Abstract
Interferon-gamma (IFNG), a pro-inflammatory cytokine, increases concentrations of neopterin, a stable pteridine derivative, due to IFNG-induced transcriptional activation of the rate-limiting enzyme of pteridines biosynthesis. Neopterin concentrations were reported to correlate with metabolic syndrome (MetS), the cause of increased mortality risk, in subjects of European ancestry. We were interested to assessed neopterin correlations with clinical markers of MetS and mortality risk in population with a different genetic background, i.e., Puerto Ricans residents of Boston. Since inflammation is associated with pyridoxal-5′-phosphate (PLP) deficiency, we assessed correlations of neopterin with PLP. Plasma neopterin concentrations were evaluated in 592 adult (45–75 years of age) participants of Boston Puerto Rican Health Study. Neopterin concentrations correlated with abdominal obesity (waist circumference, r = 0.085, p < 0.038), HDL cholesterol (r = −0.15, p < 0.0001), insulin resistance (HOMA-IR, r = 0.08, P < 0.03), and plasma pyridoxal-5′-phosphate (PLP (r = −0.13, P = 0.002). Neopterin concentrations of >16 nmol/L at baseline were associated with the increased risk of mortality in 113 subjects followed for 6 years. The present results together with previously reported data in European subjects suggest a similar pattern of neopterin correlations with MetS and mortality risk in population with different genetic backgrounds. PLP is a cofactor of IFNG-induced key enzymes of tryptophan-kynurenine metabolism. Since PLP deficiency is associated with the increased production of diabetogenic kynurenine derivative, xanthurenic acid, our results suggest that up-regulated IFNG production might contribute to the development of insulin resistance. Assessment of neopterin concentrations might help to monitor the activity of IFNG-inducible inflammation associated with aging-associated medical and psychiatric disorders.
Keywords: Interferon-Gamma, Neopterin, Obesity, Pyridoxal-5′-phosphate, Aging-Associated Medical and Psychiatric Disorders, Interferon-Gamma-Inducible Inflammation Cascade
1. INTRODUCTION
Low-grade chronic inflammation in the periphery and brain is associated with metabolic syndrome (MetS).1 Converging evidence suggests the involvement of the pro-inflammatory cytokine interferon-gamma (IFNG) in mechanisms of MetS and/or aging and age-associated disorders.2–4 IFNG is produced by microglia and macrophages. After release into the circulation, however, IFNG becomes rapidly neutralized by soluble receptors or binds to target structures. Consequently, the half-life of circulating IFNG is short, and its activity cannot be reliably evaluated by systemic measurements, e.g., IFNG concentrations in blood.6 The more reliable method to assess the rate of IFNG production is the evaluation of concentrations of neopterin, a stable by-product of pteridines biosynthesis. IFNG transcriptionally induces the rate-limiting enzyme of pteridine biosynthesis, guanosine triphosphate cyclohydrolase 1 (GTPCH).5 In humans, IFNG-induced stimulation of GTPCH results in accumulation of 7,8-dihydroneopterin (BH2) and its stable metabolite, neopterin.6 Elevation of neopterin, as a consequence of up-regulation of IFNG production, has been shown to correlate with MetS, aging, and total and disease-specific mortality in populations of European ancestry.7–9 We found that that neopterin production is affected by a polymorphism of the IFNG (+874) T/A gene,10 and, therefore, the genetic composition of the studied population may impact neopterin concentrations. Puerto Ricans represent a mixture of genetic backgrounds,11 and the population is characterized by high prevalence of MetS.12 Therefore, we assessed neopterin concentration in adults of Puerto Rican origin residing in the Boston area and analyzed correlations between neopterin and previously assessed markers of inflammation and MetS12, 13 and with mortality risk in this population. Since pyridoxal-5′-phosphate (PLP) deficiency was associated with inflammation,13 we assessed neopterin correlation with PLP in the same population.
2. METHODS
2.1. Subjects
2.1.1. Study 1
Neopterin concentrations were measured in 592 participants of the Boston Puerto Rican Health Study (45–75 years of age). Study was approved by Tufts Medical Center IRB, and written consents were obtained for participation in the study. The majority of the sample was female (70%). Mean ages were 57.2 years for men and 57.9 years for women.12 They have high prevalence of MetS relative to the general US population. Major characteristics of this population and their metabolic status have been described elsewhere.12, 13
2.1.2. Study 2
Mortality rate of 113 (out of 592) participants of the Boston Puerto Rican Health Study These participants was assessed during 6 years observation period.
