Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disease thought to be mediated by dysfunctional innate and/or adaptive immunity. This aberrant immune response leads to the secretion of harmful cytokines that destroy the epithelium of the gastrointestinal tract leading to further inflammation. IL-22 is a Th17 T cell associated cytokine that is bi-functional with both pro-inflammatory and protective effects on tissues depending on the inflammatory context. We show herein that IL-22 protects mice from IBD. Interestingly, this protection is not only mediated by CD4 T cells, but IL-22 expressing NK cells also confer protection. In addition, IL-22 expression is differentially regulated between NK cell subsets. Thus, both the innate and adaptive immune responses have developed protective mechanisms to counteract the damaging effects of inflammation on tissues.
Introduction
Interleukin-22 (IL-22) is a member of the IL-10 related family of cytokines, which also includes IL-19, IL-20, IL-24 and in humans, IL-26, which are expressed during chronic inflammation. These cytokines share 20–30% amino acid identity and also have homologous secondary structures (Kotenko, 2002). IL-22 signals through a heterodimeric receptor that consists of IL-22R and IL-10Rβ, whereas IL-10 signals through IL-10Rα and IL-10Rβ (Kotenko et al., 2001). Since IL-10Rβ is ubiquitously expressed, signaling specificity is conferred by IL-10Rα and IL-22R expression; IL-10Rα is limited to cells of the immune system, whereas IL-22R expression is limited to tissue cells, such as epithelial cells (Wolk et al., 2004). Just as IL-10 protects the immune system from itself, IL-22 is proposed to protect the tissues during inflammation via a Stat3-mediated mechanism. IL-22 has recently been shown to be protective during acute inflammation using a hepatitis model (Radaeva et al., 2004; Zenewicz et al., 2007). On the other hand, IL-22 has been shown to mediate dermal inflammation (Ma et al., 2008; Zheng et al., 2007). The dual nature of this cytokine, protective versus inflammatory, likely depends on the inflammatory context, which includes, but is not limited to, the duration and amount of IL-22 present, the overall cytokine milieu and the involved tissues.
IL-22 is highly expressed by Th17 cells and is strongly linked to chronic inflammation (Chung et al., 2006; Liang et al., 2006; Zheng et al., 2007). Th17 cells were first defined by their expression of IL-17A, but have since been shown to also preferentially express IL-22, as well as IL-17F and IL-21 (Korn et al., 2007; Liang et al., 2006; Nurieva et al., 2007; Weaver et al., 2006). In mice, IL-23 was once thought to control Th17 differentiation but it now appears that its role is in survival and expansion of Th17 cells (Aggarwal et al., 2003) (Cua et al., 2003; Veldhoen et al., 2006). Differentiation in mice appears to be directed by the presence of both TGF-β and inflammatory cytokines, such as IL-6 or IL-21 that activate Stat3 signaling pathways in the T cells (Korn et al., 2007; Nurieva et al., 2007; Veldhoen et al., 2006). However, it is becoming increasingly apparent that the IL-22 expression profile differs from that of IL-17A. Whereas TGF-β and IL-6 are both necessary for induction of IL-17A, IL-22 can be induced via IL-6 alone and increasing levels of TGF-β are actually inhibitory to its expression ((Zheng et al., 2007) and our unpublished observations).
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract due to aberrant innate and/or adaptive immune responses (Podolsky, 2002). IBD has long been described as a Th1-mediated disease since IFNγ is essential for disease progression (Powrie et al., 1994b). However, the recent discovery of Th17 cells has led to a reevaluation of the role of T cells in disease. IL-23, important for the maintenance of Th17 cells, is essential for development of IBD in mouse models (Kullberg et al., 2006) and protective IL-23R polymorphisms in the human population have been identified through a genome association study (Duerr et al., 2006). However, the role of individual Th17 cytokines in IBD, such as IL-22, remains elusive. IL-22 has been shown to be highly upregulated in the sera and lesions of patients with either Crohn’s disease or ulcerative colitis (Andoh et al., 2005). IL-22 can have pro-inflammatory effects on colon epithelial cells and induce secretion of IL-6 and IL-8, as well as activate NF-κB and AP-1 (Andoh et al., 2005). On the other hand, ectopic expression of IL-22 in the gastrointestinal tract by targeted micro-injection is protective to colitis (Sugimoto et al., 2008). However, it remains to be determined if the immune response itself has this protection mechanism during IBD.
