Abstract
Introduction
Cytologic detection of peritoneal gastric cancer cells by Papanicolaou staining offers important prognostic information but has low sensitivity. We evaluated a novel detection technique using Newcastle disease virus expressing the enhanced green fluorescent protein (NDV-GFP) gene.
Methods
NDV-GFP was tested on MKN-1 human gastric adenocarcinoma cells plated upon rat hepatocytes to determine tumor-specific infection and GFP expression. Malignant ascites infected with increasing doses of virus was evaluated for NDV-GFP dose determination. Peritoneal lavage samples from 30 patients with gastric adenocarcinoma undergoing staging laparoscopy were evaluated with NDV-GFP.
Results
NDV-GFP can specifically detect one MKN-1 cell among one million benign rat hepatocytes. NDV-GFP at 5×106 plaque-forming units (PFU) produced optimal GFP expression in malignant ascites. Noncancerous cells were non-GFP expressing. GFP-expressing cells counterstained positive for carcinoembryonic antigen expression, confirming their cancerous origin. Furthermore, in patients with advanced gastric cancer, GFP expression was markedly enhanced over cytology. Of six patients with M1 disease discovered during laparoscopy, only 50% were cytology positive. All six, however, were NDV-GFP positive. Cytology was positive in 9% of patients with T3 disease, 8% with N1 disease, and 50% with N2 disease. In contrast, NDV-GFP was positive in 95% of T3 patients and 100% of patients with N1 or N2 disease.
Conclusions
NDV-GFP can specifically infect and detect peritoneal gastric cancer cells and offers a more sensitive method compared with conventional cytology. This novel modality may offer enhanced detection of intraperitoneal cancer spread and provide important prognostic information.
Keywords: Newcastle disease virus, Detection, Gastric cancer, Peritoneal lavage
Introduction
Gastric cancer is the fourth most common cancer globally1 and is particularly prevalent in Asian countries. Within the USA, over 21,000 new cases were estimated to occur in 2008 alone.2 Staging of gastric cancer is currently done by endoscopy with biopsy and various imaging modalities, including endoscopic ultrasound (EUS) and computed tomography (CT).3,4 Diagnostic laparoscopy has emerged as a better means of excluding metastases in the peritoneum while also enabling the procurement of lavage washings for cytologic examination. Positive cytologic washings, as determined by the Papanicolaou (Pap) stain, confer the same poor prognosis as overt metastases.5–7 Cytology, however, is not always positive in cases of obvious metastatic disease and has a low sensitivity of 54–59%.8,9 A more sensitive means of diagnosing free peritoneal cancer cells would allow for identification of those patients who may benefit from adjuvant therapy.
Newcastle disease virus (NDV) is a member of the Paramyxoviridae family, is replication competent, and contains a nonsegmented, negative-stranded RNA genome.10 It is known to be pathogenic in birds but does not cause toxicity in humans. Several trials have noted clinical benefit after administration of naturally occurring NDV in metastatic or malignant tumors refractory to standard care, such as melanoma and various solid tumors.11,12 The recent establishment of the reverse-genetics system has allowed for genetic manipulation of the NDV virus, thereby enhancing its oncolytic effect, as well as inserting a reporter gene, such as the enhanced green fluorescent protein (eGFP).13,14 The NDV containing the eGFP marker gene, NDV-GFP, may offer a novel diagnostic modality in evaluation of peritoneal lavage fluid from patients with gastric cancer.
This study set out to determine whether the recombinant NDV-GFP virus, which has been designed to specifically target and infect gastric cancer cells, could be used diagnostically. Virally mediated detection of free peritoneal cancer cells from patients undergoing staging diagnostic laparoscopy for gastric cancer was then investigated as a means of detection and compared with cytologic examination by the Pap stain.
Materials and Methods
Cell Culture
The MKN-1 cell line, an adenosquamous cell carcinoma, was kindly provided by Dr. T. Suzuki (Fukushima Medical College, Japan) and was cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin.