2.2. Measurement of Neopterin
Blood was drawn into EDTA tubes between 7:00 a.m. and 10:00 a.m. after overnight and morning fasting. For measurement of neopterin, blood was protected from light, centrifuged to obtain plasma, and frozen at −40 °C before blinded assay in duplicate by sandwich ELISA using commercially available kits (American Research Products, Inc., Belmont, MA). Duplicate measurements were performed for each undiluted sample. The inter-and intra-assay coefficients of variation were <10% and <10%, respectively. Neopterin concentration is expressed in nmol/L.
Plasma high density lipoprotein (HDL)-cholesterol, waist circumferences, plasma PLP and homeostasis model assessment of insulin resistance (HOMA-IR) (calculated according to equation: glucose × insulin/405) were assessed in this population previously.12,13
2.3. Statistical Analysis
Results of neopterin analysis are expressed as mean± SD. Pearson correlations coefficients were calculated between plasma neopterin and waist circumference; PLP concentrations; HDL-cholesterol and HOMA-IR (adjusted for age and gender). Association with mortality was assessed using Cox proportional hazards models.
3. RESULTS
3.1. Study 1
3.1.1. Baseline values
Mean plasma neopterin concentrations ranged from 4 to 30 nmol/L (10.26 ± 7.35).
Waist circumferences (an index of abdominal obesity) ranged from 86 to 121 cm.12
The HDL-cholesterol values ranged between 39 to 46 mg/dL.12
HOMA-IR values varied from 4.1 to 12.5.13
Plasma PLP concentrations ranged from 5.5 to 737 nmol/L.12
3.1.2. Correlations
Neopterin concentrations correlated with waist circumference (r = 0.08, P < 0.04), HDL-cholesterol (r = −0.15, P < 0.0001), and HOMA-IR (r = 0.09, P = 0.03), suggesting association of inflammation with obesity, dyslipidemia and increased insulin resistance.
Neopterin concentrations correlated with plasma levels of PLP (r = −0.13, P = 0.002) suggesting association of inflammation (elevated neopterin concentrations) with PLP deficiency.
Neopterin/PLP ratio (index of combination of increased inflammation with PLP deficiency) correlated with HOMA-IR (r = 0.15, P = 0.0002) suggesting association of combined inflammation and PLP deficiency with insulin resistance.
3.2. Study 2
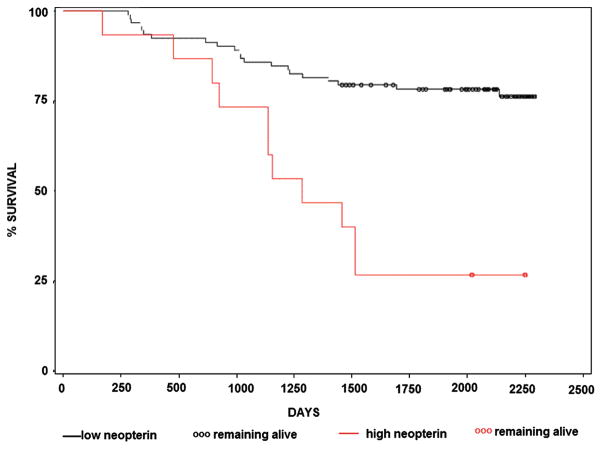
113 participants were followed for 6 years. At the end of observation period 81 participants were alive and 32 deceased. Neopterin concentrations of >16 nmol/L (mean ± two standard deviations) at the entry of the study were associated with the increased risk of mortality (Fig. 1).
Fig. 1.
Kaplan-Meier survivor curves of subjects with plasma neopterin concentration higher than 16 nmol/L (red line) and lower than 16 nmol/L (black line) (P < 0.02). Out of 113 study participants, 81 were alive and 32 deceased six years after the entry of the study.
