Abstract
Hexane (HEX), dichloromethane (DM), ethyl acetate (EA) and methanol (M) extracts (0.1, 0.2 and 0.4mg/ml) were obtained via Soxhlet from Plathymenia reticulata barks (Pr). These extracts were evaluated against the myotoxicity (58%) and the irreversible in vitro neuromuscular blockade of Bothrops jararacussu (Bjssu) venom (40μg/ml) in a mouse phrenic-nerve diaphragm preparation, by using light-microscopy and conventional myographic techniques. Thin layer chromatography (TLC) was used to access the basic composition of extracts. The efficacy of the extracts was analyzed by Student's t-test or repeated measures ANOVA. The significance level was set at 5%. The Pr extracts showed a higher polyphenols content (3.75%), from which tannins take part, around 20 times more than flavonoids content (0.16%). Qualitatively, via TLC, DM and EA extracts showed higher tannins concentration than the HEX and M extracts. Pharmacologically, at 0.4mg/ml, DM was more effective (92 ± 6.2%) than EA (81.3 ±10%) = HEX, 77.2 ±4.7%) > M (54 ±10%) against the toxic effects of the venom. Morphologically, DM extract preserved intact 52.8% of the muscle fibers in the presence of the venom. We concluded that P. reticulata extracts are able to inhibit toxic effects of B. jararacussu venom, whose protective mechanism could be mediated by tannins.
Keywords: Bothrops jararacussu, neuromuscular preparation, snake venom, tannin, polyphenol, flavonoid, Plathymenia reticulata
INTRODUCTION
Plathymenia reticulata Benth (Fabacea, Mimosoideae) genus biometry was recently related by Lopes et al (2010). This genus is represented by tropical trees from South America and is found in Brazil, Bolivia, north of Paraguay and Suriname (Lewis and Warwick, 2003). The P. reticulata (Pr) species, popularly known as “vinhático-do-cerrado”, is found in open formations of Brazilian “cerrado”, since Amapá state (extreme north) up to São Paulo state (southeast), being found in all states of west-central region (Almeida et al, 1998). It was classified by Brazilian Agricultural Research Corporation (EMBRAPA) as one of the most important and useful plant species from “cerrado” due to its high quality wood and its potential use for the recovery of degraded areas (Heringer and Ferreira, 1972).
Besides the economical importance, recent studies have shown its medicinal value for treating some inflammatory and infectious diseases in folk medicine (Pott et al, 2004). P. reticulata-bark infusions are used as medicinal bath for many illnesses, such as hemorrhage and insects/ticks bites (Pott and Pott, 1994). Some P. reticulata extracts also showed in vitro antimicrobial activity against Streptococcus mutans and Staphylococcus sp by Fernandes et al (2005), and in Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Candida parapsilosis, promastigote forms of Leishmania amazonensis, and poliovirus by de Toledo et al (2011). An antiophidian property of P. reticulata extracts was also previously suggested (Melo et al, 2009). The main constituents of P. reticulata extracts are tannins and flavonoids (Fernandes et al, 2005).
In 2007, the ophidic accidents were included as a neglected tropical disease by World Health Organization (WHO, 2007), since “Envenoming resulting from snake bites is a particularly important public health problem in rural areas of tropical and subtropical countries situated in Africa, Asia, Oceania and Latin America” (WHO, 2011). Oliveira et al (2010) observed approximately 27,000 ophidic accidents in 2008 from Brazilian official databases. Bothrops and Bothropoides snakes were responsible for about 70% of these cases. The main symptoms are local tissue damage (hemorrhage, necrosis, and edema) and alterations in blood coagulation that are induced by a complex mixture of toxic enzymes and proteins in the crude snake venoms (Fernandes et al, 2011). Serum therapy is the most efficacious treatment against lethality induced by snakebites, but it has limited action against the local myotoxic action (Cardoso et al, 2003). Thus, the search for new alternatives is necessary, mainly in biodiversity-rich and poor countries.
The aim of the present study was to observe the ability to neutralize the in vitro neuromuscular blockade and myotoxicity of B. jararacussu venom of P. reticulata bark extracts.
MATERIALS AND METHODS
Plathymenia reticulata
The barks from P. reticulata were collected in December 2007 in Miracema city, Tocantins State, Brazil. A specimen was deposited (protocol NRHTO 3327 Coord. 48º 23′ 39″ S - 9º 41′ 34″ W) at the herbarium of Federal University of Tocantins (UFT). The barks were dehydrated at 37ºC (Fanen® incubator) for 48hr, and pulverized in a grinder of knives and hammers (Marconi®). Part of the powdered sample was extracted in a hydroalcoholic extract in a 20% (m/v) final concentration (Farmacopéia Portuguesa, 2002) for further chemical characterization. The powdered sample was also extracted with solvents of crescent polarities (hexane, dichloromethane, ethyl acetate and methanol) in Soxhlet apparatus, and evaporated in a water bath.
