Abstract
The management of chronic myelogenous leukemia during pregnancy requires balancing the well-being of the mother with that of the fetus. We report a case of a 26-year-old lady who was diagnosed with chronic myelogenous leukemia (CML) at 15 weeks gestation and who had an atypical chromosome t(9;22;11) (q34;q11.2;q13) translocation. She was observed through the remainder of the pregnancy and the disease remained stable; she delivered a normal boy. Treatment with imatinib mesylate was initiated shortly after delivery and she went into molecular complete remission. We discuss the course of the disease and suggest guidelines for managing pregnancy with respect to the currently available agents imatinib, dasatinib and nilotinib.
Key words: medicine, hematology, leukemia, CML.
Introduction
Chronic myelogenous leukemia (CML) is a clonal proliferation of progenitor blast cells, resulting from a reciprocal translocation between the long arms of chromosomes 9 and 22. The chimeric BCR-ABL fusion protein synthesized from this translocation has tyrosine kinase activity that activates a number of intracellular proteins and is felt to be responsible for the disease. Until recent years no effective therapy has been available for CML and patients typically died within 6 years of diagnosis.1 Hydroxyurea was of some value in reducing peripheral blood counts, but had no effect on the bone marrow blast count. In the 1980s interferon alpha (IFN-α) was shown to reduce the number of marrow blast progenitor cells and to induce partial remissions. More recently imatinib mesylate, an inhibitor of the BCR-ABL tyrosine kinase, has made dramatic changes in the therapeutic approach to CML. Two newer congeners, desatinib and nilotinib are now also available and others are under development. At the same time the use of tyrosine kinase inhibitors (TKIs) during pregnancy presents problems. Here we discuss the case of a 26-year-old lady who presented with CML expressing an atypical t(9;22;11) (q34;q11.2;q13) chromosome translocation at gestational age 15 weeks. The management of CML in pregnancy is considered with respect to all of the currently available tyrosine kinase inhibitors.
Case Report
A 26-year-old white female in her 15th week of gestation was referred to hematology for evaluation of an elevated white cell count. At initial evaluation her CBC showed WBC 18100 cells/mm3, hemoglobin 11.8 g/dL, Hct 36% and platelets 562,000/mm3. MCV was 95. Differential was 87% polymorphonuclear leukocytes, 10% lymphocytes, 1% monocytes, 1% eosinophils and 1% basophils. Other laboratory studies, including electrolytes and liver function tests, were entirely normal. Leukocyte alkaline phosphatase was normal. Physical examination was unremarkable except for a 15 week pregnancy. FISH cytogenetics showed a BCR-ABL fusion in 50.2% of the nuclei examined and metaphase cytogenetics confirmed an atypical BCR-ABL translocation t(9;22;11) (q34;q11.2;q13). Because of the pregnancy and also her relatively low white cell count, a decision was made to observe her closely and to postpone bone marrow examination. Her pregnancy proceeded uneventfully and her white count did not rise. The highest white cell count observed before delivery was 18,500 cells/mm3 and the highest platelet count was 562,000 mm.3 In May, 2006 she delivered a normal boy at term. His peripheral blood cytogenetics were completely normal, 46XY. A bone marrow examination on the patient was performed 2 weeks after delivery and showed morphology consistent with chronic myelogenous leukemia. She was counseled about the use of imatinib mesylate in pregnancy and elected to undergo tubal ligation, as she already had two normal children. She was started on imatinib mesylate 400 mg daily. Six months later RT-PCR analysis for BCR-ABL showed no amplifiable mRNA with the translocation. She has remained in molecular complete remission ever since. The most recent RT-PCR exam was in 04/2011.
Materials and Methods
Bone marrow samples were processed at Mayo Clinic Laboratories for cytogenetic analysis, FISH and quantitative RT-PCR.