4. DISCUSSION
To our knowledge, the present results are the first observation of correlation between plasma neopterin concentration and waist circumference, HDL-cholesterol and mortality risk in subjects of Puerto Rican origin, a population with unique genetic composition11 and high prevalence of MetS.12
Neopterin is used as a marker of up-regulated production of IFNG.6 We have found that neopterin production is impacted by a polymorphism of the IFNG (+874) T/A gene: carriers of the high producer (T) allele have higher neopterin concentrations than do carriers of the low producer (A) allele.10 Therefore, genetic composition of the studied population may impact neopterin concentrations. Puerto Rican population represents a mixture of genetic backgrounds.11 Associations between neopterin and clinical markers of MetS, HDL-cholesterol and mortality risk have been described in European population.7–9 The present results suggest that genetic differences between European and Puerto Ricans populations did not affect neopterin association with above mentioned markers and with mortality risk.
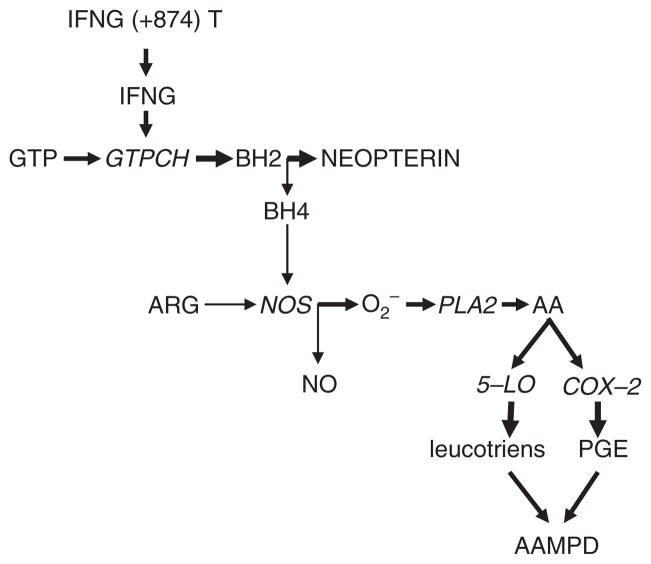
Development of MetS and risk of mortality are associated with aging. Converging evidence suggests the involvement of pro-inflammatory cytokine, IFNG, in mechanisms of aging and aging-associated medical3 and psychiatric14 disorders (AAMPD). Interferon-related genes have been identified among six pathways regulating senescence/immortalization: the cell cycle pRB/p53, cytoskeletal, insulin growth factor-related, MAP kinase and oxidative stress pathway.15 Prolonged treatment with IFNG induces cellular senescence in human vascular endothelial cells via up-regulation of senescence-associated genes.16 Age-dependent increases in IFNG production have been reported in in vitro (17) and in vivo studies, with minor changes in the remaining evaluated cytokines in senescence-accelerated mice.18 IFNG-induced accumulation of neopterin occurs at the expense of formation of tetrahydropteridine (BH4), an essential cofactor of nitric oxide synthase (NOS).6 Deficiency of BH4 results in NOS uncoupling, leading to production of superoxide anions19 and hydrogen peroxide20 rather than NO from arginine. The consequent formation of free radicals triggers arachidonic acid metabolism, with increased production of inflammatory prostaglandins, via activation of cycloxygenase (COX), and leucotrienes, via activation of arachidonate 5-lipoxygenase (5-LO)21 (Fig. 2).
Fig. 2.
Interferon-induced up-regulation of neopterin formation and aging-associated medical and psychiatric disorders.
 up-regulation
up-regulation
 down-regulation of corresponding metabolic pathways (BH4 deficiency leads to increased formation of oxygen radicals).
down-regulation of corresponding metabolic pathways (BH4 deficiency leads to increased formation of oxygen radicals).
Abbreviations: GTP—guanosine triphosphate; GTPCH—GTP cyclohydrolase 1; BH2—7,8-dihydroneopterin; BH4—tetrahydrobiopterine; NOS—NO synthase; PLA—phospholipase 2; AA—arachidonic acid; COX—cyclooxygenase; 5-LO—arachidonate 5-lipoxygenase; PGE—prostaglandins; AAMPD—aging-associated medical and psychiatric disorders.
The other findings of our study are:
Correlation (negative) between elevated neopterin and decreased PLP concentrations. To the best of our knowledge, this is the first report on such a correlation;
Correlation between increased insulin resistance (the precursor of type 2 diabetes) and elevated neopterin. These results are in line with the report of association of elevated glucose levels with higher neopterin concentrations in 1234 otherwise healthy outpatients, who visited the physician’s office for a medical health check-up;23
Correlation between insulin resistance and combined inflammation and PLP deficiency (neopterin/PLP ratio: elevated neopterin and decreased PLP concentrations).
