Abstract
Studies in animals showed that stress is associated with changes in hippocampal function and structure, an effect mediated through decreased neurogenesis, increased glucocorticoids, and/or decreased brain derived neurotrophic factor. Antidepressants and some anticonvulsants block the effects of stress and/or promote neurogenesis in animal studies. Patients with posttraumatic stress disorder (PTSD) have been shown to have smaller hippocampal volume on magnetic resonance imaging and deficits in hippocampal-based memory. Symptom activation is associated with decreased anterior cingulate and medial prefrontal function, which is proposed as the neural correlate of a failure of extinction seen in these patients. Treatment with antidepressants and phenytoin reverse hippocampal volume reduction and memory deficits in PTSD patients, suggesting that these agents may promote neurogenesis in humans.
EFFECTS OF STRESS ON THE INDIVIDUAL
Stress has wide ranging effects on the individual. Traumatic stressors represent the most severe range of the spectrum and are defined by the Diagnostic and Statistical Manual (DSM) as threats to life of self or significant other with intense fear, horror or helplessness [1]. Traumatic stress so defined affects at least half of the US population [2]. Childhood sexual abuse is the most common trauma in women while physical assault is most common in men [3, 4]. Traumatic stress leads to a range of mental disorders, including posttraumatic stress disorder (PTSD), depression, alcoholism, dissociation, anxiety and borderline personality disorder [5]. Based on the high overlap amongst these stress-related disorders, I have argued that they should be considered together as “trauma-spectrum disorders” [5]. Traumatic stress is also associated with a range of poor health outcomes, including increased risk of heart disease, diabetes, stroke, and asthma [6–13]. PTSD is the sine qua non trauma-related disorder, as it requires a trauma exposure for the diagnosis. PTSD is twice as common in women as in men, and affects about 8% of the US population at some time in their lives [14]. Trauma exposure leads to short-term PTSD in about a third of individuals, while 15% develop chronic PTSD [15]. In order to understand mechanisms involved in PTSD we have applied neurobiological findings from studies of the effects of stress on animals. Studies in PTSD patients corroborate findings in animals that stress is associated with changes in brain structure and function. These changes appear to be specific to PTSD, and not a non-specific effect of exposure to traumatic stress.
EFFECTS OF STRESS ON BRAIN STRESS RESPONSIVE SYSTEMS
Animal studies show that stress has lasting effects on brain circuits and systems. A network of brain regions are involved in the stress response, including hippocampus, amygdala, cingulate, and prefrontal cortex. Neurohormonal systems that play a critical role in stress include the hypothalamic-pituitary-adrenal (HPA) axis and noradrenergic systems.
Stress is associated with activation of the HPA axis. Corticotropin-releasing factor (CRF) is released from the hypothalamus, with stimulation of adrenocorticotropin hormone (ACTH) release from the pituitary. This results in glucocorticoid (cortisol) release from the adrenal, which in turn has a negative feedback effect on the axis at the level of the pituitary as well as central brain sites including hypothalamus and hippocampus. In addition to its role in triggering the HPA axis, CRF acts centrally to mediate fear-related behaviors [16] and triggers other neurochemical responses to stress such as the noradrenergic system via the brainstem locus coeruleus [17]. A variety of early stressors, including maternal deprivation, result in increased glucocorticoid response to subsequent stressors [18, 19]].
The noradrenergic system also plays a critical role in stress [20, 21]. The majority of noradrenergic cell bodies are located in the locus coeruleus, a nucleus in the dorsal pons region of the brainstem, with a dense network of axons that extend throughout the cerebral cortex and to multiple cortical and subcortical areas, including hippocampus, amygdala, thalamus and hypothalamus, bed nucleus of stria terminalis, nucleus accumbens, as well as descending projections which synapse at the level of the thoracic spinal cord [22]. Exposure to stressors results in activation of the locus coeruleus, with release of norepinephrine throughout the brain [23]. Acute stressors such as a cat seeing a dog or another aggressive cat result in an acute increase in firing of neurons in the locus coeruleus [24] with increased release of norepinephrine in the hippocampus and medial prefrontal cortex [25]. Chronic stress is associated with potentiated release of norepinephrine in the hippocampus with exposure to subsequent stressors [20, 26].
Studies have also looked at the long-term neurobiological effects of trauma in patients with PTSD. Baseline CRF concentrations are elevated in the cerebrospinal fluid in PTSD [27, 28]. Studies have shown either low [29–38] no different [28, 39–42] or increased cortisol [43–49] in baseline measurements in blood or urine in PTSD. Adult patients with PTSD showed increased suppression of cortisol with low dose (0.5 mg) dexamethasone [50, 51]. We performed a comprehensive assessment of the HPA axis in women with diagnoses of PTSD, with and without a history of childhood abuse, including measurement of cortisol in plasma every 15 minutes over a 24 hour period. Abused women with PTSD had lower levels of cortisol in the afternoon (12–8 pm) compared to the other groups (p<0.05). We have also developed methods for assessment of neuroendocrine responses to stress in stress-related neuropsychiatric disorders. In an initial study, we looked at male and female PTSD patients with a range of primary traumas, using a cognitive stress challenge with problem solving under time pressure and with negative feedback. PTSD patients had increased cortisol levels at baseline in the pre-stress period consistent with anticipatory anxiety, although their 24 hour cortisol during a resting period was low relative to controls. During the challenge both groups had an increase in cortisol, with patients continuing to be higher than controls, but returning to control levels in the post-stress phase [52]. We assessed cortisol response to traumatic reminders in women with abuse-related PTSD using personalized scripts of their childhood trauma. Women with PTSD had 4 fold higher increases in cortisol with the traumatic scripts compared to abused non-PTSD women. Stress-induced elevations in cortisol were correlated with baseline PTSD symptom levels measured with the Clinician Administered PTSD Scale (CAPS) [53]. Adult women with depression and a history of early childhood abuse had an increased cortisol response to a stressful cognitive challenge relative to controls [54] and a blunted ACTH response to CRF challenge [55].
PTSD is also associated with increased noradrenergic function [20, 21]. Studies have shown increased norepinephrine in blood and urine at baseline and with traumatic reminders [44, 56–58]. Studies also show increased EMG, heart rate and skin conductance responses during exposure to traumatic scripts [59]. Administration of the alpha-2 adrenergic antagonist, yohimbine, which causes norepinephrine release in the brain, is associated with increased PTSD symptoms, plasma norepinephrine metabolites [57, 58], and decreased yohimbine induced hippocampal and frontal function (consistent with increased norepinephrine release) [58] in PTSD. These findings are consistent with increased sympathetic nervous system activity in PTSD.