In this study, we have investigated the role of IL-22 during IBD. Using both innate and T cell-driven colitis animal models we have found a protective role for IL-22 during IBD. Our data suggest that IL-22 secretion by not only CD4 T cells, but surprisingly also NK cells in the colon, mediates this protection.
Results
Colon epithelial cells are responsive to IL-22
The IL-10 related cytokines (IL-10, IL-19, IL-20, IL-22, IL-24) share use of a family of heterodimeric cytokine receptors (Kotenko, 2002; Moore et al., 2001). To begin to understand which of these cytokines play a role in IBD, we compared expression of the different receptor subunits in the gastrointestinal tract to other tissues, such as the liver, spleen and skin (Supplemental Figure 1A). IL-10Rα, which is only used by IL-10 and whose expression is primarily limited to immune cells and not tissues, was highly expressed in the spleen compared to other tissues. IL-10Rβ, the β chain for both the IL-10 and IL-22 receptor, was ubiquitously expressed in all tissues, as previously reported. IL-22R, the α chain for the IL-22 receptor, was highly expressed in the colon and small intestine and to a lesser extent in the other tissues we examined. We additionally examined expression of IL-20Rα and IL-20Rβ, which recognize IL-19, IL-20 and IL-24, and found that both of these chains were most highly expressed in the skin, which agrees with published data that these cytokines primary target is keratinocytes (Blumberg et al., 2001; Boniface et al., 2005).
The expression of the IL-22 receptor in the gut suggested that the gastrointestinal system should be highly responsive to IL-22. To further examine to which IL-10 family cytokine members colon cells are responsive, and confirm expression of functional receptor, we stimulated the human colon epithelial cell line, Caco-2, with recombinant human IL-10, IL-19, IL-20 or IL-22. Upon recognition of the appropriate receptor, each of these cytokines activates Stat3 in responsive cell types (Blumberg et al., 2001; Dumoutier et al., 2001; Dumoutier et al., 2000). IL-22, but not IL-10, IL-19 nor IL-20, induced detectable phosphorylation of Stat3 (Supplemental Figure 1B). Thus, colon epithelial cells are a target for IL-22 activity.
IL-22 is induced in the colon during IBD
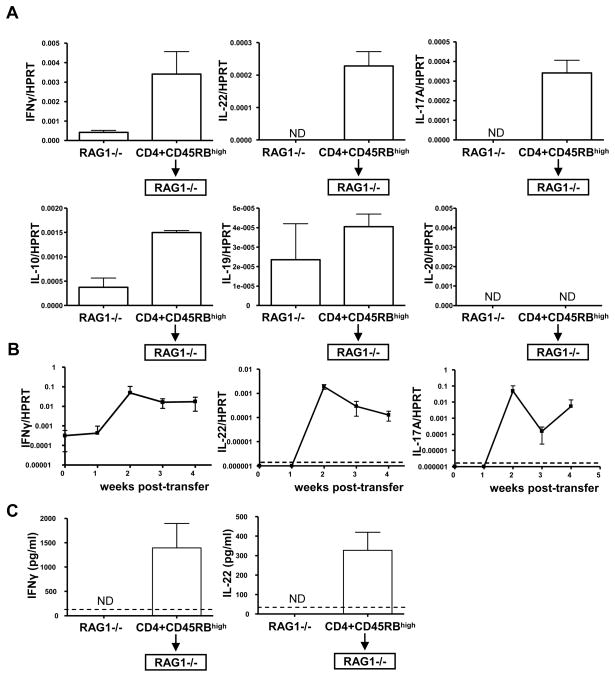
To examine which cytokines are expressed during IBD in mice, we used the CD45RBhigh transfer model of colitis. This T cell-dependent model involves the transfer of purified naïve CD4 T cells (CD45RBhigh) into RAG−/− mice, which lack T cells (Morrissey et al., 1993; Powrie et al., 1994a). In the absence of both host and regulatory T cells, these cells rapidly expand and gain effector functions, such as IFNγ secretion. In the absence of regulatory T cells, these functions are