- ADCS-ADL
- Alzheimer's Disease Cooperative Study Activities of Daily Living scale
- ADCS-CGIC
- Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change
- AE
- adverse effect
- ANCOVA
- analysis of covariance
- APP
- amyloid precursor protein
- LOCF
- last observation carried forward
- MMSE
- Mini-Mental State Examination
- NPI
- Neuropsychiatric Inventory
- SAE
- serious adverse effect.
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Jin, Dr. Thomas, and Dr. Aisen.
COINVESTIGATORS
Alzheimer's Disease Cooperative Study Group: Joseph Quinn, MD (Oregon Health & Science University, Portland, Site Investigator); Lon S. Schneider, MD (University of Southern California, Los Angeles, Site Investigator); Linda Harrell, MD, PhD (University of Alabama at Birmingham, Site Investigator); Hillel Grossman, MD (Mount Sinai School of Medicine, Bronx, NY, Site Investigator); Neelum Aggarwal, MD (Rush University Medical Center, Chicago, IL, Site Investigator); Steven H. Ferris, PhD (New York University Medical Center, NY, Site Investigator); Steven T. DeKosky, MD (University of Pittsburgh, Pittsburgh, Pennsylvania, Site Investigator); Saleem M. Ismail, MD (University of Rochester, Rochester, NY, Site Investigator); Ruth A. Mulnard, RN, DNSc (University of California, Irvine, Site Investigator); Myron Weiner, MD (University of Texas, Southwestern Medical Center at Dallas, Site Investigator); Allan Levey, MD, PhD (Emory University, Atlanta, GA, Site Investigator); Charles DeCarli, MD (University of California, Davis, Site Investigator); Charles Bernick, MD (University of Nevada, Las Vegas, Site Investigator); Jacobo E. Mintzer, MD (Medical University of South Carolina, North Charleston, Site Investigator); Carl Sadowdky, MD, Teresa Villena, MD, Walter C. Martinez, MD (Premiere Research Institute, West Palm Beach, FL, Site Investigator); Thomas Obisesan, MD, MPH (Howard University, Washington, DC, Site Investigator); Jody Corey-Bloom (University of California, San Diego, Site Investigator); Pierre Tariot, MD (Banner Alzheimer's Institute Phoenix, Site investigator); Earl Zimmerman, MD (Albany Medical College, Site Investigator).
DISCLOSURE
Dr. Rafii serves on the advisory board of Medical Care Corporation, Vivity Labs, Inc., and serves on the speakers' bureau for Novartis. Dr. Walsh reports no disclosures. Dr. Little received salary support from Neuro-Hitech, Inc. K. Behan, B. Reynolds, Dr. Jin, and Dr. Thomas report no disclosures. Dr. Aisen serves on a scientific advisory board for NeuroPhage and Novartis; serves on the editorial boards of BMC Medicine and Alzheimer's Research & Therapy; is listed as inventor on a patent re: DHA therapy for apolipoprotein E4 negative Alzheimer's disease (potential royalties assigned in full to UCSD); serves as a consultant to Elan Corporation, Wyeth, Eisai Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, Bayer Schering Pharma, Astellas Pharma Inc., Dainippon Sumitomo Pharma, BioMarin Pharmaceutical Inc., Solvay Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo, AstraZeneca, Janssen, and Medivation, Inc.; receives research support from Pfizer Inc, Baxter International Inc., and the NIH/NIA; and has received stock options from Medivation, Inc. and NeuroPhage.
REFERENCES
- 1. Wang YE, Yue DX, Tang XC. [Anti-cholinesterase activity of huperzine A.] Chung Kuo Yao Li Hsueh Pao 1986;7:110–113 [PubMed] [Google Scholar]
- 2. Ashani Y, Peggins JO, Doctor BP. Mechanism of inhibition of cholinesterases by huperzine A. Biochem Biophys Res Commun 1992;184:719–726 [DOI] [PubMed] [Google Scholar]
- 3. Saxena A, Qian N, Kovach IM, et al. Identification of amino acid residues involved in the binding of huperzine A to cholinesterases. Protein Sci 1994;3:1770–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camps P, Muñoz-Torrero D. Tacrine-huperzine A hybrids (huprines): a new class of highly potent and selective acetylcholinesterase inhibitors of interest for the treatment of Alzheimer's disease. Mini Rev Med Chem 2001;1:163–174 [DOI] [PubMed] [Google Scholar]
- 5. Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 2006;27:1–26 [DOI] [PubMed] [Google Scholar]
- 6. Laganière S, Corey J, Tang XC, Wülfert E, Hanin I. Acute and chronic studies with the anticholinesterase huperzine A: effect on central nervous system cholinergic parameters. Neuropharmacology 1991;30:763–768 [DOI] [PubMed] [Google Scholar]
- 7. Peng Y, Lee DY, Jiang L, Ma Z, Schachter SC, Lemere CA. Huperzine A regulates amyloid precursor protein processing via protein kinase C and mitogen-activated protein kinase pathways in neuroblastoma SK-N-SH cells over-expressing wild type human amyloid precursor protein 695. Neuroscience 2007;150:386–395 [DOI] [PubMed] [Google Scholar]
- 8. Ved HS, Koenig ML, Dave JR, Doctor BP. Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. Neuroreport 1997;8:963–968 [DOI] [PubMed] [Google Scholar]
- 9. Little JT, Walsh S, Aisen PS. An update on huperzine A as a treatment for Alzheimer's disease. Expert Opin Investig Drugs 2008;17:209–215 [DOI] [PubMed] [Google Scholar]
- 10. Rosen W, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364 [DOI] [PubMed] [Google Scholar]
- 11. Folstein M, Folstein S, McHugh P. Mini Mental State Exam (MMSE): The Mini Mental State Examination. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 12. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord 1997;11(suppl 2):S33–S39 [PubMed] [Google Scholar]
- 13. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314 [DOI] [PubMed] [Google Scholar]
- 14. Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change: The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(suppl 2):S22–S32 [DOI] [PubMed] [Google Scholar]
- 15. Aisen PS, Schneider LS, Sano M, et al. Alzheimer Disease Cooperative Study High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 2008;300:15:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev 2006;1:CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aisen PS, Davis KL, Berg JD, et al. A randomized controlled trial of prednisone in Alzheimer's disease: Alzheimer's Disease Cooperative Study. Neurology 2000;54:588–593 [DOI] [PubMed] [Google Scholar]
- 18. R Development Core Team R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing v. 2.3.1.11. Available at: http://www.R-project.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.