EFFECT OF STRESSORS ON BRAIN STRUCTURE AND FUNCTION
When we first initiated research in the area of PTSD we applied studies in animals of the effects of stress on the brain. These studies showed that stress is associated with alterations in the hippocampus, which plays a key role in memory. Stress induced changes in hippocampal structure are associated with deficits in memory function [60–64], an effect related to elevated levels of glucocorticoids [65–68], inhibition of neurogenesis [69–71], increased glutamate [72] or CRF [73], and/or decreased levels of Brain Derived Nerve Growth Factor (NGF) [74–76]. Changes in the environment, e.g. social enrichment or learning, can also enhance neurogenesis, and slow the normal age-related decline in neurogenesis [77, 78] and treatment with antidepressants or anticonvulsants promotes neurogenesis, blocks the effects of stress, and/or reduces apoptosis in the hippocampus [69, 76, 79–83]. Stress induced changes in memory are related to alterations in hippocampal structure [84], in part related to glucocorticoid elevations [61, 63, 64, 85–87]. Although originally conceived as a cognition oriented structure, the hippocampus may play a greater role in behavior than we previously thought. It has long been known that the hippocampus mediates emotional responses to the context of a situation [88, 89]. More recently, studies have suggested that antidepressant induced neurogenesis is required for the behavioral effects of antidepressants [90], although debate continues on this topic [91].
Brain areas involved in the stress circuit (amygdala, pre-frontal cortex, and hippocampus) share in common the fact that they mediate different aspects of memory and visuospatial processing. The amygdala plays a central role in conditioned fear responses [92, 93]. Stress is associated with increased dendritic arborization in the amygdala [94, 95]. Medial prefrontal cortex consists of several related areas, including orbitofrontal cortex, anterior cingulate (area 25-subcallosal gyrus, and Area 32), and anterior prefrontal cortex (Area 9) [96, 97]. The medial prefrontal dopaminergic system is one of the most sensitive areas in the brain to even mild stressors [98]. Lesions in this area result in a failure to mount the peripheral cortisol and sympathetic response to stress [96, 97]. Recently, stress has been associated with a reduction in dendritic branching in this area [99]. This area also has important inhibitory inputs to the amygdala that mediate extinction to fear responding [100, 101]. Animals with lesions of the medial prefrontal cortex are unable to extinguish fear responses after trials of fear conditioning [101, 102]. Formation of extinction memories appears to involve a process of independent memory formation that is separate from the development of fear memory [103, 104]. Human subjects with lesions of the prefrontal cortex show dysfunction of normal emotions and an inability to relate in social situations that require correct interpretation of the emotional expressions of others [105]. These findings suggest that dysfunction of medial prefrontal cortex may play a role in pathological emotions that sometimes follow exposure to extreme stressors such as childhood sexual abuse.
BRAIN IMAGING OF TRAUMA
Work from the studies reviewed above introduced the possibility that stress may lead to damage to the hippocampus in human subjects [5, 106, 107]. The first neuroimaging study in PTSD was performed using magnetic resonance imaging (MRI) to measure the volume of the hippocampus [108]. This study showed an 8% decrease in MRI-based measurement of right hippocampal volume in patients with combat-related PTSD (N=26) in comparison to matched controls (N=22) (p<0.05). Decreases in right hippocampal volume in the PTSD patients were associated with deficits in short-term memory [108]. Findings of smaller hippocampal volume and/or a reduction in N-acetyl aspartate (NAA, a marker of neuronal integrity) in the hippocampus in adults with chronic, long-standing PTSD have been replicated several times in the published literature [109–115]. One study used a specific cognitive task to probe hippocampal function and demonstrated a failure of left hippocampal activation with a memory task in women with abuse-related PTSD. This was significant after controlling for differences in hippocampal volume measured on MRI in the same subjects. Women with PTSD had smaller hippocampal volume than both abused non-PTSD and non-abused non-PTSD women [116]. Studies in children [117–119] and new onset PTSD [120, 121] have not shown smaller hippocampal volume in PTSD, suggesting that chronic PTSD is required for the effect. One study showed a correlation between PTSD symptoms and hippocampal volume in unaffected twin brothers, suggesting a genetic contribution to smaller hippocampal volume [122], however our own unpublished twin study of twins discordant for PTSD shows smaller hippocampal volume in a pattern consistent with a combined genetic and environmental effect.
Studies have also shown that PTSD is associated with deficits in hippocampal based memory, as tested by paragraph recall and word learning tasks [123]. Several studies from our group and others found a reduction in MRI-based hippocampus volume in men with combat-related PTSD [109, 122, 124–126]. Studies have also found smaller hippocampal volume in men and women with early “abuse-related” PTSD [111] and in women with early abuse related PTSD [113]. A study in women with PTSD related to domestic violence in adulthood did not show smaller hippocampal volume [120]. We found smaller hippocampal volume in women with abuse and PTSD compared to women with abuse without PTSD, and non-abused non-PTSD women [116]. There were no differences in whole brain volumes in adult women with abuse-related PTSD [116]. Studies of hippocampal volume in depression, a common outcome of early abuse in women, have been conflicting [127]. In a recent study, we found that smaller hippocampal volume in women was specific to depression with a history of early childhood sexual abuse. There were no changes in women with depression without a history of early abuse [128]. Other studies showed smaller anterior cingulate volume [129, 130] and corpus callosum volume [131] in adults with PTSD. In summary, there are several studies in PTSD showing smaller volume of the hippocampus, at least in patients with chronic and severe illness.
There has long been an interest in the relationship between exposure to psychological trauma and deficits in memory function [132, 133]. Danish survivors of the WWII concentration camps were noted to have subjective complaints of memory problems in a large number of cases [134]. American POWs from the Korean War had deficits in verbal declarative memory function, with a relative preservation of IQ [135]. These studies occurred before the development of the diagnostic category of PTSD, leaving unanswered the question of whether verbal declarative memory deficits are specifically associated with stress-related psychiatric disorders including PTSD.
Subsequent studies have demonstrated verbal declarative memory deficits in PTSD consistent with hippocampal dysfunction [132, 133, 136, 137]. Several studies, using a variety of measures (including the Wechsler Memory Scale, the visual and verbal components of the Selective Reminding Test, the Auditory Verbal Learning Test, the California Verbal New Learning Test, and the Rivermead Behavioral Memory Test), found specific deficits in verbal declarative memory function, with a relative sparing of visual memory and IQ [123, 124, 138–149]. These studies have been conducted in both patients with PTSD related to Vietnam combat [123, 138, 141–146, 148, 149], rape [139], adults with early childhood abuse [124] and traumatized children [140]. One study in adult rape survivors showed that verbal declarative memory are specifically associated with PTSD, and are not a non-specific effect of trauma exposure [139]. Another study of women with early childhood sexual abuse in which some, but not all, of the patients had PTSD, showed no difference between abused and non-abused women [150], while another study was not able to show a difference between Vietnam veterans with and without PTSD [151]. Other types of memory disturbances studies in PTSD include gaps in memory for everyday events (dissociative amnesia) [152], deficits in autobiographical memory [153], an attentional bias for trauma-related material [154–162], and frontal lobe-related impairments [163]. These studies suggest that traumas such as early abuse with associated PTSD result in deficits in verbal declarative memory. More recently we showed that cognitive deficits in early abuse survivors are specific to PTSD and are not related to the non-specific effects of abuse [164].