unchecked and result in massive inflammation of primarily the gut, but eventually other organs such as the liver and skin. Five weeks post-transfer, when mice had lost considerable body mass, we examined cytokine expression in their inflamed colons. IFNγ has long been associated with IBD and accordingly we found that IFNγ mRNA was highly induced in the inflamed colons compared to colons from mice that did not receive T cells (Figure 1A). On the other hand, expression of Th17 associated cytokines is less well characterized; however, elevated IL-17A levels have been observed in several other mouse models of IBD (Hue et al., 2006; Kullberg et al., 2006). We found that both IL-22 and IL-17A mRNA were highly expressed in the colons of RAG1−/− mice that received naïve T cells (Figure 1A). A closer examination of the kinetics of the induction of the mRNA of these cytokines revealed a 10–1000 fold increase by two weeks post-transfer which remained elevated throughout the examined disease course (Figure 1B). In addition, when purified via FACS sorting, the population of transferred CD4+ T cells recovered from the inflamed colons expressed mRNA for these cytokines (data not shown). At the protein level, secreted IL-22 was detected in the supernatant of excised colons cultured in vitro for three days, as well as IFNγ (Figure 1C). Thus, IL-22 is expressed in the colon by cells including at least CD4+ T cells in the colon, during IBD.
Figure 1. IL-22 is expressed in the inflamed colon.
(A) C57BL/6 CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. to RAG1−/− mice and five weeks post-transfer, when clinical signs of IBD were evident, cytokine mRNA in the colon was assessed by real-time RT-PCR. As a control, cytokine in the colons of RAG1−/− that did not receive T cells was also assessed. Bars represent the mean±SD expression of the cytokine gene to HPRT using the ΔΔCT method. Experiment was performed two times with similar results. 5–7 mice/group. ND=not detected.
(B) C57BL/6 CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. to RAG1−/− mice and at different weeks post-transfer (0, 1, 2, 3 and 4 weeks) cytokine mRNA expressed in the colon was semi-quantitated by real-time RT-PCR. Mean±SD; dashed line indicates limit of detection. 4 mice/group.
(C) From the colon samples in (A), a 1 cm section of the ascending colons of the above mice were cultured ex vivo for three days. Secreted IFNγ (left) or IL-22 (right) from the supernatants were quantiated by ELISA, mean±SD of 5–7 mice/group. ND=not detected, dashed line indicates limit of detection.
IL-22 is protective during IBD
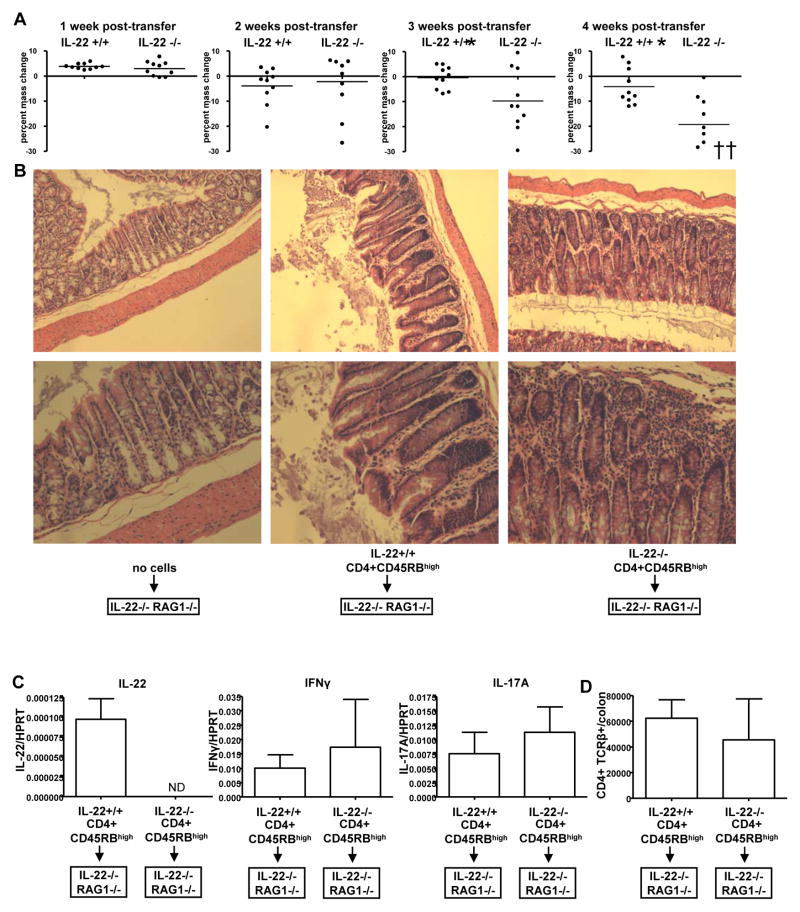