Results
This patient exhibited a complex three-way translocation in each metaphase from the chromosome study, t(9;22;11)(q34;q11.2;q13). The gene/gene region involved on chromosome 11q13 is unknown. This complex translocation still resulted in a typical Philadelphia chromosome, which demonstrated typical BCR/ABL fusion. This implies that the tyrosine kinase binding site also had the typical structure associated with CML. While the chromosome studies indicated an apparently balanced three-way translocation based on the banding pattern, the FISH results indicated there was concurrent deletion on the abnormal chromosome 9, resulting in a deletion of the residual ABL gene signal and a deletion of the ASS gene at 9q34. In addition, a deletion of the portion of the BCR gene that was supposed to translocate to chromosome 11q13 was also observed by the FISH test. Clinically this patient had a relatively low elevation in WBC count for CML at presentation. Furthermore her WBC count did not change significantly during her pregnancy and the disease followed a stable and somewhat indolent course over that period. This greatly simplified her management. After she delivered and was placed on imatinib mesylate, she went into complete molecular remission in 6 months. This represents a 6 log reduction in the number of neoplastic cells, estimating that 1012 cells existed at presentation and 106 were present in molecular complete remission.2 This raises the question of whether the inclusion of the fragment of chromosome 11 in any way contributed to the slow progression of the disease.
Discussion
Clinical progression of case
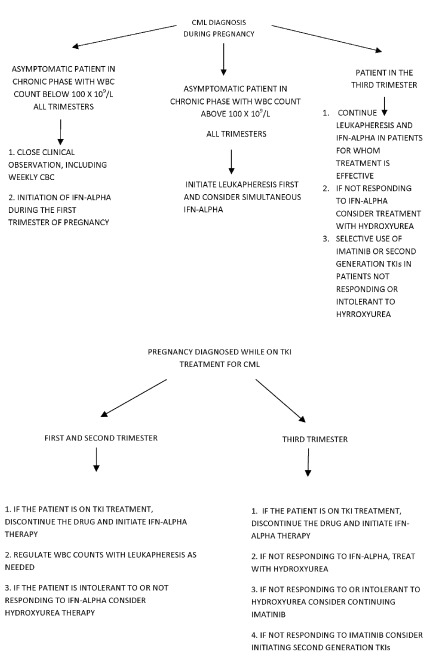
The development of tyrosine kinase inhibitors has markedly changed the prognosis of patients with CML. With the currently available drugs, survivals in excess of six years are common. The incidence of CML in pregnancy is 1–2 per 100,000 pregnancies/year.3,4 Although most patients carry the t(9;22) translocation, atypical translocations are found in approximately 5–8% of cases.5,6 It is plausible that those variations could affect the course of the disease by altering the structure of the tyrosine kinase ligand binding site. Several authors have reported atypical t(9;22;11) translocations in CML and some have commented on the clinical course of the disease associated with the translocation.6,7 Gahrton et al. noted in 1977 that a patient was alive 10 months after diagnosis on busulfan therapy.8 In 1985, Sessarego et al.6 described four cases of variant Philadelphia chromosome translocations in CML. Cytogenetic analysis of one case revealed a complex three-way translocation t(9;11;22)(q34;q13;q11). The disease eventually progressed to blast crisis. The unusual translocation did not change either the expected survival or the course of the disease. However, Belli et al.7 reported a variant translocation t(9;22;11)(q34;q11.2;p15) inv(9) (p13q34) without progression to an accelerated phase after 7 years. The present case provides a somewhat more detailed description of the clinical disease features associated with a similar translocation. CML in pregnancy has been the subject of previous discussions.9 Questions that arise include: i) how women whose CML is diagnosed in pregnancy should be managed; ii) whether women who are on treatment with tyrosine kinase inhibitors for CML should become pregnant; iii) whether there is a risk of fetal damage if the sexual partner of a normal woman is being treated for the disease, and iv) whether women who are taking tyrosine kinase inhibitors should breastfeed. These questions have been addressed for imatinib mesylate,9–17 but a discussion including dasatinib and nilotinib is now pertinent. An algorithm relating to the issues discussed is presented in Figure 1.
Figure 1.
Algorithm for chronic myelogenous leukemia treatment during pregnancy.