Based on findings related to the effects of antidepressants on neurogenesis, we assessed the effects of the selective serotonin reuptake inhibitor (SSRI) paroxetine on outcomes related to function of the hippocampus. We studied 28 patients with PTSD and treated them for up to a year with variable doses of paroxetine. Twenty three patients completed the course of treatment, and MRI post treatment was obtained in 20 patients. Patients who did not complete treatment stopped because of a relapse of substance abuse, or were lost to followup (possibly because of a treatment non response). Neuropsychological testing was used to assess hippocampal-based declarative memory function and MRI to assess hippocampal volume before and after treatment. Declarative memory was assessed with the Wechsler Memory Scale–Revised and Selective Reminding Test. Patients with PTSD showed a significant improvement in PTSD symptoms with treatment. Treatment resulted in significant improvements in verbal declarative memory and a 4.6% increase in mean hippocampal volume. These findings suggested that long-term treatment with paroxetine is associated with improvement of verbal declarative memory deficits and an increase in hippocampal volume in PTSD [165]. Phenytoin also blocks the effects of stress on the hippocampus in animal studies through modulation of glutamatergic function [166]. We recently found that phenytoin increased hippocampal and whole brain volume in PTSD [167, 168].
Studies of brain structure have typically found hippocampal volume reduction in adults with PTSD, but not in children. Two studies have found reductions in brain volume in children with trauma and PTSD symptoms [117, 118], although studies have not found reductions in hippocampal volume in boys and girls with PTSD either at baseline or over a longitudinal period [117–119]. One study used single voxel proton magnetic resonance spectroscopy (proton MRS) to measure relative concentration of NAA and creatinine (a marker of neuronal viability) in the anterior cingulate of 11 children with maltreatment-related PTSD and 11 controls. The authors found a reduction in the ratio of NAA to creatinine in PTSD relative to controls [169]. Studies have also found smaller size of the corpus callosum in children with abuse and PTSD relative to controls [118] as well as larger volume of the superior temporal gyrus [170]. In a study of abused children in whom diagnosis was not specified, there was an increase in T2 relaxation time in the cerebellar vermis, suggesting dysfunction in this brain region [171].
MAPPING THE NEURAL CIRCUITRY OF PTSD
Functional neuroimaging studies have been performed to map out the neural circuitry of PTSD [172, 173]. These studies are consistent with dysfunction in a network of related brain areas including medial prefrontal cortex and hippocampus. We measured brain blood flow with PET and [15O]H 2O during exposure to personalized scripts of childhood sexual abuse. Twenty two women with a history of childhood sexual abuse underwent injection of H2[15O] followed by positron emission tomography (PET) imaging of the brain while listening to neutral and traumatic (personalized childhood sexual abuse events) scripts. Brain blood flow during exposure to traumatic versus neutral scripts was compared between sexually abused women with and without PTSD. Memories of childhood sexual abuse were associated with greater increases in blood flow in portions of anterior prefrontal cortex (superior and middle frontal gyri-Areas 6 and 9), posterior cingulate (area 31), and motor cortex in sexually abused women with PTSD compared to sexually abused women without PTSD. Abuse memories were associated with alterations in blood flow in medial prefrontal cortex, with decreased blood flow in subcallosal gyrus-area 25, and a failure of activation in anterior cingulate-area 32. There was also decreased blood flow in right hippocampus, fusiform/inferior temporal gyrus, supra-marginal gyrus, and visual association cortex in PTSD relative to non-PTSD women [174]. This study replicated findings of decreased function in medial prefrontal cortex and increased function in posterior cingulate in combat-related PTSD during exposure to combat-related slides and sounds [175]. In another study 8 women with childhood sexual abuse and PTSD were compared to 8 women with abuse without PTSD using PET during exposure to script-driven imagery of childhood abuse. The authors found increases in orbitofrontal cortex and anterior temporal pole in both groups of subjects, with greater increases in these areas in the PTSD group. PTSD patients showed a relative failure of anterior cingulate/medial pre-frontal cortex activation compared to controls. The PTSD patients (but not controls) showed decreased blood flow in anteromedial portions of prefrontal cortex and left inferior frontal gyrus [176]. Several other studies have shown a failure of medial prefrontal cortical activation in PTSD related to other traumas including combat [58, 176–184].
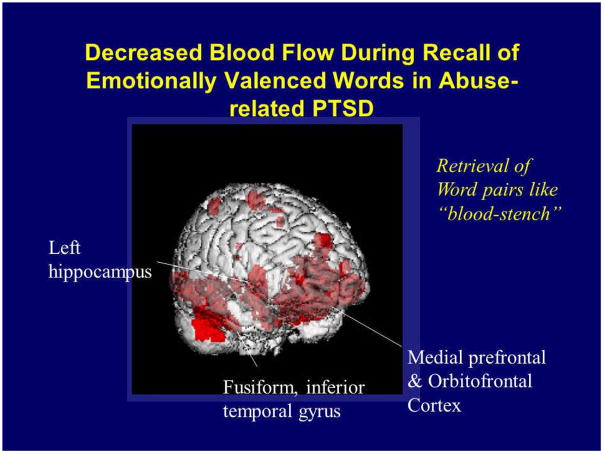
These studies have relied on specific traumatic cues to activate personalized traumatic memories and PTSD symptoms in patients with PTSD. Another method to probe neural circuits in PTSD is to assess neural correlates of retrieval of emotionally valenced declarative memory. In this type of paradigm, instead of using a traditional declarative memory task, such as retrieval of word pairs like “gold-west”, which has been the standard of memory research for several decades, words with emotional valence, such as “stench-fear” are utilized [185]. Although there has been relatively little research on retrieval of emotionally valenced words, it is of interest from the standpoint of PTSD as a method for activating neural pathways relevant to trauma and memory. If PTSD patients demonstrate a pattern of brain activation during retrieval of emotionally valenced declarative memory that is similar to that seen during exposure to other tasks that stimulate brain networks mediating PTSD symptoms, such as exposure to personalized scripts of childhood trauma, or exposure to trauma-related pictures and sounds, then that would provide convergent evidence for dysfunction of a specific neural circuit in the processing of emotional memory in PTSD. We recently used PET in the examination of neural correlates of retrieval of emotionally valenced declarative memory in 10 women with a history of childhood sexual abuse and the diagnosis of PTSD and 11 women without abuse or PTSD. We hypothesized that retrieval of emotionally valenced words would result in an altered pattern of brain activation in patients with PTSD similar to that seen in prior studies of exposure to cues of personalized traumatic memories. Specifically we hypothesized that retrieval of emotionally valenced words in PTSD patients relative to non-PTSD would result in decreased blood flow in medial prefrontal cortex (subcallosal gyrus and other parts of anterior cingulate), hippocampus, and fusiform gyrus/inferior temporal cortex (Fig. 3), with increased blood flow in posterior cingulate, motor and parietal cortex, and dorsolateral prefrontal cortex. PTSD patients during retrieval of emotionally valenced word pairs showed greater decreases in blood flow in an extensive area which included orbitofrontal cortex, anterior cingulate, and medial prefrontal cortex (Brodmann’s areas 25, 32, 9), left hippocampus, and fusiform gyrus/inferior temporal gyrus, with increased activation in posterior cingulate, left inferior parietal cortex, left middle frontal gyrus, and visual association and motor cortex. There were no differences in patterns of brain activation during retrieval of neutral word pairs between patients and controls. These findings were similar to prior imaging studies in PTSD from our group using trauma-specific stimuli for symptom provocation, adding further supportive evidence for a dysfunctional network of brain areas involved in memory, including hippocampus, medial prefrontal cortex and cingulate, in PTSD [180].