Virus
The attenuated NDV Hitchner B1 strain (NDV-B1) was modified with the reverse-genetics system.15,16 To generate NDV expressing GFP, a GFP DNA fragment flanked by the appropriate NDV-specific RNA transcriptional signals was inserted into the XbaI site created between the P and M genes of pT7NDV of F3aa. Viruses were rescued from complementary cDNA using methods described previously and sequenced by reverse transcription–polymerase chain reaction for insert fidelity.
Rat Hepatocyte Study
All animal studies were done in accordance with Memorial Sloan–Kettering Cancer Center’s (MSKCC’s) Institute of Animal Care and Use Committee under an approved protocol. Adult male Sprague Dawley rats were anesthetized using 2% inhalational isoflurane mixed with 3 l of oxygen. A midline laparotomy was performed and the liver isolated. The portal vein was cannulated and perfused with warm liver perfusion medium followed by Liver Digest Medium (Gibco, Grand Island, NY, USA). Rat hepatocytes were isolated and cultured according to the manufacturer’s protocol on six-well plates coated with 1% rat tail collagen and incubated at 37°C. Four hours after plating the hepatocytes, MKN-1 cells were added at a ratio of one cancer cell against a background of one million rat hepatocytes. Twenty-four hours later, the plates were infected with NDV-GFP at a dose of 5×106 PFU and evaluated for GFP expression.
Patient Study
All patient samples were collected under an Institutional-Review-Board-approved tissue collection protocol with patient consent. Thirty patients underwent diagnostic laparoscopy at MSKCC for biopsy-proven gastric adenocarcinoma. Normal saline was instilled into the peritoneal cavity, and lavage samples were collected from the right upper quadrant, left upper quadrant, and pelvis. Duplicate samples from each site were obtained from every patient; half were sent to the pathology department for evaluation, and the other half were transported to the laboratory on ice. The lavage fluid was combined and placed in 50-ml conicals and centrifuged at 800 rpm for 5 min to obtain a cell pellet. This pellet was then washed once with phosphate-buffered saline (PBS), resuspended in 1-ml RPMI media containing 10% FBS and 1% penicillin and streptomycin, plated in four-well chamber slides, and incubated at 37°C.
NDV-GFP Dose Optimization
A sample of grossly malignant ascites was obtained from the operating room and processed in the above-described fashion. Wells were incubated with single doses of NDV-GFP ranging from 5×103 to 5×107 PFU. Fluorescence microscopy was performed after 12 h of incubation to evaluate for number of green fluorescent cells visualized per high-powered field.
Viral Infection and Costaining for CEA
After a minimum of 6 h and up to 24 h of incubation, 500 μl of media was aspirated carefully from each well of the chamber slide. NDV-GFP 5×106 PFU was added to the remaining media and left at room temperature. Five hundred microliters of media was added back after 30 min, and the chamber slide returned to 37°C. Samples were also counter-stained with phycoerythrin–anti-carcinoembryonic antigen (CEA; BD Pharmingen, Franklin Lakes, NJ, USA).
Fluorescence Microscopy
All samples were evaluated with an inverted microscope (Nikon Eclipse TE300, Nikon, Tokyo, Japan) using phase contrast and fluorescence microscopy. A GFP emission filter was used to detect green fluorescence and a TRIT-C filter for the red fluorescent CEA antibody. Images were obtained using NIS software.
Immunofluorescent Staining
Samples were washed with PBS and fixed with 4% paraformaldehyde for 20 min. The cells were lysed with 1% Triton-X and incubated in rabbit anti-NDV antibody for 2 h. Incubation for 1 h with Alex-fluor 532 secondary antibody followed. The slide was then mounted with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) and evaluated with fluorescence microscopy.
Results
Detection of MKN-1 Cells Against Benign Hepatocytes
To confirm that NDV-GFP infects cancer cells but not cells of noncancerous origin, one MKN-1 cell was plated on a background of one million benign rat hepatocytes and infected with NDV-GFP. At a dose of 5×106 PFU, NDV-GFP was able to detect one cancer cell against a background of one million benign rat hepatocytes (Fig. 1). GFP expression was seen as early as 6 h and continued for over 24 h. This demonstrated that NDV was both sensitive and specific for gastric cancer cell detection.
Figure 1.