Management of Chronic myelogenous leukemia diagnosed during pregnancy
Pregnancy does not alter the natural course of CML. Appropriate management of pregnant patients with CML requires attention to the health of both mother and fetus. The risks to the mother are rapid progression of the disease and thrombotic events, while those to the fetus involve exposure to potentially teratogenic tyrosine kinase inhibitors, fetal prematurity, placental insufficiency and subnormal birth weight.9 Successful pregnancies have been reported with close observation, leuka-pheresis, hydroxyurea, IFN-α, and tyrosine kinase inhibitors.3,4,18–29
Imatinib mesylate and its congeners dasatinib and nilotinib have a potent effect on progression of CML and have improved overall disease survival. Animal studies suggest that imatinib has teratogenic effects.9 Imatinib was found to cause fetal abnormalities, including decreased birth weight, abortion and mortality in rats and rabbits at exposures lower than those expected in humans.9 Nilotinib was not considered teratogenic in these systems.30 Pye et al.31 evaluated the effects of imatinib in 180 women exposed to the drug during pregnancy. Outcomes were available for 125 women, of whom 50% delivered healthy infants. Twelve pregnancies resulted in fetal abnormalities. Cole et al.19 reviewed the cases of women who became pregnant while on imatinib therapy. Complications included low birth weight, hypospadias, meningocele, premature closure of skull sutures, pyloric stenosis, exomphalos and kidney abnormalities. These findings emphasize the teratogenicity of imatinib mesylate and by extension seem to apply to dasatinib and nilotinib. In humans, they have all been classified as pregnancy category D drugs, which means that there is evidence of fetal adverse reactions, but potential drug benefits could warrant use during pregnancy. We conclude that continuing these drugs during pregnancy is undesirable, and that although normal pregnancies can occur, the risk of fetal abnormalities is unacceptably high.
Various alternatives to the use of TKIs in this situation exist. The optimal approach is close clinical observation for suitable patients. This includes the subset of asymptomatic patients with laboratory values close to the normal range. Cole et al.19 reported a 21-years-old female who was diagnosed with CML in early pregnancy. A decision was made to follow her closely during the course of the pregnancy and she delivered a normal baby. Therapy with dasatinib was initiated 4 weeks after delivery. In the present case the patient was monitored closely throughout the duration of her pregnancy and delivered a normal boy with normal chromosomes.
If clinical observation is not feasible, it may be possible to control peripheral white counts with leukapheresis. This method has been widely advocated as the treatment of choice for patients at or near the first trimester to rapidly decrease WBC counts and to control high risks of vascular occlusion.26,32 An obvious advantage to leukapheresis is that it avoids the teratogenic effects of drug administration.25–28,33 Some authors propose that leukapheresis can be used as a treatment throughout pregnancy.26,28 It can lead to hemodynamic instability, but this can be controlled with care in administration. If cytoreductive drugs are necessary, the two leading candidates are IFN-α and hydroxyurea. Of the two, IFN-α is the more desirable. It does not inhibit DNA synthesis directly and it crosses the placenta to a minimal extent4,29,32 Many references describe administration of IFN-α during pregnancy.4,22,29,32,34,35 All pregnancies resulted in healthy babies without congenital abnormalities. Interferon is relatively safe in pregnancy and could be considered a drug of choice.32,34 A limitation to its use could be its relatively long period of onset to maximum effect. However, it could be used in combination with leukapheresis. A number of cases of hydroxyurea administration during pregnancy have been reported.23,24,36 The drug has teratogenic effects both in animals36 and in humans. Robinet et al.36 examined pregnancy outcomes in 31 women exposed to hydroxyurea. Malformations such as hip dysplasia, unilateral renal dilatation and pilonidal sinus were observed; the causal relationship was unclear. These malformations could be regarded as minor but are still greater in frequency than with interferon α.
Should women who are being treated with a TKI become pregnant?