Fig. 3.
Another study examined neural correlates of the Stroop task in sexually abused women with PTSD. The Stroop task involves color naming semantically incongruent words (e.g., name the color of the word green printed in the color red). The Stroop task has been consistently found to be associated with activation of the anterior cingulate in normal subjects, an effect attributed to the divided attention or inhibition of responses involved in the task. Emotional Stroop tasks (e.g. name the color of a trauma specific word like rape) in abused women with PTSD have also been shown to be associated with a delay in color naming in PTSD [155]. Women with early childhood sexual abuse-related PTSD (n=12) and women with abuse but without PTSD (n=9) underwent positron emission tomographic measurement of cerebral blood flow during exposure to control, color Stroop, and emotional Stroop conditions. Women with abuse with PTSD (but not abused non-PTSD women) had a relative decrease in anterior cingulate blood flow during exposure to the emotional (but not color) classic Stroop task. During the color Stroop there were also relatively greater increases in blood flow in non-PTSD compared with PTSD women in right visual association cortex, cuneus, and right inferior parietal lobule. These findings were consistent with dysfunction of the anterior cingulate/medial prefrontal cortex in women with early abuse-related PTSD [186].
We compared hippocampal function and structure in 33 women with and without early childhood sexual abuse and PTSD. Women with abuse with and without PTSD were studied during encoding of a verbal memory paragraph compared to a control in conjunction with measurement of brain blood flow with PET. Subjects underwent four PET scans using methods described by us previously in detail [174, 175] in conjunction with encoding of a control task and an active paragraph encoding. There were no differences in blood flow during the control task between groups, however there were significantly greater increases in blood flow during verbal memory encoding in the hippocampus in non-PTSD abused women relative to PTSD women (F=14.93; df 1,20; p<0.001). PTSD women also had smaller left hippocampal volume on MRI volumetrics compared to abused women without PTSD and non-abused non-PTSD women. Differences in hippocampal activation were statistically significant after covarying for left hippocampal volume, suggesting that failure of activation was not secondary to smaller hippocampal volume in patients with PTSD. There was a significant relationship between increased dissociative states as measured with the Clinician-Administered Dissociative States Scale (CADSS) and smaller left hippocampal volume as measured with MRI in abused women as measured with logistic regression (R Squared=0.30, F=3.90; df=1; p<.05) [116]. Another study in men and women with Vietnam service and PTSD found a failure of hippocampal activation with a word stem completion memory task [126].
In addition to a failure of hippocampal activation with cognitive tasks, studies found decreased hippocampal activation with symptom provocation in PTSD. Studies have found decreased hippocampal function during traumatic remembrance stimulated with trauma-specific scripts [174], stimulation of PTSD symptoms with yohimbine [58], or during recall of emotionally negative words in PTSD [180], although increased function was seen during counting of combat words [182]. Increased dissociation and flashbacks during these tasks may lead to (or be caused by) decreased hippocampal function, leading to the divergence from normal declarative memories that can often occur in these states [136].
Although some studies have demonstrated increased amygdala function in PTSD [187], the experience to date suggests that increased amygdala involvement is not necessarily seen in all of the study paradigms applied to PTSD. While some studies found amygdala activation with trauma-specific stimuli [188] a larger number did not [58, 174–176, 181, 184]. It is more likely that specific tasks are required to show increased amygdala function in PTSD. For instance, Rauch et al. found that exposure to masked fearful faces was associated with greater amygdala activation in PTSD [189], and we found increased amygdala activation during acquisition of fear in a classical fear conditioning paradigm (Bremner et al in press). In summary, increased amygdala function has not been shown to be non-specifically associated with traumatic remembrance in PTSD, however there are suggestions that alterations in amygdala activity do play a role in PTSD, probably related to specific mechanisms of the disorder. Future studies are required in this area.
Imaging studies that involved provocation of PTSD symptoms in adults with PTSD are also consistent with dysfunction in medial prefrontal cortex/anterior cingulate [190]. In an earlier study PTSD symptoms were stimulated through activation of the brain norepinephrine system yohimbine (an alpha-2 noradrenergic receptor antagonist which stimulates norepinephrine release in the brain) in conjunction with PET imaging of brain metabolism with FDG. PTSD patients showed decreased function in orbitofrontal cortex, relative to controls [58]. Decreased baseline blood flow during an attentional task was seen in medial prefrontal cortex in patients with PTSD and substance abuse [187]. Other studies showed dysfunction in various subregions of medial prefrontal cortex/anterior cingulate (Areas 32, 24, and 25), including a failure of activation and decreased function relative to controls during exposure to traumatic scripts [174, 176, 178, 181, 191, 192], combat-related slides and/or sounds [175, 184] a trauma-specific counting Stroop task [182] and an emotional Stroop task [186]. In the other parts of medial prefrontal cortex (orbitofrontal cortex (Area 11), Area 9 and 10), the findings have been mixed [183], with about an equal number of studies showing increases as decreases. In conclusion, it is reasonable to postulate that exposure to standard materials such as traumatic scripts and slides is associated with a relative failure of function in the “extended anterior cingulate” portion of medial prefrontal cortex (Areas 24, 32, 25), however more studies are required to confirm this finding.
Studies have begun to use neuroimaging to examine central receptor function in PTSD. Animal studies showed that chronic stress leads to a decrease in benzodiazepine receptor binding in frontal cortex. We used SPECT with [123I]Iomazenil to quantitate benzodiazepine receptor binding in patients with combat-related PTSD and healthy controls. In this study we found a decrease in benzodiazepine receptor binding in medial prefrontal cortex (Brodmann’s area 9) in 13 patients with combat-related PTSD compared to 13 case-matched healthy controls [193]. These findings were consistent with animal studies of stress showing decreased binding in frontal lobe.
Functionally, the cingulate has been divided into an anterior portion involved in emotion and selection for action, and a posterior portion involved in visuospatial processing [97]. Recent imaging studies in humans, however, have been consistent with a role for the posterior cingulate in processing of emotional and traumatic material in normal individuals [194]. Multiple PET studies found increased posterior cingulate function during stimulation of traumatic memories in PTSD [174, 175, 181, 182, 195]. These findings corroborate the inclusion of the entire cingulate in the original limbic model.