Determination of the flavonoids
The content of flavonoids in P. reticulata hydroalcoholic extract was determined as described previously (Mori, 1997; Harborne, 1998), based on the UV absorption of Al-Cl3-flavonoid complexes and expressed as the total content of quercetin. The percentage of flavonoids (%) was calculated from a standard curve of quercetin prepared in methanol (0, 4, 8, 12, and 16μg/ml).
Determination of Polyphenols
The content of polyphenols was determined in the hydroalcoholic extract of P. reticulata barks as described by Reicher et al (1982). The percentage of polyphenols (%) was determined from a standard curve (5, 10, 15, 20, 25, 30, 35, and 40μg/ml) of pyrogallol (Sigma®, USA).
Thin layer chromatography (TLC)
Aliquots of hexane (HEX), dichloromethane (DM), ethyl acetate (EA) and methanol (M) extracts from the powder of P. reticulata barks were spotted onto 0.3mm thick silica-gel or GF254 plates (Merck®) along with appropriate standards (Harbone, 1998; Simões et al, 2004). The TLC system for running the extracts consisted of system acetone:chloroform:formic acid (10:75:8, v/v) with NP/PEG as follows: 5% (v/v) ethanolic NP (diphenylboric acid 2-aminoethyl ester, Sigma®), followed by 5% (v/v) ethanolic PEG4000 (polyethylene glycol 4000, Synth®), being visualized under UV light at 360nm. The presence of phytochemical groups such as phenolic acids and flavonoids was investigated by comparison with the following standards: Caffeic acid, chlorogenic acid, and tannic acid, all solubilized in methanol (1mg/ml), with or without chromogenic agent. The retention factor (Rf) of each standard was compared with spots exhibited by P. reticulata extracts.
Qualitative determination of tannins
The powder of P. reticulata barks showed the aroma and color (pink), which are characteristic of tannin-rich compounds (Costa, 1987). A hot-water extract was obtained from 2gm powder of P. reticulata barks followed by 40ml distilled water. The mixture was boiled for 2min and was filtered; the process was repeated by a second addition of 20ml distilled water to the residue. Four general assays were carried out by using 5ml of the extract added of three drops of each one of the following reagents (% in w/v): Ferric chloride 2%, plumbum acetate 10%, cuprum acetate 5% and gelatin 2%. An assay to detect hydrolysable tannins was also performed by adding 5ml of 10% (v/v) glacial acetic acid and 3ml of 10% (w/v) plumbum acetate to 5ml of the aqueous extract. Condensed tannins were accessed by adding 5ml of p-dimethyl aminobenzaldehyde (Wasick reagent) to the extract. Catechin was verified by using a toothpick added into 5ml of the extract. The toothpick was then boiled for 2min and received a few drops of concentrated chloridric acid.
Protein precipitation assay and tannin determination
The proteins in the extracts were precipitated (Hagerman and Butler 1989) with 1.0mg/ml bovine serum albumin (BSA, fraction V, Sigma) solution in 0.2M acetate buffer (pH 4.9). The tannin concentration measurement was carried out by using the methanol extract due its solubility. A standard curve using tannic acid was obtained by a polynomial regression (y=0.673x-0.173, R=0.997) and was used to estimate tannin concentrations. All tests were performed in triplicates.
Animals
Male Swiss white mice (26-32gm) supplied by Animais de Laboratório (Anilab, Paulínia, Brazil) were housed at 25 ±3°C on a 12hr light/dark cycle and had access to food and water ad libitum. This project was approved by the institutional Committee for Ethics in Research from University of Vale do Paraiba (protocol number noA22 - UNIVAP - CEP/2008), and the experiments were performed within the guidelines of the Brazilian College for Animal Experimentation.
Bothrops jararacussu venom
Crude venom was obtained from an adult B. jararacussu (Bjssu) snake (Nature Studies Centre's Serpentary) and was certified by Professor Dr José Carlos Cogo of Vale do Paraíba University (UNIVAP, São José dos Campos, SP, Brazil).
Mouse phrenic nerve-diaphragm muscle (PND) preparation
The phrenic nerve-diaphragm (Bülbring, 1946) was obtained from mice anesthetized with halotane and sacrificed by exsanguination. The diaphragm was removed and mounted under a tension of 5gm in a 5ml organ bath containing aerated Tyrode solution (control) of the following composition (mM): NaCl 137; KCl 2.7; CaCl2 1.8; MgCl2 0.49; NaH2PO4 0.42; NaHCO3 11.9; and glucose 11.1. After equilibration with 95% O2/5% CO2 (v/v), the pH of this solution was 7.0. The PND myographic recording was performed according to Melo et al (2009). PND were allowed to stabilize for at least 20min before addition of the following reagents: Tyrode solution (control, n=7); P. reticulata extracts (HEX, DM, EA and M) at three different concentrations (mg/ml) of 0.1; 0.2 and 0.4 (with n=3-5); Bjssu venom alone (40μg/ml, n=7) and pre-incubation of Bjssu (40μg/ml) + HEX, DM, EA and M extracts (at 0.1; 0.2; or 0.4mg/ml) mixtures during 15min (with n=3-7). The concentration of Bjssu venom was chosen after corroborating its pharmacological action.