At this point in time, treatment of CML with imatinib or other TKIs commits a patient to a lifetime of therapy. No good guidelines exist for discontinuation of the drug, except in the context of a clinical trial. Even patients in major molecular remission may have 104–106 residual neoplastic bone marrow cells which are sources of possible relapse although they are below the level of detection by PCR amplification. Relapse after discontinuing imatinib mesylate may be followed by failure to reachieve remission when the drug is resumed.32 This raises the question of whether a patient in complete remission, even major molecular remission, on imatinib or another TKI, should become pregnant. The primary recommendation for all three of these drugs is to avoid pregnancy, and if a woman becomes pregnant, the drug should be discontinued. She should be clearly apprised of the issues and be followed by a hematologist familiar with this type of situation and its implications. It is not clear at this point if the teratogenic risk of TKIs extends throughout the entire pregnancy. They should certainly be avoided during the organ-forming period of the first and mid-second trimesters. If a pregnant woman in complete remission has had imatinib discontinued, the drug will be held so long as she remains in complete remission and would be restarted after delivery. If she showed evidence of relapse, then attempts would be made to delay re-initiation until at least the 6th month. The alternatives of leukapheresis, hydroxyurea and IFN-α remain possibilities and can be used on an individualized basis. These considerations apply to all three drugs.
Data on the use of nilotinib in pregnancy are limited. Conchon et al.3 reported a 30-years-old female with CML whose second pregnancy was identified at 7.4 weeks of gestation, while she was on 200 mg of nilotinib; treatment was discontinued during the remainder of her pregnancy. A healthy infant was delivered without complications. After delivery the patient lost molecular, cytogenetic and hematologic remission and was started on dasatinib. The authors nonetheless concluded that if a patient becomes pregnant while on nilotinib, the drug should be discontinued.
Conchon et al.18 described a case of an 18-years-old female who became pregnant while in hematologic remission on dasatinib therapy. The pregnancy was discovered during the first trimester of gestation (after 4 weeks of amenorrhea). Dasatinib treatment was discontinued. The patient relapsed during pregnancy and was treated with IFN-α without complete hematologic response. She delivered a healthy infant without any abnormalities detected through 8 months of age. This patient was exposed to the drug 8 weeks post conception, during the critical period of embryogenesis.
We conclude that although cases of successful pregnancies have been reported with patients on each of these drugs, the elevated teratogenic risk remains. To the extent possible, women on treatment with these TKIs should not become pregnant. Any drugs of this class should be discontinued in a woman found to be pregnant and the patient should be observed until either the pregnancy is complete or the disease starts to progress.
If the sexual partner of an unaffected woman is taking a TKI, will it affect the pregnancy?
Several reports of successful pregnancies conceived by men taking imatinib mesylate exist.11,12,37 These authors report a total of 19 pregnancies conceived from 17 men. Eighteen of these resulted in normal children, without birth defects and one ended in a spontaneous abortion. Examination of the sperm in two patients did not reveal an effect on its motility or count.37 A single recent report has described a male taking dasatinib who fathered a healthy baby.38 In preclinical experiments utilizing drug doses five times higher than those used in humans, nilotinib has not been shown to affect sperm count, motility or fertility in rats.30 We conclude that at this time there is no convincing evidence that treatment of a male sexual partner with imatinib mesylate produces a deleterious effect on pregnancy outcomes.
Should women taking TKIs breast feed infants?