The dorsolateral prefrontal cortex, which includes areas such as middle and inferior frontal gyri, is involved in cognitive functions, language and speech [196]. Prefrontal cortex plays an important role in the activation of memory pathways and sustained attention that are elicited during the stress response. This area has functional connections with other regions mediating cognitive and emotional responses to stress, including motor cortex, parietal cortex, cingulate, hippocampus and amygdala. Disruption of circuits between the dorsolateral prefrontal cortex and other regions involved in emotion and the stress response (e.g. limbic regions) may lead to disconnection between cognitive and emotional processing and responses to traumatic events.
There have in fact been several studies showing altered function in dorsolateral prefrontal cortex with PTSD symptom provocation. Studies found decreased function in either inferior or middle frontal gyri [58, 174, 176, 188, 195]. Dysfunction in this area may be involved in the dysfunction of memory, speech and cognition seen in PTSD patients, especially during periods of stress or traumatic reminders. A functional disconnection between “higher” prefrontal cortical areas involved in abstract thought, language and cognition and “lower” limbic areas that govern primary emotions may underlie unregulated emotions, traumatic dissociative memory recall in PTSD, and difficulties in verbalization of traumatic experiences.
The parietal cortex plays a critical role in visuospatial processing that is involved in the response to threat [197, 198]. Two or more studies have found decreased function with traumatic remembrance in visual association cortex [174, 195], while increases and decreases were seen in the precuneus (which plays a role in processing of visual memory). Several studies found decreased function in parietal cortex [58, 175, 176, 188, 195] with a smaller number of studies showing an increase [174, 182]. These studies are consistent with alterations in parietal and visual association cortical function in PTSD, probably mediating alterations in cognitive functions associated with these areas.
BRAIN IMAGING OF TRAUMA SPECTRUM DISORDERS
I have argued for a viewpoint of psychiatric diagnosis to include a trauma spectrum group of disorders including PTSD, depression, borderline personality disorder (BPD), and dissociative disorders [5]. This is a departure from the DSM approach of having multiple disorders grouped by symptoms without any theoretical foundation. The DSM view would state that neurobiology should help us to parcel out different disorders. However these disorders have high comorbidity and overlapping descriptive language. Neuroimaging has shown common deficits in anterior cingulate/medial prefrontal cortex and hippocampus in these disorders. For instance we studied women sexually abused in childhood with the primary diagnosis of borderline personality disorder (BPD). About 50% of these women had comorbid PTSD [199]. Our group [200] and others [201] found smaller hippocampal and amygdala volume in women with abuse and BPD. A consistent finding in these studies is decreased amygdala function. We have developed a paradigm involving exposure to scripts of an abandonment situation, which is specific to the psychopathology of BPD, and which differentially affects women with BPD compared to PTSD as measured by psychophysiological responding [202]. We assessed cerebral blood flow with PET in 20 abused women with and without BPD while they listened to scripts describing neutral and personal abandonment events. Memories of abandonment were associated with greater increases in blood flow in bilateral dorsolateral prefrontal cortex and right cuneus, and greater decreases in anterior cingulate in women with BPD compared to women without BPD [203]. These findings show some overlap between abused women with BPD and PTSD, specifically in the area of decreased anterior cingulate/medial prefrontal cortical function.
SUMMARY AND CONCLUSIONS
In a series of studies our group and others have assessed changes in the brain in PTSD. Several studies have shown smaller hippocampal volume and memory deficits in adults with chronic PTSD. Although baseline cortisol in adult women with abuse-related PTSD appears to be low, traumatic reminders and other stressors result in exaggerated release of cortisol. There is also evidence for increased catecholaminergic function in PTSD. Brain imaging studies have consistently found decreased anterior cingulate/medial prefrontal cortical function in PTSD during recall of traumatic memories. Other less well replicated findings include increased dorsolateral prefrontal cortex and posterior cingulate function, and decreased hippocampal and left inferior frontal gyrus function.
Studies to date suggest that developmental epoch during which trauma occurs and other factors such as chronicity of trauma exposure and illness are important factors to consider in trauma research. For instance, the brain continues to develop in early childhood and adolescence, with increasing volume of the amygdala and hippocampus and decreasing frontal cortex volume. Studies have found that a history of trauma in childhood can increase the risk of PTSD following exposure to a trauma in adulthood, such as combat trauma in Vietnam, by as much as 4-fold, even in individuals without any history of psychiatric disorder before entering the military [204]. Patients with long-standing and chronic PTSD do not respond as well to treatment as patients with more acute onset PTSD [205]. These findings are convergent with research in animal studies [206] suggesting that once traumatic memories have become established as indelible memories in the brain, they are resistant to subsequent modification and alteration. Patients with early onset PTSD differ from patients with adult onset PTSD, showing increased depression, substance abuse and character pathology, while adult onset PTSD is characterized by a greater degree of classical PTSD symptoms, including increased anxiety and hyperarousal [207]. One might speculate that these differences are related to effects on different brain structures (e.g. depression is linked to prefrontal dysfunction), however we have little evidence on which to base these speculations. The emerging evidence suggests that early trauma with BPD is associated with smaller amygdala volume, the opposite of depression, and different from what might be expected from animal studies.
Animal studies showed that environmental influences early in development can have effects on the brain and neurohormonal systems that persist throughout life. For example, as noted above, animals exposed to stressors early in life show a heightened responding (e.g. increased glucocorticoid responses) to subsequent stressors that persists throughout the lifespan. Although it was previously thought that humans do not have the capacity to grow new neurons in adulthood (neurogenesis), recently the capacity for neurogenesis in the human hippocampus was discovered. It is thought that the creation of new neurons is related to the processes of new learning and memory. Deprived versus enriched and learning enhanced environments early in life can affect hippocampal neurogenesis for the rest of the lifespan [78, 208]. The implications of these findings are that early abuse may inhibit neurogenesis and result in life long problems in learning, as well as promoting some of the PTSD symptoms that may be mediated by the hippocampus.
Fig. 1.

Smaller hippocampal volume in a PTSD patient seen on a coronal MRI (outlined in red). From Bremner JD, Does Stress Damage the Brain? WW Norton, NY, 2002, Fig 4.2, p. 116.
Fig. 2.