Quantitative histological study
Preparations resulting from pharmacological assays were analyzed by quantitative morphometry. At the end of the experiments (120min time period), the preparations used in Tyrode (control), DM (0.4mg/ml), Bjssu venom (40μg/ml) and DM (0.4mg/ml) + Bjssu venom (40μg/ml) were fixed in Bouin solution and processed by routine morphological techniques. Cross-sections (5μm thick) of diaphragm muscle were stained with 0.5% (w/v) hematoxylin-eosin for microscopy examination. Tissue damage (edema, intense myonecrosis characterized by atrophy of the muscle fibers, hyaline aspect, sarcolemmal disruption and lysis of the myofibrils) was expressed in myotoxicity index (MI), i.e., the percentage of damaged muscle cells number divided by the total number of cells in three non-overlapping, non-adjacent areas of each preparation (de Jesus Reis Rosa et al, 2010).
Statistical analysis
Results were expressed as the mean ± standard error mean (±SEM). The Student's t-test or repeated measures ANOVA were used for statistical comparison of the data. The significance level was set at 5%.
RESULTS
The phytochemical characterization of initial hydroalcoholic extract from P. reticulata barks showed 0.16% of flavonoids and 3.75% of polyphenols. The four general reactions of qualitative determination of tannins were positive: A violet-blue color, the precipitation with plumbum-acetate, cuprum-acetate and gelatin, showed hydrolysable tannins. Specific reactions for hydrolysable and condensed tannins were also positive for gallic acid presence and by a pink color development, respectively. The catechin assay was positive, being a wine-red color observed on the toothpick. The efficiency of P. reticulata extracts production was 1.09g% for hexane, 1.15g% for dichloromethane, 1.98g% for ethyl acetate, and 1.39g% for methanol solvents. The tannin concentration in methanol extract was 1.2%.
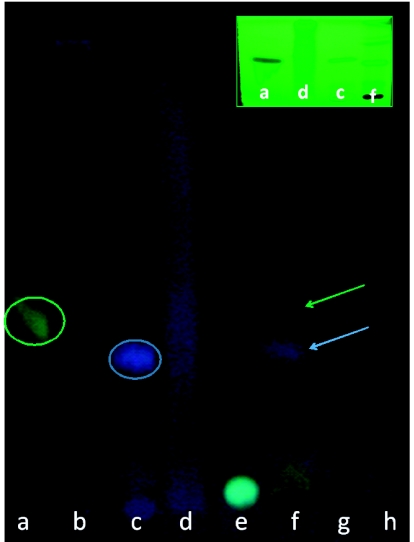
Figure 1 illustrates the chromatographic profile of HEX (b), DM (d), EA (f), and M (h) extracts obtained from P. reticulata barks, compared with the commercial phytochemicals. The retention factor (Rf) of each commercial phytochemical was 0.3 for caffeic acid, 0.25 for tannic acid, 0.2 for chlorogenic acid and 0 for rutin. Note that the solvent system did not permit the migration of constituents from HEX and M extracts. However, it did allow us to identify phenolic compounds by the blue and green stains in the DM and EA extracts, which are suggestive of caffeic acid and tannic acid, respectively (also indicated by green and blue arrows, respectively). This Figure also shows (see top, right panel) a GF254 plate visualized under UV light, with DM and EA extracts along with caffeic and tannic acids, corroborating that the extracts contained these phytochemicals. Note the expressive blue stain in DM extract revealing its tannin-rich content.
Figure 1.
Thin layer chromatography of P. reticulata extracts. Solvent system (v/v): acetone (10%), chloroform (75%) and formic acid (8%). Developer: NP/PEG. Extracts: hexane along (b), dichloromethane (d), ethyl acetate (f) and methanol (h). Standards: caffeic acid (a), tannic acid (c) (both are circled), chlorogenic acid (e) and rutin (g). Chromatography image (prepared on GF254 plate) is shown on the top right of the figures to indicate the caffeic and tannic acids in DM and EA extracts, under UV light and with no developer. Arrows indicate the phytochemicals, caffeic acid (green) and tannic acid (blue), in ethyl acetate extract. Note that dichloromethane extract shows a blue stain, suggestive of high tannin-content.