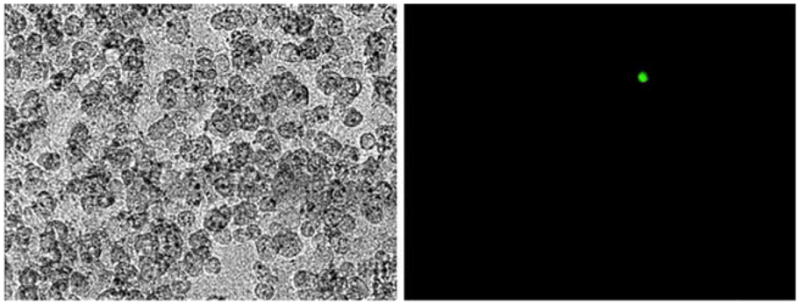
Representative phase contrast and GFP images of MKN-1 gastric adenocarcinoma cells against benign rat hepatocytes after infection with NDV-GFP for 24 h. NDV-GFP can detect one MKN-1 cancer cell against a background of one million benign hepatocytes.
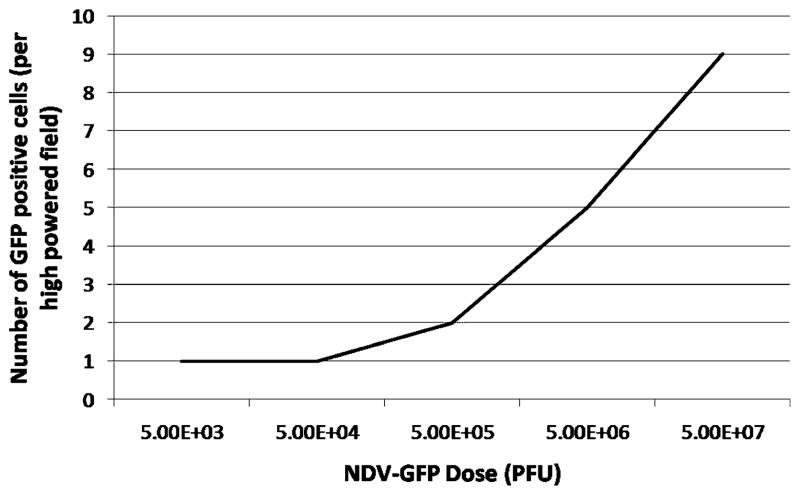
Dose Optimization
To optimize the dose of NDV for maximum detection benefit, grossly positive ascites were infected with different doses of NDV, and the numbers of cancer cells that were detectable by GFP expression among the different groups were compared. NDV-GFP produced detectable GFP expression with as low a dose as 5×103 PFU after 12 h of incubation. The number of GFP-positive cells increased with increasing doses of virus from one GFP-positive cell per high-powered field at a dose of 5×103 PFU to nine GFP-positive cells per high-powered field with a dose of 5×107 PFU (Fig. 2). A dose of 5×106 PFU, which produced five GFP-positive cells per high-powered field, was chosen as the dose of NDV-GFP with which to proceed, since this dose was the most practical and provided the best balance of viral dose and number of green-fluorescent-positive cells. Also noted were that noncancerous cells, such as erythrocytes and fibroblasts, determined by phenotypic appearance, were also non-GFP expressing.
Figure 2.
Dose optimization of NDV-GFP. A grossly malignant sample of ascites was processed and divided into multiple aliquots, incubated with increasing doses of virus, and evaluated with fluorescence microscopy after 12 h.