A final question regarding perinatal drug exposure is whether women taking TKIs should breast feed. The data on that subject pertain mostly to imatinib mesylate. Imatinib is known to be secreted in breast milk.10,13–15 The literature on women who breast fed while taking imatinib is very limited. In one case of a woman who breast fed no adverse effect occurred in the newborn.13 Concentrations of the drug and its metabolites were measured in both plasma and breast milk. Plasma steady state concentrations were approximately 3.0 mcg/mL and 1.0 mcg/mL between 1 and 9 hours after oral administration of 400 mg of the drug. Concentrations in breast milk were between 1.1 and 1.4 mcg/mL. The authors concluded that mothers with CML could safely breast feed. Other reports have focused on the excretion of imatinib in breast milk.10,14,15 Ali et al.10 reported a 27-years-old female who was treated with imatinib from the 21st to the 39th weeks of gestation. The infant was a healthy male. Imatinib was detected in the umbilical cord blood, the infant’s peripheral blood and the mother’s breast milk. Two other reports14,15 have shown very similar results. No human data are available for nilotinib and dasatinib. However, one study demonstrated nilotinib transfer into the milk of lactating rats after administration of a dose of 20 mg/kg.39 It seems likely, although it is not yet proven, that these drugs would also be transferred into human milk. Many sources recommend against breast feeding, but it is not possible at this time to make a definitive statement about whether the low levels to which an infant would be exposed are truly harmful.
Conclusions
Pregnancy in CML is an infrequent, but real problem. The number of agents available for the treatment of CML is increasing. The data regarding teratogenicity and abortion rates in patients treated with these drugs are incomplete. Based on the review of the existing data certain generalizations tend to appear. All of these drugs have some teratogenic potential and possibly some abortificient potential as well. To the extent possible, their use should be avoided during the organ forming period of the first five months of pregnancy. If treatment is necessary, leukapheresis or IFN-α alone or in combination appears to be the safest. The same considerations apply to a female who is already on imatinib and wishes to become pregnant. If at all possible, pregnancy should be avoided. Based on the limited evidence available, men who are on imatinib mesylate would be able to safely conceive children. Imatinib is clearly excreted in human breast milk. It is not clear that these levels pose a risk to the child, but if possible breast feeding should be avoided. The same applies to dasatinib and nilotinib, but the data are very limited. Continued attention to this problem in the future is important in order to allow clinicians to make informed decisions.
Acknowledgements:
the authors thank Dr. Adib Khouzami and Dr. John Lister for critical review of the manuscript. Dr. Rhett Ketterling of the Cytogenetics Laboratory at Mayo Clinic provided very helpful discussion on the cytogenetic abnormalities. They also thank the staff of the Medical Library at Conemaugh Memorial Medical Center for their help in assembling bibliographic material.
References
- 1.Sokal JE. Evaluation of survival data for chronic myelocytic leukemia. Am J Hematol. 1976;1:493–500. doi: 10.1002/ajh.2830010414. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PW, Salmon SE. Kinetics of tumor growth and regression in IgG multiple myeloma. J Clin Invest. 1972;51:1697–1708. doi: 10.1172/JCI106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conchon M, Sanabani S, Bendit I. Two successful pregnancies in a woman with chronic myeloid leukemia exposed to nilotinib during the first trimester of her second pregnancy: case study. J Hematol Oncol. 2009;2:42–42. doi: 10.1186/1756-8722-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesquita MM, Pestana A, Mota A. Successful pregnancy occurring with interferon-alpha therapy in chronic myeloid leukemia. Acta Obstet Gynecol Scand. 2005;84:300–1. doi: 10.1111/j.0001-6349.2005.0358b.x. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Lee SJ. DeVita VT, Lawrence TS, Rosenberg SA, et al. Cancer: Principles & Practice of Oncology. (ed 8) Lippincott Williams & Wilkins; Philadelphia: 2008. Chronic leukemias; pp. 2269–2276. [Google Scholar]