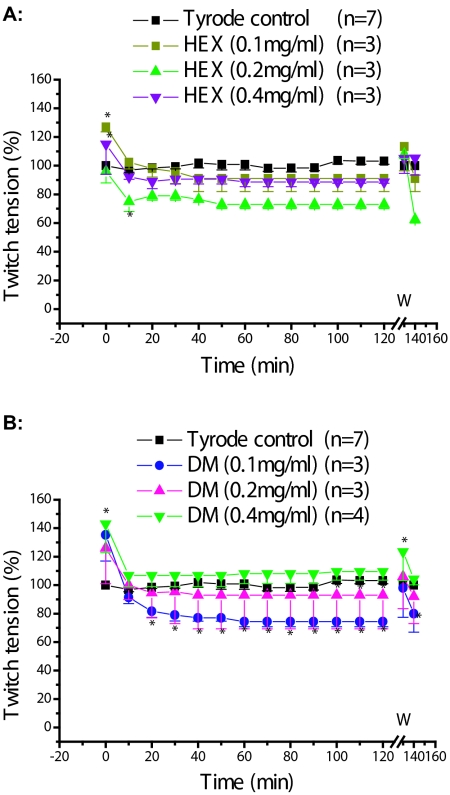
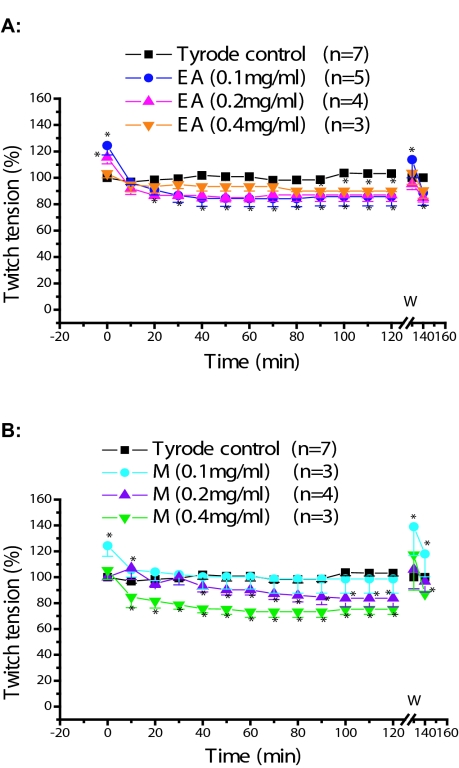
Figure 2 shows the pharmacological effects of HEX (A) and DM (B) extracts at 0.1, 0.2, and 0.4mg/ml concentrations. The results were statistically compared to nutritive solution (Tyrode control) until the end of experiment (120min). No statistically significant differences (p>0.05) were observed in 2A, except for the periods 0 (for HEX 0.1 and 0.4mg/ml) and 10min (for 0.2mg/ml). The effect of EA and M extracts is shown in Figure 3A and 3B, respectively.
Figure 2.
Phrenic nerve-diaphragm preparation, indirect stimulation: Pharmacological effects of P. reticulata extracts; A and B represent hexane (HEX) and dichoromethane (DM) extracts, respectively, at various concentrations as indicated. Each point represents the mean ±SEM of the number of experiments (n). * p<0.05; W = washing.
Figure 3.
Phrenic nerve-diaphragm preparation, indirect stimulation: Pharmacological effects of P. reticulata extracts. A and B are ethyl acetate (EA) and methanol (M) extracts, respectively, at concentrations (mg/ml) of 0.1, 0.2 and 0.4. Each point represents the mean ±SEM of the number of experiments (n). * p<0.05; W = washing.
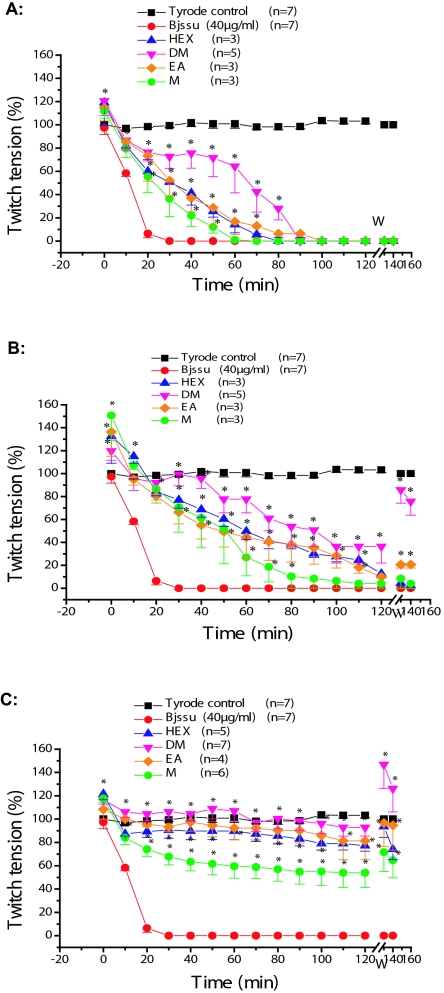
The neutralizing ability against the neuromuscular blockade-induced by Bjssu venom (40μg/ml) of HEX, DM, EA and M extracts at concentrations of (A) 0.1mg/ml, (B) 0.2mg/ml and (C) 0.4mg/ml are shown in Figure 4. Figure 4A shows that none of the 0.1mg/ml extracts was able to neutralize Bjssu venom. At 0.2mg/ml concentration (Figure 4B), DM extract showed protective effect (p<0.05) when compared to the crude Bjssu-venom. Figure 4C shows that the concentration of 0.4mg/ml was the ideal concentration to neutralize the venom effect. DM extract showed no statistical difference when compared to Tyrode solution (control). None statistically significant difference (p>0.05) was observed between the effects of HEX and EA extracts. In resume, the protection of all extracts against the paralysis of Bjssu venom was (in decreasing order): DM (92 ± 6.2%) > EA (81.3 ±10%) = HEX, 77.2 ±4.7%) > M (54 ±10%).