Since IL-22R is highly expressed in the gastrointestinal tract and IL-22 expression is highly induced during inflammation of these tissues, we sought to examine the role of IL-22 in IBD. IL-22 deficient mice housed under specific-pathogen free conditions for up to one year did not develop spontaneous colitis indicating that IL-22 is not necessary for maintenance of normal intestinal homeostasis. We transferred IL-22 deficient or control wild-type CD45RBhigh CD4 T cells into IL-22−/− RAG1−/− double deficient mice. Over the course of several weeks, all mice that received T cells developed colitis. However, mice that received IL-22 deficient T cells lost significantly more mass than mice that received wild-type cells by three weeks post-transfer, and a greater difference was observed at 4 weeks (Figure 2A) (p value week 3 = 0.0367, week 4 = 0.0013). A significant difference was also observed at 5 and 6 weeks post-transfer, as well as greater mortality of mice that received IL-22 deficient cells (Supplemental Figure 2). At 3 weeks post-transfer these mice had greater morphological changes in their ascending colons, the main site of disease, as assessed by histology (Figure 2B). As a control, no histopathology was noted in IL-22−/− RAG1−/− double deficient mice that did not receive transferred cells (Figure 2B). Although in the absence of IL-22, there was greater loss of body mass and destruction of the colonic tissue, we observed little difference in the total number of infiltrated CD4 T cells in the inflamed colon (Figure 2D) or cytokine expression (Figure 2C); with the exception of IL-22, agreeing with our previous data that IL-22 deficient T cells do not have a defect in migration or cytokine expression (Zenewicz et al., 2007). Thus, IL-22 is protective to the colonic tissues during IBD.
Figure 2. IL-22 protects the colon during CD4 T cell-mediated colitis.
IL-22 +/+ or IL-22 −/− CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. into IL-22−/− RAG1−/− double knockout mice.
(A) Mice were massed weekly and percent change from week 0 was calculated. Each dot represents one mouse, bar indicates mean, crosses represent dead mice or mice that reached 30% mass loss and were euthanized according to protocol. For statistics, dead mice were assigned mass loss of −30%. * = p value of <0.05 by an unpaired two-tailed Student’s t test.
(B) Histology of H&E stained sections from the ascending colons of the indicated mice, 3 weeks post-transfer. Shown are representative sections from one mouse of 3–4 mice/group. Bottom image is a higher magnification of the top row image.
(C) At four weeks post-transfer, cytokine mRNA expression was assessed in the colons of the mice by real-time RT-PCR. Bars represent the mean±SD expression of the cytokine gene to HPRT using the ΔΔCT method. ND=not detected. Experiment was performed two times with similar results.
(D) Total numbers of CD4+ TCRβ+ cells in the inflamed colons as determined by FACS. Mean±SD.