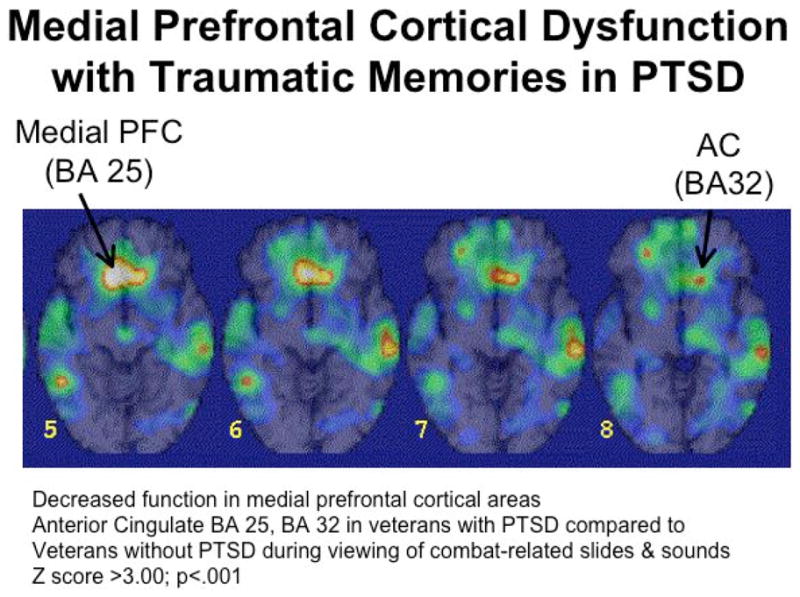
Failure of medial prefrontal and anterior cingulate activation with exposure to traumatic reminders in the form of combat related slides and sounds in PTSD. From Bremner JD, Does Stress Damage the Brain? WW Norton, NY, 2002, Fig. 4.5, p. 113.
References
- 1.APA. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, D.C: 2000. [Google Scholar]
- 2.Kessler RC. J Clin Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- 3.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EG. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 4.MacMillan HL, Fleming JE, Trocme N, Boyle MH, Wong M, Racine YA, Beardslee WR, Offord DR. JAMA. 1997;278:131–135. [PubMed] [Google Scholar]
- 5.Bremner JD. Understanding Trauma-related Disorders from a Mind-Body Perspective. W.W. Norton; New York: 2002. Does Stress Damage the Brain? [Google Scholar]
- 6.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 7.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. Preventive Medicine. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 8.Wagner AW, Wolfe J, Rotnitsky A, Proctor SP, Erickson DJ. J Traumatic Stress. 2000;13:41–55. doi: 10.1023/A:1007716813407. [DOI] [PubMed] [Google Scholar]
- 9.Engel CC, Liu X, McCarthy BD, Miller RF, Ursano R. Psychosom Med. 2000;62:739–745. doi: 10.1097/00006842-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Boscarino JA. Psychosom Med. 1997;59:605–615. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, Stewart A, Schlenger WE, Wells KB. Am J Psychiat. 1997;154:1690–1695. doi: 10.1176/ajp.154.12.1690. [DOI] [PubMed] [Google Scholar]
- 12.Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti VJ. Int J Obesity. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS, Stellar E. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 14.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 15.Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; New York: 1990. [Google Scholar]
- 16.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 17.Melia KR, Duman RS. Proc Natl Acad Sci USA. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanton ME, Gutierrez YR, Levine S. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 19.Levine S, Weiner SG, Coe CL. Psychoneuroendocrinology. 1993;4:297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- 20.Bremner JD, Krystal JH, Southwick SM, Charney DS. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Bremner JD, Krystal JH, Southwick SM, Charney DS. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Foote SL, Bloom FE, Aston-Jones G. Physiol Behav. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 23.Abercrombie ED, Jacobs BL. J Neurosci. 1987;7:2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine ES, Litto WJ, Jacobs BL. Brain Res. 1990;531:189–195. doi: 10.1016/0006-8993(90)90773-5. [DOI] [PubMed] [Google Scholar]
- 25.Finlay JM, Zigmond MJ, Abercrombie ED. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 26.Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. J Neurosci. 1991;11:1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens M, Southwick SM, Nemeroff CB, Charney DS. Am J Psychiat. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker DB, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD. Am J Psychiat. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 29.Anisman H, Griffiths J, Matheson K, Ravindran AV, Merali Z. Am J Psychiat. 2001;158:1509–1511. doi: 10.1176/appi.ajp.158.9.1509. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 31.Yehuda R, Teicher MH, Levengood RA, Trestman RL, Siever LJ. Ann NY Acad Sci. 1994:378–380. doi: 10.1111/j.1749-6632.1994.tb39260.x. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, Southwick SM, Nussbaum EL, Giller EL, Mason JW. J Nerv Ment Dis. 1991;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Yehuda R, Kahana B, Binder-Brynes K, Southwick S, Mason JW, Giller EL. Am J Psychiat. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- 34.Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, Cooper TB, Malone KM, John MJ. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- 35.Kanter ED, Wilkinson CW, Radant AD, Petrie EC, Dobie DJ, McFall ME, Peskind ER, Raskind MA. Biol Psychiatry. 2001;50:238–245. doi: 10.1016/s0006-3223(01)01158-1. [DOI] [PubMed] [Google Scholar]
- 36.Glover DA, Poland RE. Psychoneuroendocrinology. 2002;27:805–819. doi: 10.1016/s0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
- 37.Delahanty DL, Raimonde AJ, Spoonster E, Cullado M. J Anxiety Disord. 2003;17:149–164. doi: 10.1016/s0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 38.Seedat S, Stein MB, Kennedy CM, Hauger RL. Psychoneuroendocrinology. 2003;28:796–808. doi: 10.1016/s0306-4530(02)00086-0. [DOI] [PubMed] [Google Scholar]
- 39.Mason J, Wang S, Yehuda R, Lubin H, Johnson D, Bremner JD, Riney S, Charney DS, Southwick SM. Psychosom Med. 2002;64:238–246. doi: 10.1097/00006842-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Young EA, Breslau N. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- 41.Young EA, Breslau N. Biol Psychiatry. 2004;56:205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Young EA, Toman T, Witkowski K, Kaplan G. Biol Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Cicchetti D, Rogosch FA. Dev Psychopathol. 2001;13:783–804. [PubMed] [Google Scholar]
- 44.De Bellis MD, Baum AS, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 45.Gunnar MR, Morison SJ, Chisolm K, Schuder M. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 46.Lemieux AM, Coe CL. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmans E, Kenis G, Scharpe S. Acta Psychiatr Scand. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- 48.Pico-Alfonso MA, Garcia-Linares MI, Celda-Navarro N, Herbert J, Martinez M. Biol Psychiatry. 2004;56:233–240. doi: 10.1016/j.biopsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Pitman RK, Orr SP. Biol Psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- 50.Stein MB, Yehuda R, Koverola C, Hanna C. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- 51.Yehuda R, Southwick SM, Krystal JH, Bremner JD, Charney DS, Mason J. Am J Psychiat. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 52.Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Anderson G, Heninger GR, Southwick SM, Charney DS. Psychoneuroendocrinology. 2002 doi: 10.1016/s0306-4530(02)00067-7. (in press) [DOI] [PubMed] [Google Scholar]
- 53.Elzinga BM, Schmahl CS, Vermetten E, van Dyck R, Bremner JD. Neuropsychopharmacology. 2002 doi: 10.1038/sj.npp.1300226. (in press) [DOI] [PubMed] [Google Scholar]
- 54.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 55.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Am J Psychiat. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 56.De Bellis MD, Lefter L, Trickett PK, Putnam FW. J Am Acad Child Adolesc Psychiatry. 1994;33:320–327. doi: 10.1097/00004583-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Southwick SM, Krystal JH, Morgan CA, Johnson DR, Nagy LM, Nicolaou AL, Heninger GR, Charney DS. Arch Gen Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 58.Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, Bronen R, Zubal G, Rich D, Krystal JH, Dey H, Soufer R, Charney DS. Arch Gen Psychiatry. 1997;54:246–256. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 59.Orr SP, Lasko NB, Metzger LJ, Ahern CE, Berry NJ, Pitman RK. J Consult Clin Psychol. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- 60.Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 61.Luine V, Villages M, Martinex C, McEwen BS. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 62.Arbel I, Kadar T, Silberman M, Levy A. Brain Res. 1994;657:227–235. doi: 10.1016/0006-8993(94)90972-5. [DOI] [PubMed] [Google Scholar]