Evaluation of Peritoneal Washing Samples
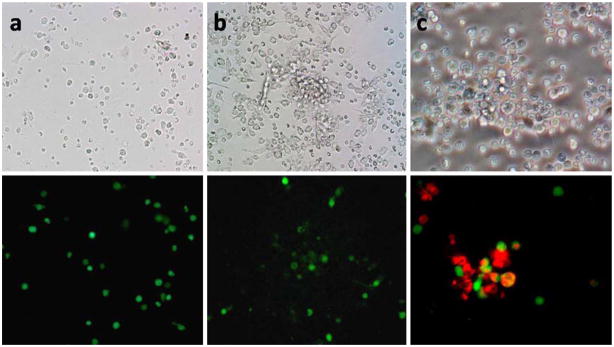
We proceeded to evaluate the detection of gastric cancer cells in peritoneal washings using the NDV-GFP virus. GFP-positive cells were found in both cytology-negative and cytology-positive samples (Fig. 3a, b). Other cell types, such as red blood cells and dendritic cells, were GFP negative. GFP-positive cells were detected in 29 of 30 (97%) samples. Cytology, in comparison, was positive in three of 30 (10%). Of the six patients found to have metastatic disease at laparoscopy, GFP-positive cells were found in all washing samples. Cytology, however, was positive in only half of these cases. Overall, NDV-GFP offered a greater sensitivity in detecting gross disease, p< 0.01 (Table 1).
Figure 3.
Phase contrast and GFP images of human peritoneal washing samples after infection with NDV-GFP and counterstaining for CEA. Peritoneal washings processed with NDV-GFP from a cytology-negative patient (a) and from a cytology-positive patient (b). Both samples demonstrated GFP-positive cells. Counterstaining for CEA (red) confirms the GFP-positive cells are cancer cells (c).
Table 1.
Relationship Between NDV-GFP and Cytology Detection of Cancer Cells in Peritoneal Washings and Clinical Features of the Gastric Cancer Cohort
| Clinicopathologic features | NDV-GFP+ N=29/30 |
NDV-GFP+/cytology+ N=3/30 |
|---|---|---|
| Gender | ||
| Male | 67% | 10% |
| Location | ||
| GE junction | 17% | 7% |
| Cardia | 13% | 0% |
| Fundus | 3% | 0% |
| Body | 33% | 3% |
| Antrum | 30% | 0% |
| Differentiation | ||
| Well | 7% | 0% |
| Moderate | 33% | 3% |
| Poor | 57% | 7% |
| EUS/CT/laparoscopy | ||
| Stages 1–2 | 92% | 0% |
| Stages 3–4 | 100% | 18% |
| T1/T2 (N=6) | 100% | 0% |
| T3/T4 (N=22) | 95% | 9% |
| N1/N215 | 100% | 13% |
| M1 (N=6) | 100% | 50% |
GE gastroesophageal, EUS endoscopic ultrasound, CT computed tomography
There was no correlation between patient gender or tumor location and the detection of free peritoneal cancer cells, either by cytology or by NDV-GFP (Table 1). Based on EUS, CT, and diagnostic laparoscopy findings, 13 patients were considered stages 1 or 2, while 17 patients were considered stages 3 or 4. NDV-GFP was positive in 100% of stage 3–4 patients, while cytology was positive in only 18% (Table 1). Additionally, 22 patients were determined to have T3 disease or tumors that penetrated the serosa. Of these, two of 22 (9%) were cytology positive, while 21 of 22 (95%) were found to be NDV-GFP positive. Fifteen patients were determined to have N1 or N2 disease. Of these, two of 15 (13%) were cytology positive, while all 15 (100%) of patients were NDV-GFP positive (Table 1).
After diagnostic laparoscopy, 23 patients ultimately underwent resection. Of these, 22 of 23 (96%) had GFP-positive cells as detected by NDV-GFP. None of the patients were cytology positive. Of the 17 patients found to have vascular invasion on pathology, all were NDV-GFP positive (Table 2). Similarly, all nine patients found to have perineural invasion and all seven patients found to have positive margins on pathologic examination were also found to be NDV-GFP positive and cytology negative.
Table 2.
Relationship of NDV-GFP Detection and Clinical Indicators of Poor Prognosis in Gastric Cancer Patients at the Time of Resection
| Pathologic features of resected specimens | NDV-GFP+ N=22/23 |
Cytology+ N=0/23 |
|---|---|---|
| Vascular invasion | 17/17 (100%) | 0/17 (0%) |
| Perineural invasion | 9/9 (100%) | 0/9 (0%) |
| Positive margins | 7/7 (100%) | 0/7 (0%) |
Counterstaining for CEA
To confirm that these GFP-positive cells were indeed cancer cells, the samples were costained with anti-CEA antibody. Using fluorescence microscopy, the GFP-positive cells were shown to costain for CEA (Fig. 3c), further confirming their cancerous origin.