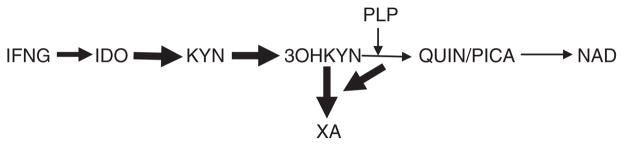
Considering that neopterin is a marker of IFNG-inducible inflammation cascade,2 present results suggest that PLP deficiency might contribute to the development of insulin resistance (and type 2 diabetes) in subjects suffering from conditions associated with chronic inflammation (e.g., depression, aging).3, 14, 24 Such a suggestion is in line with the observations of increased incidence of diabetes among depressed patients25 and association of PLP deficiency with symptoms of depression in elderly.26 Pro-inflammatory cytokine, IFNG, concurrently up-regulates formation of neopterin from guanosine triphosphate and kynurenine from tryptophan, and neopterin levels strongly correlate with kynurenine levels.6 Therefore, elevation of neopterin levels indirectly suggests increased of kynurenine production during inflammation. Kynurenine is further metabolized into its derivatives. Since PLP is a cofactor of the key enzymes of post-kynurenine metabolism, PLP deficiency results in increased formation of xanthurenic acid (XA) at the expense of other kynurenine derivatives27, 28 (Fig. 3). Clinical and experimental data suggest a diabetogenic effect of XA. Urine XA concentrations were higher in type 2 diabetes patients than in healthy subjects.29 XA induced experimental diabetes in rats.30 XA may contribute to the development of insulin resistance by formation of chelate complexes with insulin (XA-In). XA-In complex is antigenetically indistinguishable from insulin but it activity was 49% lower than activity of pure insulin.30 In addition, XA may exert a toxic effect in isolated pancreatic islets because of formation of complexes with Zn++-ions in β-cells.31 A recent metabolomic study found changes in a mouse model of insulin resistance compatible with increased formation of XA.32
Fig. 3.
Shift of post-KYN metabolism towards formation of XA in vitamin B6 deficiency.
Abbreviations. IDO—indoleamine 2,3,-dioxygenase; PLP—pyridoxal-5′-phosphate; KYN—kynurenine; 3OHKYN—3-hydroxyKYN; QUIN—quinolinic acid; PICA—picolinic acid; XA—xanthurenic acid; NAD—nicotinamide adenine dinucleotide.
 up-regulation
up-regulation
 down-regulation of corresponding metabolic pathways (PLP deficiency resulted in increased formation of XA).
down-regulation of corresponding metabolic pathways (PLP deficiency resulted in increased formation of XA).
Present results of correlation between PLP deficiency, insulin resistance and elevated neopterin, indirectly suggest that PLP deficiency might facilitate the development of insulin resistance (and type 2 diabetes) in conditions associated with IFNG-mediated chronic inflammation (e.g., aging, depression). Further studies have to assess the role of increased XA combined with PLP deficiency in the development of insulin resistance.
Acknowledgments
Authors highly appreciate the excellent help with statistical treatment provided by Mr. Ning Qiao.