Figure 4.
Phrenic nerve-diaphragm preparation, indirect stimulation: Neutralization assays using different concentrations of P. reticulata extracts. Note the blockade-induced by Bjssu venom alone (40μg/ml) and the protection level of each extract. A. each extract at 0.1mg/ml; B. each extract at 0.2mg/m, and; C. each extract at 0.4mg/ml. Each point represents the mean ±SEM of the number of experiments (n). *p<0.05 at the point indicated and subsequent to others, in relation to Bjssu venom. Bjssu = B. jararacussu.
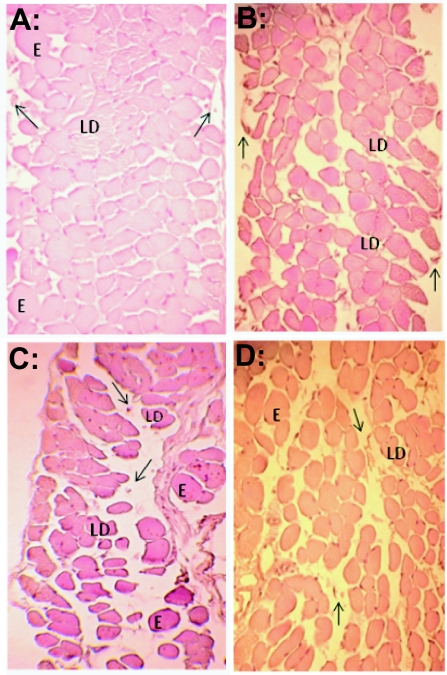
Among the extracts, DM extract was selected for the morphological assay. Muscles (n=3) exposed for 120min to Tyrode solution (control), Bjssu venom (40μg/ml), DM extract (0.4mg/ml), and 40μg/ml venom plus 0.4mg/ml DM were evaluated morphologically by light microscopy.
Muscles incubated with Tyrode solution (Figure 5A) and DM extract (Figure 5B) showed muscle fibers organized in polygonal rearrangement, alternating with some small voids and losing some nuclei (arrow, ghost cells). Some lesions, such as edema (E), delta lesion (DL) and condensation of myofibrils were observed. The myotoxicity index (MI) for Tyrode solution was 15.9 ±0.8% and for DM extract was 29.1 ±11%. The animals submitted to Bjssu venom (40μg/ml, Figure 5C) showed empty muscle spaces representing the loss of fiber caused by intense myotoxic action (MI = 58.4 ±1%). The percentage of working muscle cells (58.2 ±1%) resulting from the exposure to pre-incubated mixture (40μg/ml Bjssu venom plus 0.4mg/ml DM extract, Figure 5D) shows the protection level of DM extract.
Figure 5.
Cross-section of preparation incubated with: A. Tyrode solution (control); B. (DM extract 0.4mg/ml); C. (Bjssu venom, 40μg/ml) and; D. (Bjssu 40μg/ml + DM extract 0.4mg/ml). E, edema. LD, lesion delta type. Arrow, nuclei loss and membrane disruption. DM, dichloromethane extract. Bars=50 μm. Ojective 40x. Zoom camera Canon PowerShot A620 7.1 mega pixels 4x optical zoom.
DISCUSSION
The exploitation of plant species with medicinal potential in Brazil has been relatively insignificant. A previous study reported that 99.6% of the 55,000 angiosperm species estimated in the Brazilian territory were still unknown to the scientific community, considering their phytochemical and pharmacological properties. Despite of this significance, the situation remains practically unchanged (Rodrigues and Carlini, 2004).
Many plants from Brazilian cerrado suffer deforesting threat (IBGE, 2004). The encouragement for agricultural development, improvement of pasture for high-density cattle-grazing and charcoal production for steel industry has threaten the cerrado area, which is one of the most important Brazilian biomes, leading to the extinction of many potential species with unexplored pharmacological properties (Ratter et al, 1997). The medicinal use of a given plant is a manner of adding value and controlling the natural resource devastation, as result of popular consciousness. Besides, the snake envenoming is a worldwide health problem, particularly in the Latin American countries (Warrel, 2004).
Like plants, snakes venom can also be considered a sophisticated laboratory of biotechnology (Mahmood et al, 2005), since both are able to produce many pharmacologically bioactive substances. However, it is necessary to identify and isolate the chemical compounds responsible for each pharmacological effect of those substances. Thus, the use of solvents for separating the main classes of plant constituents from each other is usually required. Hexane (HE) is used to extract apolar constituents, such as terpenes and chlorophils; dichloromethane (DM) is employed to remove chlorophils and components with low polarity; ethyl acetate (EA) removes low chlorophil containing-components, and methanol (M) separates some polar compounds (Cintra-Francischinelli et al, 2008).