- 63.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diamond DM, Fleshner M, Ingersoll N, Rose GM. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 65.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woolley CS, Gould E, McEwen BS. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 67.McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley CS. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 68.Sapolsky RM. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 69.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. J Comparative Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 72.Moghaddam B, Adams B, Verma A, Daly D. J Neurosci. 1997;17:2912–2127. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nibuya M, Morinobu S, Duman RS. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith MA, Makino S, Kvetnansky R, Post RM. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duman RS, Heninger GR, Nestler EJ. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 77.Kempermann G, Kuhn HG, Gage FH. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 80.Duman RS, Malberg JE, Nakagawa S. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 81.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H, Kim JW, Yim SV, Kim MJ, Kim SA, Kin YJ, Din CJ, Chung JH. Mol Psychiatry. 2001;6:725–728. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- 83.Lucassen PJ, Fuchs E, Czeh B. Eur J Neurosci. 2004;14:161–166. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 84.van der Beek EM, Wiegant VM, Schouten WGP, van Eerdenburg FJCM, Loijens LWS, van der Plas C, Benning MA, de Vries H, de Kloet ER, Lucassen PJ. Hippocampus. 2004;14:688–700. doi: 10.1002/hipo.10213. [DOI] [PubMed] [Google Scholar]
- 85.Bowman RE, Ferguson D, Luine VN. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 86.de Kloet ER, Oitzl MS, Joels M. Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 87.Lemaire V, Koehl M, Le Moal M, Abrous DN. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JJ, Fanselow MS. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 89.Phillips RG, LeDoux JE. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 90.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Aranico O, Belzung C, Hen R. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 91.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 92.LeDoux JL. Ann NY Acad Sci. 1993:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- 93.Davis M. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 94.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vyas A, Pillai AG, Chattarji S. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 96.Vogt BA, Finch DM, Olson CR. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 97.Devinsky O, Morrell MJ, Vogt BA. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 98.Roth RH, Tam SY, Ida Y, Yang JX, Deutch AY. Ann NY Acad Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- 99.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Quirk GJ. Learning & Memory. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morgan CA, LeDoux JE. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 102.Morgan CA, Romanski LM, LeDoux JE. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 103.Milad MR, Quirk GJ. Nature. 2002;420:70–73. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 104.Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JDE. Science. 2004;303:232–234. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 105.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 106.Pitman RK. J Clin Psychiatry. 2001;62:47–54. [PubMed] [Google Scholar]
- 107.Bremner JD. Psych Annal. 1998;28:445–450. [Google Scholar]
- 108.Bremner JD, Randall PR, Scott TM, Bronen RA, Delaney RC, Seibyl JP, Southwick SM, McCarthy G, Charney DS, Innis RB. Am J Psychiat. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gurvits TG, Shenton MR, Hokama H, Ohta H, Lasko NB, Gilbertson MB, Orr SP, Kikinis R, Lolesz FA. Biol Psychiatry. 1996;40:192–199. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Villareale G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Biological Psychiatry. 2002;15:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 111.Bremner JD, Randall PR, Vermetten E, Staib L, Bronen RA, Mazure CM, Capelli S, McCarthy G, Innis RB, Charney DS. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg P, Ng CK, Staib LH, Duncan JS, Charney DS. Am J Psychiat. 2002 (in press) [Google Scholar]
- 113.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 114.Freeman TW, Cardwell D, Karson CN, Komoroski RA. Magnetic Resonance in Medicine. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 115.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg P, Ng CK, Staib LH, Duncan JS, Charney DS. Am J Psychiat. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 117.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 118.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 119.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 120.Notestine CF, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Biol Psychiatry. 2002;51:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 121.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Am J Psychiat. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. J Traumatic Stress. 2001;14:413–420. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- 124.Bremner JD, Randall PR, Capelli S, Scott TM, McCarthy G, Charney DS. Psychiatry Res. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- 125.Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 126.Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- 127.Bremner JD. CNS Spectrums. 2002;7:129–139. doi: 10.1017/s1092852900017442. [DOI] [PubMed] [Google Scholar]
- 128.Vythilingam M, Heim C, Newport CD, Miller AH, Vermetten E, Anderson E, Bronen R, Staib L, Charney DS, Nemeroff CB, Bremner JD. Am J Psychiat. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Proc Natl Acad Sci USA. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 131.Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, Petropoulos H, Brooks WM. Psych Res : Neuroimaging. 2004;131:227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 132.Elzinga BM, Bremner JD. J Affective Disord. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buckley TC, Blanchard EB, Neill WT. Clin Psychol Rev. 2000;28:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 134.Thygesen P, Hermann K, Willanger R. Danish Medical Bulletin. 1970;17:65–108. [PubMed] [Google Scholar]
- 135.Sutker PB, Winstead DK, Galina ZH, Allain AN. Am J Psychiat. 1991;148:67–72. doi: 10.1176/ajp.148.1.67. [DOI] [PubMed] [Google Scholar]
- 136.Brewin CR. Behav Res Ther. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 137.Golier J, Yehuda R. Dev Psychopathol. 1998;10:857–869. doi: 10.1017/s0954579498001904. [DOI] [PubMed] [Google Scholar]
- 138.Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Am J Psychiat. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 139.Jenkins MA, Langlais PJ, Delis D, Cohen R. Am J Psychiat. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- 140.Moradi AR, Doost HT, Taghavi MR, Yule W, Dalgleish T. J Child Psychol Psychiat. 1999;40:357–361. [PubMed] [Google Scholar]
- 141.Roca V, Freeman TW. Am J Psychiat. 2001;158:1738. doi: 10.1176/appi.ajp.158.10.1738-a. [DOI] [PubMed] [Google Scholar]
- 142.Uddo M, Vasterling JJ, Braily K, Sutker PB. J Psychopathol Behavioral Assess. 1993;15:43–52. [Google Scholar]