References and Notes
- 1.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 2.Oxenkrug GF. Genetic and hormonal regulation of the kynurenine pathway of tryptophan metabolism: New target for clinical intervention in vascular dementia, depression and aging. Ann NY Acad Sci. 2007;1122:35. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- 3.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders and dysregulation of tryptophan–kynurenine pathway metabolism. Ann NY Acad Sci. 2010a;1199:1. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- 4.Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: Implications for aging and aging-associated medical and psychiatric disorders. J Neural Transm. 2011;118:75. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoedon G, Troppmair J, Adolf G, Huber C, Niederwieser A. Interferon-gamma enhances biosynthesis of pterins in peripheral blood mononuclear cells by induction of GTP-cyclohydrolase I activity. J Interferon Res. 1986;6:697. doi: 10.1089/jir.1986.6.697. [DOI] [PubMed] [Google Scholar]
- 6.Fuch D, Avanzas P, Arroyo-Espliquero R, Jenny M, Consuegra-Sanchez L, Kaski JC. The role of neopterin in atherosclerosis and cardiovascular risk assesment. Current Medicinal Chemistry. 2009;16:4644. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, Braunwald E. Long-term prognostic value of neopterin: A novel marker of monocyte activation in patients with acute coronary syndrome. Circulation. 2007;115:3071. doi: 10.1161/CIRCULATIONAHA.106.666511. [DOI] [PubMed] [Google Scholar]
- 8.Grammer TB, Fuch D, Boehm BO, Winkelmann BR, Maerz W. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2009;55:1135. doi: 10.1373/clinchem.2008.118844. [DOI] [PubMed] [Google Scholar]
- 9.Solichova D, Melichar B, Blaha V, Kleina M, Vavrona J, Palicha V, Zadak Z. Biochemical profile and survival in nonagenarians. Clin Biochem. 2001;34:563. doi: 10.1016/s0009-9120(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 10.Perianayagam M, Oxenkrug G, Summergrad P. Interferon-gamma (+874) single nucleotide polymorphism and human plasma neopterin levels (submitted) [Google Scholar]
- 11.Mattei J, Parnell LD, Lai CQ, Garcia-Bailo B, Adiconis X, Shen J, Arnett D, Demissie S, Tucker KL, Ordovas JM. Disparities in allele frequencies and population differentiation for 101 disease-associated single nucleotide polymorphisms between Puerto Ricans and non-Hispanic whites. BMC Genet. 2009;10:45. doi: 10.1186/1471-2156-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker KL, Mattei J, Noel SE, Collado BM, Nelson J, Griffith J, Ordovas JM, Falcon LM. The boston puerto rican health study, a longitudinal cohort study on health disparities in Puerto Rican adults: Challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: The Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxenkrug GF. Tryptophan–kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: Serotonin hypothesis revisited 40 years later. Israel J Psychiatry. 2010b;47:56. [PMC free article] [PubMed] [Google Scholar]
- 15.Fridman AL, Tainky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KS, Kang KW, Seu YB, et al. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev. 2009;130:179. doi: 10.1016/j.mad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Poynter ME, Daynes RA. Age-associated alterations in splenic iNOS regulation: Influence of constitutively expressed IFN-gamma and correction following supplementation with PPARalpha activators or vitamin E. Cell Immunol. 1999;195:127. doi: 10.1006/cimm.1999.1525. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez MI, Escames G, López LC, Lopez A, Garcia JA, Ortiz F, Acuna-Castroviejo D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J Pineal Res. 2007;42:272. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 19.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173. [PubMed] [Google Scholar]
- 20.Heinzel B, John M, Klatt P, et al. Ca+2/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart JC, et al. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 22.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledochowski M, Murr C, Widner B, Fuchs D. Association between insulin resistance, body mass and neopterin concentrations. Clin Chim Acta. 1999;282:115. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 24.Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1:32. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- 25.Campayo A, de Jonge P, Roy JF, Saz P, de la Camara C, Quintamilla MA, Marcos G, Santabárbara J. Lobo A: Depressive disorder and incident diabetes mellitus: The effect of characteristics of depression. Am J Psychiatry. 2010;167:580. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]
- 26.Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27:421. doi: 10.1080/07315724.2008.10719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender DA, Njagi EN, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr. 1990;63:27. doi: 10.1079/bjn19900089. [DOI] [PubMed] [Google Scholar]
- 28.Chiang EP, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283. doi: 10.1016/s0002-9343(02)01528-0. [DOI] [PubMed] [Google Scholar]
- 29.Hattori M, Kotake Y, Kotake Y. Studies on the urinary excretion of xanthurenic acid in diabetics. Acta Vitaminol Enzymol. 1984;6:221. [PubMed] [Google Scholar]
- 30.Kotaki Y, Ueda T, Mori T, Igaki S, Hattori M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA) Acta Vitaminol Enzymol. 1075;29:236. [PubMed] [Google Scholar]
- 31.Meyramov G, Korchin V, Kocheryzkina N. Diabetogenic activity of xanturenic acid determined by its chelating properties? Acta Vitaminol Enzymol. 1984;6:221. doi: 10.1016/s0041-1345(98)00788-x. [DOI] [PubMed] [Google Scholar]
- 32.Li LO, Hu YF, Wang L, Mitchell M, Berger A, Coleman RA. Early hepatic insulin resistance in mice: A metabolomics analysis. Mol Endocrinol. 2010;24:657. doi: 10.1210/me.2009-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]