It is known that P. reticulata is a plant that contains tannins and flavonoids (Fernandes et al, 2005). The level of vegetable tannins normally found in most plant tissues, such as fruit and leaves, is in the range 2-5% of the wet-weight (Haslam, 2007). In the present study, the methanolic extract of P. reticulata barks showed similar concentration (1.2%) of tannins. The chromatographic profile showed that caffeic and tannic acids in DM and EA extracts. These phytochemicals (blue colour, phenolic group), but not rutin (orange colour, flavonoids group), were eluted in the chromatography assay. Probably the extraction method, which used Soxhlet – a hot process, did not achieve complete separation of the constituents, and the same compounds could be recovered in several extracts (Harborne, 1998).
B. jararacussu venom causes irreversible in vitro neuromuscular blockade that was first described by Rodrigues-Simioni et al (1983). Subsequently, Homsi-Brandeburgo et al (1988) isolated the bothropstoxin-I, the major myotoxin of crude venom that is able to reproduce the principal toxic effects of venom, i.e., in vitro paralysis and extensive myonecrosis. These toxic effects were chosen in the present study to evaluate the protective ability of the P. reticulata extracts. A progressive protective level that is directly proportional to the increase of extract concentration is shown in Figure 4. A total protection was achieved by DM extract. In opposite to other plants, such as Casearia sylvestris (Cintra-Francischinelli et al, 2008) and Dipteryx alata (Nazato et al, 2010), P. reticulata methanolic extract was not the most efficacious agent against the paralysis induced by B. jararacussu venom.
In the present study, dichloromethane extract was the most efficacious against the blockade-induced by Bjssu venom. Our results, as clearly indicated by TLC, show that tannins are present in DM and EA extracts, among other unidentified constituents. The mechanisms by which plants can act against the snake venoms have been extensively studied. Among them, the phytochemicals as catequines, flavones, anthocyanines and condensated tannins were related in their abilities in the chelation of the zinc required for the catalytic activity of venom's hemorrhagic metalloproteinases (Castro et al, 1999).
Tannins in the aqueous extract prepared from Schizolobium parahyba (Sp) leaves, a native plant from Atlantic Forest (Brazil), together with other compounds, displayed specific inhibitory abilities against some biological (lethality, blood incoagulability, haemorrhagic and indirect haemolytic activities) and enzymatic (fibrigenolitic) activities of B. alternatus and B. moojeni venoms (Vale et al, 2008).
Melo et al (2009) have shown the effect of tannins against B. jararacussu and Crotalus durissus terrificus venoms, with visible venom protein precipitation and loss of toxicity. In agreement with the data of Melo et al (2009), our results also suggest that tannins present in DM and EA extracts were responsible by the best inhibition of neuromuscular blockade of venom. Related to myotoxic effect, DM extract also minimized the cell damage induced by Bjssu venom to 58%, which is comparable to the effect of a commercial antibothropic-venom (about of 62% of protection) (Oshima-Franco et al, 2000). Besides, those remaining non-damaged cells maintained 92±6.2% of the contractile ability, showing the well-known robustness of the neuromuscular preparations (Theodorou and Valero-Cuevas, 2010).
The medicinal potential of P. reticulata, particularly the mutagenic effect of its hydroalcoholic extract, has been previously related (Della Torre et al, 2011). The mutagenic effect, initially considered a toxic effect, can be also considered as an anti-tumoral property, as described previously (Gali et al, 1992; Perchellet et al, 1992).
Future studies must address the fractioning of the DM extract in order to identify the bioactive compound, and the development of pharmaceutical formulations (cream or gel) for treating lesions induced by bothropic venom.
CONCLUSIONS
Dichloromethane extract from P. reticulata barks, which contain high polyphenols content, was able to reverse the neuromuscular blockade and myotoxic effects of B. jararacussu venom, probably by protein precipitation induced by tannins.
Acknowledgments
This work was supported by a research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Proc. 04/09705-8; 07/53883-6, 08/52643-4), and PROBIC/UNISO.
LIST OF ABBREVIATIONS
- Bjssu
Bothrops jararacussu
- BSA
Bovine serum albumin
- DM
Dichloromethane
- EA
Ethyl acetate
- HEX
Hexane
- MI
Myotoxicity index
- Rf
Retention factor
- TLC
Thin layer chromatography
COMPETING INTERESTS
None declared.