- 6.Sessarego M, Ajmar F, Bianchi Scarra GL, et al. Unusual Ph translocations in CML: four new cases. Cancer Genet Cytogenet. 1985;15:199–207. doi: 10.1016/0165-4608(85)90163-3. [DOI] [PubMed] [Google Scholar]
- 7.Belli C, Alu MF, Alfonso G, et al. Novel variant Ph translocation t(9;22;11)(q34;q11.2;p15)inv(9)(p13q34) in chronic myeloid leukemia involving a one-step mechanism. Cytogenet Genome Res. 2011;132:304–8. doi: 10.1159/000322824. [DOI] [PubMed] [Google Scholar]
- 8.Gahrton G, Friberg K, Zech L. A new translocation involving three chromosomes in chronic myelocytic leukemia, 46,XY,t(9;11;22) Cytogenet Cell Genet. 1977;18:75–81. doi: 10.1159/000130750. [DOI] [PubMed] [Google Scholar]
- 9.East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011. Gleevec [package insert] [Google Scholar]
- 10.Ali R, Ozkalemkas F, Kimya Y, et al. Imatinib use during pregnancy and breast feeding: a case report and review of literature. Arch Gynecol Obstet. 2009;280:169–75. doi: 10.1007/s00404-008-0861-7. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy K, Hayden J, Lim Z, et al. Successful pregnancies involving men with chronic myeloid leukemia on imatinib therapy. Br J Haematol. 2007;137:374–5. doi: 10.1111/j.1365-2141.2007.06542.x. [DOI] [PubMed] [Google Scholar]
- 12.Ault P, Kantarjian H, O’Brien S, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24:1204–8. doi: 10.1200/JCO.2005.04.6557. [DOI] [PubMed] [Google Scholar]
- 13.Gambacorti-Passerini CB, Tornaghi L, Marangon E, et al. Imatinib concentrations in human milk. Blood. 2007;109:1790–1790. doi: 10.1182/blood-2006-08-039545. [DOI] [PubMed] [Google Scholar]
- 14.Russell MA, Carpenter MW, Akhtar MS, et al. Imatinib mesylate and metabolite concentrations in maternal blood, umbilical cord blood, placenta and breast milk. J Perinatol. 2007;27:241–3. doi: 10.1038/sj.jp.7211665. [DOI] [PubMed] [Google Scholar]
- 15.Kronenberger R, Schleyer E, Bornhauser M, et al. Imatinib in breast milk. Ann Hematol. 2009;88:1265–6. doi: 10.1007/s00277-009-0754-2. [DOI] [PubMed] [Google Scholar]
- 16.Apperley J. CML in pregnancy and childhood. Best Pract Res Clin Haematol. 2009;22:455–74. doi: 10.1016/j.beha.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Apperley J. Issues of imatinib and pregnancy outcome. J Natl Compr Canc Netw. 2009;7:1050–8. doi: 10.6004/jnccn.2009.0069. [DOI] [PubMed] [Google Scholar]
- 18.Conchon M, Sanabani SS, Serpa M, et al. Successful pregnancy and delivery in a patient with chronic myelogenous leukemia while on dasatinib therapy. Adv Hematol. 2010;2010:136252–136252. doi: 10.1155/2010/136252. [Epub 2010, Mar 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole S, Kantarjian H, Ault P, et al. Successful completion of pregnancy in a patient with chronic myeloid leukemia without active intervention: a case report and review of literature. Clin Lymphoma Myeloma. 2009;9:324–7. doi: 10.3816/CLM.2009.n.064. [DOI] [PubMed] [Google Scholar]
- 20.Tsuzuki M, Inaguma Y, Handa K, et al. Successful pregnancy in a patient with chronic myeloid leukemia under treatment with imatinib. Inter Med. 2009;48:1433–5. doi: 10.2169/internalmedicine.48.2084. [DOI] [PubMed] [Google Scholar]
- 21.Garderet L, Santacruz R, Barbuv, et al. Two successful pregnancies in a chronic myeloid leukemia patient treated with imatinib. Haematologica. 2007;92:e9–10. doi: 10.3324/haematol.10935. [DOI] [PubMed] [Google Scholar]