Immunofluorescent Staining
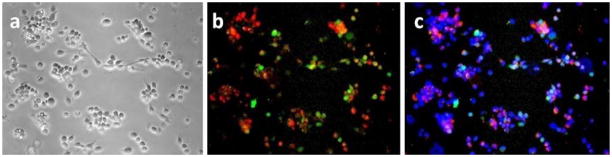
To confirm that the GFP-positive cells were fluorescent due to NDV-GFP infection, human samples were processed in the manner described above, evaluated with fluorescent microscopy for GFP and CEA expression, and then fixed with 4% paraformaldehyde. These samples were then stained with anti-NDV antibody, anti-CEA antibody, and appropriate secondary antibodies. As can be seen in Fig. 4, cells that were stained for NDV antigens also costained for CEA, confirming that only cancer cells were infected with NDV.
Figure 4.
Immunofluorescent staining for Newcastle disease virus and CEA. Peritoneal washings from a cytology-positive patient were processed and fixed with 4% paraformaldehyde. Phase-contrast image (a), immunofluorescent staining for CEA (red) and NDV (green) show double-positive staining (b), and overlay with DAPI stain for nuclei (c).
Discussion
Gastric cancer is the fourth most common cancer globally.2 While metastatic disease still carries a median survival of less than a year with chemotherapy,17 patients who can be preoperatively identified as having high risk for advanced disease would benefit from a treatment paradigm that differs from the treatment plan for patients with early-stage disease. Diagnostic laparoscopy has proven to be both a useful tool in diagnosing subradiologic metastatic disease, as well as providing a means to evaluate peritoneal lavage cytology.9 Multiple studies have demonstrated that positive peritoneal cytology, as determined by the Pap stain, confers the same prognosis as does gross metastatic disease.5,7
NDV has been studied for its natural tumor specificity and oncolytic properties since the late 1950s.18 The establishment of the reverse-genetics system for the virus has allowed for modifications enhancing cancer specificity and incorporation of marker genes, such as GFP, thereby allowing for tracking of viral replication.14 The current study set out to determine if virally mediated detection of free peritoneal cancer cells in patients undergoing diagnostic laparoscopy for biopsy-proven gastric adenocarcinoma would offer a more sensitive method of detection, as compared to conventional Pap staining.
The results of this study demonstrated that NDV-GFP was able to specifically detect cancer cells and express GFP upon a background of benign rat hepatocytes, as well as in human peritoneal lavage samples. Noncancerous cells, such red blood cells and fibroblasts, were non-GFP expressing. Counterstaining for CEA in the human lavage samples confirmed the cancerous origin of the GFP-positive cells. NDV-GFP-mediated detection offers significantly more sensitivity compared with conventional cytology. Even in patients who were found to have gross peritoneal disease during laparoscopy, NDV-GFP detected positive cells in all cases, while Pap staining was positive in only 50% of those patients.
NDV-GFP was also able to identify free peritoneal gastric cancer cells in the majority of those patients found to have more advanced disease, such as stage 3–4 disease, T3, and N1 or N2 tumors. Positive gastric cancer cells were also identified in the peritoneal washings of all patients found to have vascular invasion, perineural invasion, and positive margins within the resected specimen, while Pap staining was negative. These results suggest that NDV-GFP may better identify those patients who have risk factors for recurrence. Future clinical follow-up is needed to determine the prognostic significance of finding free peritoneal gastric cancer cells by this more sensitive, virally mediated method and how the identification of these cells may affect treatment.
Conclusion
NDV-GFP-mediated detection of gastric cancer cells offers a rapid and more sensitive method of identifying free peritoneal gastric cancer cells in peritoneal lavage fluid compared with conventional Pap staining. While positive peritoneal cytology by Pap staining confers the same poor prognosis as does metastatic disease, long-term clinical follow-up is needed to ascertain the prognostic impact of positive NDV-GFP status.