REFERENCES
- 1.Almeida SP, Proença CEB, Sano SM, Ribeiro JF. Cerrado: Espécies Vegetais Úteis. 1st Edition EMBRAPA-CPAC, Planaltina, Brazil; 1998. [Google Scholar]
- 2.Bülbring E. Observations on the isolated phrenic nerve diaphragm preparation of the rat. Br J Pharmacol. 1946;1:38–61. doi: 10.1111/j.1476-5381.1946.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso JLC, França FOS, Wen FH, Malaque CMS, Haddad V., Jr . 1st Edition Editora Sarvier; São Paulo, Brazil: 2003. Animais peçonhentos no Brasil: biologia, clínica e terapêutica dos acidentes. [Google Scholar]
- 4.Castro O, Gutiérrez JM, Barrios M, Castro I, Romero M, Umaña E. Neutralization of the hemorrhagic effect induced by Bothrops asper (Serpentes: Viperidae) venom with tropical plant extracts. Rev Biol Trop. 1999;47:605–616. [PubMed] [Google Scholar]
- 5.Cintra-Francischinelli M, Silva MG, Andréo-Filho N, Gerenutti M, Cintra ACO, Giglio JR, et al. Phytother Res. 2008;6:784–790. doi: 10.1002/ptr.2365. [DOI] [PubMed] [Google Scholar]
- 6.Costa AF. 3rd Edition Fundação Calouste Gulbenkian; Lisboa: 1987. Farmacognosia. [Google Scholar]
- 7.de Jesus Reis Rosa L, Silva GA, Filho JA, et al. The inhibitory effect of Camellia sinensis extracts against the neuromuscular blockade of Crotalus durissus terrificus venom. J Venom Res. 2010;1:1–7. [PMC free article] [PubMed] [Google Scholar]
- 8.de Toledo CE, Britta EA, Ceole LF, et al. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaça as extractor liquid. J Ethnopharmacol. 2011;133:420–425. doi: 10.1016/j.jep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Della Torre A, Albuquerque LBL, Farrapo NM, et al. Mutagenicity induced by the hydroalcoholic extract of the medicinal plant Plathymenia reticulata Benth. J Venom Anim Toxins Incl Trop Dis. 2011;17:190–198. [Google Scholar]
- 10.Farmacopéia Portuguesa. Instituto Nacional da Farmácia e do Medicamento; Infarmed, Lisboa, Portugal. 2002. [Google Scholar]
- 11.Fernandes RS, Costa TR, Marcussi S, et al. Neutralization of pharmacological and toxic activities of Bothrops jararacussu snake venom and isolated myotoxins by Serjania erecta methanolic extract and its fractions. J Venom Anim Toxins Incl Trop Dis. 2011;17:85–93. [Google Scholar]
- 12.Fernandes TT, Santos AT, Pimenta FC. Atividade antimicrobiana das plantas Plathymenia reticulata, Hymenaea courbaril e Guazuma ulmifolia. Rev Patol Trop. 2005;34:113–122. [Google Scholar]
- 13.Gali HU, Perchellet EM, Klish DS, Johnson JM, Perchellet JP. Antitumor-promoting activities of hydrolyzable tannins in mouse skin. Carcinogenesis. 1992;13:715–718. doi: 10.1093/carcin/13.4.715. [DOI] [PubMed] [Google Scholar]
- 14.Hagerman AE, Butler LG. Choosing appropriate methods and standards for assaying tannin. J Chem Ecol. 1989;15:1795–1810. doi: 10.1007/BF01012267. [DOI] [PubMed] [Google Scholar]
- 15.Harbone JB. 3rd Edition Chapman & Hall; London, England: 1998. Phytochemical Methods: A Guide to Modern Techniques of Plants Analysis. [Google Scholar]
- 16.Heringer EP, Ferreira MB. P. foliolosa Benth. e P. reticulata Benth., vinhático da mata e vinhático do campo (par vicariante) Vol. 5. Cerrado: 1972. Árvores úteis no cerrado (I): Vinhático: o gênero Plathymenia Benth; pp. 28–34. [Google Scholar]
- 17.Haslam E. 1st Edition Cambridge University Press; Cambridge, England: 1998. Practical poliphenolics: from structure to molecular recognition and physiological action. [Google Scholar]
- 18.Haslam E. Vegetable tannins – Lessons of a phytochemical lifetime. Phytochemistry. 2007;68:2713–2721. doi: 10.1016/j.phytochem.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Homsi-Brandeburgo MI, Queiroz LS, Santo-Neto H, Rodrigues-Simioni L, Giglio JR. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of bothropstoxin. Toxicon. 1988;26:615–627. doi: 10.1016/0041-0101(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 20.IBGE Instituto Brasileiro de Geografia e Estatística. Reserva ecológica do IBGE. Ambiente e Plantas Vasculares. Estudos & Pesquisas; Rio de Janeiro, Brazil. 2004. Informações Geográficas 3. [Google Scholar]
- 21.Lewis GP, Warwick MC. Revision of Plathymenia (Leguminosae-Mimosoideae) Edinb J Bot. 2003;60:111–119. [Google Scholar]
- 22.Lopes RMF, Freitas VLO, Lemos Filho JP. Biometry of fruits and seeds and germination of Plathymenia reticulata Benth. and Plathymenia foliolosa Benth. (Fabaceae - Mimosoideae). Rev Árvore. 2010;34:797–805. [Google Scholar]
- 23.Mahmood A, Ahmad M, Jabeen A, Zafar M, Nadeem S. Pharmacognostic studies of some indigenous medicinal plants of Pakistan. http://www.ethnoleaflets.com/leaflets/abid.htm. 2005 Accessed on 21 December 2011.