- 143.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 144.Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 145.Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ. Am J Psychiat. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- 146.Barrett DH, Green ML, Morris R, Giles WH, Croft JB. Am J Psychiat. 1996;153:1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- 147.Gil T, Calev A, Greenberg D, Kugelmas S, Lerer B. J Traumatic Stress. 1990;3:29–45. [Google Scholar]
- 148.Sachinvala N, vonScotti H, McGuire M, Fairbanks L, Bakst K, McGuire M, Brown N. J Nerv Ment Dis. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 149.Golier J, Yehuda R, Cornblatt B, Harvey P, Gerber D, Levengood R. Integrative Physiological & Behavioral Science. 1997;32:52–61. doi: 10.1007/BF02688613. [DOI] [PubMed] [Google Scholar]
- 150.Stein MB, Hanna C, Vaerum V, Koverola C. J Traumatic Stress. 1999;12:527–534. doi: 10.1023/A:1024775222098. [DOI] [PubMed] [Google Scholar]
- 151.Zalewski C, Thompson W, Gottesman I. Assessment. 1994;1:133–142. doi: 10.1177/1073191194001002003. [DOI] [PubMed] [Google Scholar]
- 152.Bremner JD, Steinberg M, Southwick SM, Johnson DR, Charney DS. Am J Psychiat. 1993;150:1011–1014. doi: 10.1176/ajp.150.7.1011. [DOI] [PubMed] [Google Scholar]
- 153.McNally RJ, Litz BT, Prassas A, Chin LM, Weathers FW. Cognition and Emotion. 1994;8:351–367. [Google Scholar]
- 154.Cassiday KL, McNally RJ, Zeitlin SB. Cognitive Therapy Research. 1992;16:283–295. [Google Scholar]
- 155.Foa EB, Feske U, Murdock TB, Kozak MJ, McCarthy PR. J Abnorm Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- 156.McNally RJ, Kaspi RJ, Riemann BC, Zeitlin SB. J Abnorm Psychology. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- 157.McNally RJ, English GE, Lipke HJ. J Traumatic Stress. 1993;6:33–41. [Google Scholar]
- 158.Moradi AR, Taghavi R, Neshat-Doost HT, Yule W, Dalgleish T. J Anxiety Disord. 2000;14:521–534. doi: 10.1016/s0887-6185(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 159.Bryant RA, Harvey AG. J Abnorm Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- 160.Beck JG, Freeman JB, Shipherd JC, Hamblen JL, Lackner JM. J Abnorm Psychology. 2001;110:536–543. doi: 10.1037//0021-843x.110.4.536. [DOI] [PubMed] [Google Scholar]
- 161.McNeil DW, Tucker P, Miranda R, Lewin M, Nordgren JC. J Nerv Ment Dis. 1999;187:512–516. doi: 10.1097/00005053-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 162.Thrasher SM, Dalgleish T, Yule W. Behav Res Ther. 1994;32:247–254. doi: 10.1016/0005-7967(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 163.Beckham JC, Crawford AL, Feldman ME. J Traumatic Stress. 1998;11:811–819. doi: 10.1023/A:1024409903617. [DOI] [PubMed] [Google Scholar]
- 164.Bremner JD, Vermetten E, Nafzal N, Vythilingam M. J Nerv Ment Dis. 2004;192:643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- 165.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Watanabe YE, Gould H, Cameron D, Daniels D, McEwen BS. Hippocampus. 1992;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 167.Bremner JD, Mletzko T, Welter S, Siddiq S, Reed L, Williams C, Heim CM, Nemeroff CB. J Clin Psychiatry. 2004 doi: 10.4088/jcp.v65n1120. (in press) [DOI] [PubMed] [Google Scholar]
- 168.Bremner JD, Mletzko T, Welter S, Quinn S, Williams C, Brummer M, Siddiq S, Reed L, Heim CM, Nemeroff CB. J Psychopharmacol. 2005 doi: 10.1177/0269881105048996. (in press) [DOI] [PubMed] [Google Scholar]
- 169.De Bellis MD, Keshavan MS, Spencer S, Hall J. Am J Psychiat. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 170.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J. Biol Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- 171.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 172.Bremner JD. Child Adolesc Psychiat Clin N A. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 173.Bremner JD, Vermetten E. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- 174.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Am J Psychiat. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shin LH, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Am J Psychiat. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 177.Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 178.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 179.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 180.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib L, Soufer R, Charney DS. Biol Psychiatry. 2003;53:289–299. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 181.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Am J Psychiat. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 182.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 183.Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I. J Psychiat Res. 1999;33:259–264. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 184.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 185.Bremner JD, Soufer R, McCarthy G, Delaney RC, Staib LH, Duncan JS, Charney DS. Psychopharmacol Bull. 2001;35:55–87. [PubMed] [Google Scholar]
- 186.Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga BE, Charney DS. Biological Psychiatry. 2003 doi: 10.1016/j.biopsych.2003.10.001. (in press) [DOI] [PubMed] [Google Scholar]
- 187.Semple WE, Goyer P, McCormick R, Donovan B, Muzic RF, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 188.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 189.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 190.Bremner JD. Psychopharmacol Bull. 2003;37:6–25. [PubMed] [Google Scholar]
- 191.Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RWJ, Gati JS, Menon RS. Biol Psychiatry. 2003;53:204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 192.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta MA, Neufeld RWJ, Gati JS, Menon RS. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 193.Bremner JD, Innis RB, Southwick SM, Staib LH, Zoghbi S, Charney DS. Am J Psychiat. 2000;157:1120–1126. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 194.Maddock RJ, Buonocore MH. Psychiatry Res. 1997;75:1–14. doi: 10.1016/s0925-4927(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 195.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Arch Gen Psychiatry. 1997;54:233–237. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 196.Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Proceedings of the National Academy of Sciences, USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pardo JV, Fox PT, Raichle ME. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 198.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 199.Schmahl CG, McGlashan T, Bremner JD. Psychopharmacol Bull. 2002;36:69–87. [PubMed] [Google Scholar]
- 200.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Psych Res : Neuroimaging. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 201.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Archives of General Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 202.Schmahl CG, Elzinga BM, Bremner JD. Clin Psychol Psychoth. 2002;9:271–276. [Google Scholar]
- 203.Schmahl CG, Elzinga BM, Vermetten E, Sanislow C, McGlashan TH, Bremner JD. Biol Psychiatry. 2003;54:42–51. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- 204.Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Am J Psychiat. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- 205.Meadows EA, Foa EB. In: Posttraumatic Stress Disorder: A Comprehensive Text. Saigh PA, Bremner JD, editors. Allyn & Bacon; Needham Heights, MA: 1999. pp. 376–390. [Google Scholar]
- 206.Bouton ME, Swartzentruber D. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- 207.Prigerson HG, Maciejewski PK, Rosenheck RA. J Nerv Ment Dis. 2001;189:99–108. doi: 10.1097/00005053-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 208.Kempermann G, Kuhn HG, Gage FH. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]