- 22.Mubarak AA, Kakil IR, Awidi A, et al. Normal outcome of pregnancy in chronic myeloid leukemia treated with interferon alpha in 1st trimester: report of three cases and review of literature. Am J Hematol. 2002;69:115–8. doi: 10.1002/ajh.9876. [DOI] [PubMed] [Google Scholar]
- 23.Fadilah SA, Ahmad-Zailani H, Soon-Keng C, et al. Successful treatment of chronic myeloid leukemia during pregnancy with hydroxyurea. Leukemia. 2002;16:1202–3. doi: 10.1038/sj.leu.2402494. [DOI] [PubMed] [Google Scholar]
- 24.Jackson N, Shukri A, Ali K. Hydroxyurea treatment for chronic myeloid leukemia during pregnancy. Br J Haematol. 1993;85:203–4. doi: 10.1111/j.1365-2141.1993.tb08672.x. [DOI] [PubMed] [Google Scholar]
- 25.Klaasen R, de Jong P, Wijermans PW. Successful management of chronic myeloid leukemia with leucapheresis during a twin pregnancy. Neth J Med. 2007;65:147–9. [PubMed] [Google Scholar]
- 26.Ali R, Ozkalemkas F, Ozkocaman V, et al. Successful pregnancy and delivery in a patient with chronic myelogenous leukemia (CML), and management of CML with leukapheresis during pregnancy: a case report and review of the literature. Jpn J Clin Oncol. 2004;34:215–7. doi: 10.1093/jjco/hyh038. [DOI] [PubMed] [Google Scholar]
- 27.Bazarbashi MS, Smith MR, Karanes C, et al. Successful management of Ph chromosome chronic myelogenous leukemia with leukapheresis during pregnancy. Am J Hematol. 1991;38:235–7. doi: 10.1002/ajh.2830380316. [DOI] [PubMed] [Google Scholar]
- 28.Strobl FJ, Voelkerding KV, Smith EP. Management of chronic myeloid leukemia during pregnancy with leukapheresis. J Clin Apher. 1999;14:42–4. doi: 10.1002/(sici)1098-1101(1999)14:1<42::aid-jca8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Haggstrom J, Adriansson M, Hybbinette T, et al. Two cases of CML treated with alpha-interferon during second and third trimester of pregnancy with analysis of the drug in the new-born immediately postpartum. Eur J Haematol. 1996;57:101–2. doi: 10.1111/j.1600-0609.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 30.East Hanover,NJ: Novartis Pharmaceuticals Corporation; 2011. Tasigna [package insert] [Google Scholar]
- 31.Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111:5505–8. doi: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapira T, Pereg D, Lishner M. How I treat acute and chronic leukemia in pregnancy. Blood Rev. 2008;22:247–59. doi: 10.1016/j.blre.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald D, Rowe JM, Heal J. Leukapheresis for control of chronic myelogenous leukemia during pregnancy. Am J Hematol. 1986;22:213–8. doi: 10.1002/ajh.2830220213. [DOI] [PubMed] [Google Scholar]
- 34.Kuroiwa M, Gondo H, Ashida K, et al. Interferon-alpha therapy for chronic myelogenous leukemia during pregnancy. Am J Hematol. 1998;59:101–2. doi: 10.1002/(sici)1096-8652(199809)59:1<101::aid-ajh23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 35.Al Bahar S, Pandita R, Nath SV. Pregnancy in chronic myeloid leukemia patients treated with alpha interferon. Int J Gynaecol Obstet. 2004;85:281–2. doi: 10.1016/j.ijgo.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Thauvin-Robinet C, Maingueneau C, Robert E, et al. Exposure to hydroxyurea during pregnancy:a case series. Leukemia. 2001;15:1309–11. doi: 10.1038/sj.leu.2402168. [DOI] [PubMed] [Google Scholar]
- 37.Breccia M, Cannella L, Montefusco E, et al. Male patients with chronic myeloid leukemia treated with imatinib involved in healthy pregnancies: report of five cases. Leuk Res. 2008;32:519–20. doi: 10.1016/j.leukres.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Oweini H, Otrock ZK, Mahfouz RA, et al. Successful pregnancy involving a man with chronic myeloid leukemia on dasatinib. Arch Gynecol Obstet. 2011;283:133–4. doi: 10.1007/s00404-010-1501-6. [DOI] [PubMed] [Google Scholar]
- 39.Novartis Oncology. East Hanover, NJ 07936: Data on File [AMN107-nilotinib-Investigators' Baltimore Edition5, July 13,2009] [Google Scholar]