- 24.Melo RF, Farrapo NM, Rocha Jr DS, et al. Antiophidian mechanisms of medicinal plants. In: Raymond B. Keller., editor. Flavonoids: Biosynthesis, Biological Effects and Dietary Sources. Nova Science Publishers; New York: 2009. pp. 249–262. (Org.). p. [Google Scholar]
- 25.Mori ALPM. Universidade de São Paulo; São Paulo, Faculdade de Ciências Farmacêuticas. Brazil: 1997. Própolis – identificação de flavonóides e ácidos aromáticos em tintura. Estimativa de FPS de extrato mole em base cosmética; p. 114p. Masters Dissertation. [Google Scholar]
- 26.Nazato VS, Rubem-Mauro L, Vieira NA, et al. In vitro antiophidian properties of Dipteryx alata Vogel bark extracts. Molecules. 2010;15:5956–5970. doi: 10.3390/molecules15095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira FN, Brito MT, Morais ICO, Fook SML, Albuquerque HN. Accidents caused by Bothrops and Bothropoides in the State of Paraiba: epidemiological and clinical aspects. Rev Soc Bras Med Trop. 2010;43:662–667. doi: 10.1590/s0037-86822010000600012. [DOI] [PubMed] [Google Scholar]
- 28.Oshima-Franco Y, Hyslop S, Cintra ACO, Giglio JR, Cruz-Höfling MA, Rodrigues-Simioni L. Neutralizing capacity of commercial bothropic antivenom against Bothrops jararacussu venom and bothropstoxin-I. Muscle Nerve. 2000;23:1832–1839. doi: 10.1002/1097-4598(200012)23:12<1832::aid-mus6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Perchellet JP, Gali HU, Perchellet EM, Klish DS, Armbrust AD. Antitumor-promoting activities of tannic acid, ellagic acid, and several gallic acid derivatives in mouse skin. Basic Life Sci. 1992;59:783–801. doi: 10.1007/978-1-4615-3476-1_47. [DOI] [PubMed] [Google Scholar]
- 30.Pott A, Pott VJ, Bueno Sobrinho AA. Plantas úteis à sobrevivência no Pantanal. IV Simpósio sobre Recursos Naturais e Sócio-econômicos do Pantanal; Corumbá. 2004. BR; [updated March 11; cited 2004 November 23] Access on 21 December 2011. [Google Scholar]
- 31.Pott A, Pott VJ. Brazilian Agricultural Research Corporation. Brasília, DF, Brazil: 1994. Plathymenia reticulata, Leguminosae-Mimosoideae. Plants of Pantanal. EMBRAPA-CPAP-SPI. [Google Scholar]
- 32.Ratter JA, Ribeiro JF, Bridgewater S. The Brazilian cerrado vegetation and threats to its biodiversity. Ann Bot. 1997;80:223–230. [Google Scholar]
- 33.Reicher F, Sierakowski MR, Corrêa JBC. Determinação espectrofotométrica de taninos pelo reativo fosfotungstico-fosfomolíbdico. Arq Biol Tecnol. 1982;24:407–411. [Google Scholar]
- 34.Rodrigues E, Carlini EA. Plants used by a Quilombola Group in Brazil with potential central nervous system effects. Phytother Res. 2004;18:748–753. doi: 10.1002/ptr.1535. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues-Simioni L, Borgese N, Ceccarelli B. The effects of Bothrops jararacussu venom and its components on frog nerve-muscle preparation. Neuroscience. 1983;10:475–489. doi: 10.1016/0306-4522(83)90147-1. [DOI] [PubMed] [Google Scholar]
- 36.Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR. 5th Edition UFRGS/UFSC, Porto Alegre/Florianópolis; Brazil: 2004. Farmacognosia: da Planta ao Medicamento. [Google Scholar]
- 37.Theodorou E, Valero-Cuevas FJ. Optimality in neuromuscular systems. Conf Proc IEEE Eng Med Biol Soc. 20102010:4510–4516. doi: 10.1109/IEMBS.2010.5626055. [DOI] [PubMed] [Google Scholar]
- 38.Vale LHF, Mendes MM, Hamaguchi A, Soares AM, Rodrigues VM, Homsi-Brandeburgo MI. Neutralization of pharmacological and toxic activities of Bothrops snake venoms by Schizolobium parahyba (Fabaceae) aqueous extract and its fractions. Basic Clin Pharmacol Toxicol. 2008;103:104–107. doi: 10.1111/j.1742-7843.2008.00248.x. [DOI] [PubMed] [Google Scholar]
- 39.Warrel DA. Snakebites in Central and South America: epidemiology, clinical features, and clinical management. In: Campbell JA, Lamar WW, editors. The venomous reptiles of the Western Hemisphere. 1st Edition. Comstock (Cornell University Press); Ithaca, New York, USA: 2004. pp. 709–715. (Eds) [Google Scholar]
- 40.WHO – World Health Organization Rabies and envenomings: a neglected public health issue. http://www.who.int/bloodproducts/animal_sera/Rabies.pdf. 2007 Available at. Accessed on 25 October 2011.
- 41.WHO - World Health Organization Neglected tropical diseases. http://www.who.int/neglected_diseases/diseases/snakebites/en/ Available from. Accessed on 24 July 2011.