Footnotes
Presented at 50th SSAT Annual Meeting at Digestive Disease Week, Chicago, 2009
Contributor Information
Joyce Wong, Department of Surgery, Memorial Sloan–Kettering Cancer Center, New York, NY 10065, USA.
Allison Schulman, Department of Surgery, Memorial Sloan–Kettering Cancer Center, New York, NY 10065, USA.
Kaitlyn Kelly, Department of Surgery, Memorial Sloan–Kettering Cancer Center, New York, NY 10065, USA.
Dmitriy Zamarin, Department of Surgery, Memorial Sloan–Kettering Cancer Center, New York, NY 10065, USA. Department of Medicine, Mount Sinai Medical Center, New York, NY, USA.
Peter Palese, Department of Microbiology, Mount Sinai Medical Center, New York, NY, USA.
Yuman Fong, Email: fongy@mskcc.org, Department of Surgery, Memorial Sloan–Kettering Cancer Center, New York, NY 10065, USA.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25(15):2107–2116. doi: 10.1200/JCO.2006.09.5224. [DOI] [PubMed] [Google Scholar]
- 4.Blackshaw G, Lewis WG, Hopper AN, Morgan MA, Al-Khyatt W, Edwards P, et al. Prospective comparison of endosonography, computed tomography, and histopathological stage of junctional oesophagogastric cancer. Clin Radiol. 2008;63 (10):1092–1098. doi: 10.1016/j.crad.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12(5):347–353. doi: 10.1245/ASO.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 6.Badgwell B, Cormier JN, Krishnan S, Yao J, Staerkel GA, Lupo PJ, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15(10):2684–2691. doi: 10.1245/s10434-008-0055-3. [DOI] [PubMed] [Google Scholar]
- 7.Nath J, Moorthy K, Taniere P, Hallissey M, Alderson D. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. Br J Surg. 2008;95(6):721–726. doi: 10.1002/bjs.6107. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi K, Maeda S, Kitamura K. Papillary adenoma of the gallbladder associated with regurgitation of pancreatic juice through abnormally shaped union. Case report. Acta Chirurgica Scandinavica. 1989;155(10):549–552. [PubMed] [Google Scholar]
- 9.Wilkiemeyer MB, Bieligk SC, Ashfaq R, Jones DB, Rege RV, Fleming JB. Laparoscopy alone is superior to peritoneal cytology in staging gastric and esophageal carcinoma. Surg Endosc. 2004;18(5):852–856. doi: 10.1007/s00464-003-8828-z. [DOI] [PubMed] [Google Scholar]
- 10.Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7 (2):106–119. doi: 10.1634/theoncologist.7-2-106. [DOI] [PubMed] [Google Scholar]
- 11.Batliwalla FM, Bateman BA, Serrano D, Murray D, Macphail S, Maino VC, et al. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med. 1998;4(12):783–794. [PMC free article] [PubMed] [Google Scholar]
- 12.Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20(9):2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Romer-Oberdorfer A, Mundt E, Mebatsion T, Buchholz UJ, Mettenleiter TC. Generation of recombinant lentogenic Newcastle disease virus from cDNA. J Gen Virol. 1999;80(Pt 11):2987–2995. doi: 10.1099/0022-1317-80-11-2987. [DOI] [PubMed] [Google Scholar]
- 14.Gao Q, Park MS, Palese P. Expression of transgenes from Newcastle disease virus with a segmented genome. J Virol. 2008;82(6):2692–2698. doi: 10.1128/JVI.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakaya T, Cros J, Park MS, Nakaya Y, Zheng H, Sagrera A, et al. Recombinant Newcastle disease virus as a vaccine vector. J Virol. 2001;75(23):11868–11873. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67(17):8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 17.Wilke HJ, Van CE. Current treatments and future perspectives in colorectal and gastric cancer. Ann Oncol. 2003;14(Suppl 2):ii49–ii55. doi: 10.1093/annonc/mdg730. [DOI] [PubMed] [Google Scholar]
- 18.Lambert J. Value of a dietary milk for infants in tube feeding. Sem Hop. 1969;45(27):1908–1911. [PubMed] [Google Scholar]