RAG1−/− mice express protective IL-22 in the colon during IBD
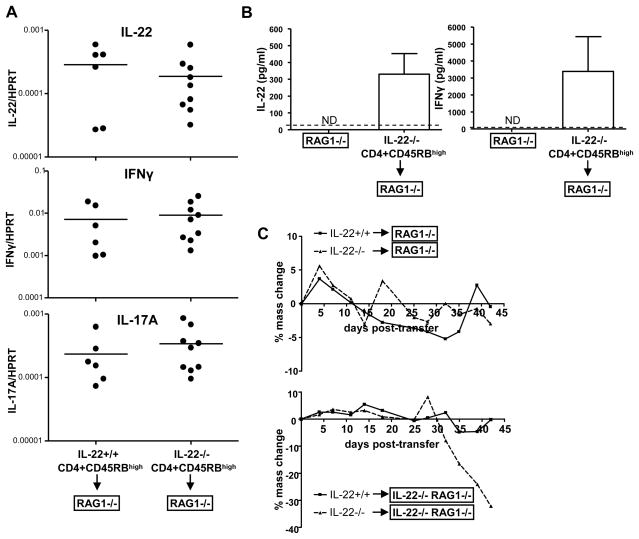
During the course of our studies, in addition to CD45RBhigh CD4 T cell transfers into IL-22−/− RAG1−/− mice, we also performed experiments using RAG1−/− mice as recipients. Upon transfer of CD45RBhigh IL-22+/+ or IL-22−/− CD4 T cells, these mice also developed colitis, as indicated by their elevated levels of both IFNγ and IL-17A mRNA in the colon (Figure 3A). Surprisingly, RAG1−/− mice that received IL-22−/− CD4 T cells also had detectable levels of IL-22 mRNA and protein in their colons (Figure 3A+B). The amount of IL-22 expressed in the colon was similar to that expressed by IL-22+/+ CD4 T cells when transferred into IL-22−/− RAG1−/− mice, where the only source of IL-22 is the transferred T cells. This suggests that there is a host-derived source of IL-22 during IBD in RAG1−/− mice.
Figure 3. RAG1−/− mice express protective IL-22 during IBD.
IL-22 +/+ or IL-22 −/−CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. into RAG1−/− mice.
(A) 6 weeks post-transfer expression of cytokines mRNA in the ascending colons of the mice was assessed by real-time RT-PCR. The cytokine gene message was compared to HPRT using the ΔΔCT method. Each dot represents one mouse, bar indicates the mean. Experiment was performed three times with similar results.
(B) Excised colon sections from either RAG−/− mice that did not receive transferred cells or RAG1−/− mice that received IL-22−/− T cells 6 weeks prior, were cultured for three days ex vivo and then cytokine levels in the supernatant were quantitated by ELISA. Bar indicates mean±SD; dashed line indicates limit of detection.
(C) Mice from (A) were massed bi-weekly and percent mass change from day 0 was calculated (top graph). At the same time, IL-22 +/+ or IL-22 −/−CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. into IL-22−/− RAG1−/− mice and their body mass was also monitored (bottom graph). Points represent the mean percent change in body mass.
IL-22 expression, whether from transferred T cells or the host, correlated with protection during IBD. Mice that completely lacked IL-22 expression (IL-22−/− CD4 T cells transferred into IL-22−/− RAG−/− mice) had the most severe colitis. There was no difference in disease, inferred from body mass reduction, of RAG1−/− mice that received CD45RBhigh IL-22+/+ or IL-22−/− CD4 T cells, in stark contrast to the difference observed when IL-22−/− RAG1−/− mice were used as the hosts (Figure 3C). Thus, in RAG1−/− mice there is a cell subset(s) that expresses IL-22 in the inflamed colon that is able to provide protection during IBD.
Differential expression of IL-22 by NK subsets
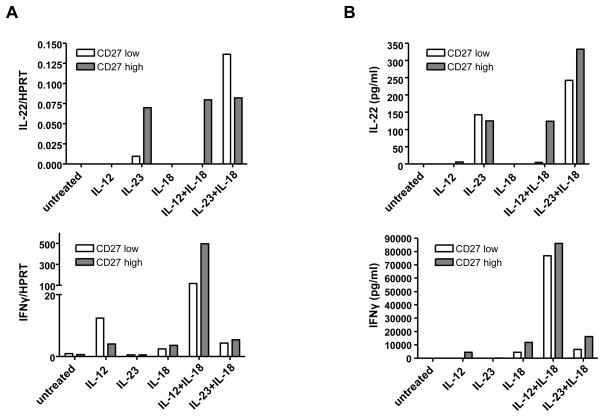
IL-22 expression is not limited to Th17 CD4 T cells. Activated NK cells, NK T cells, CD8 T cells, and γδ T cells can also express IL-22 (Wolk et al., 2002; Zheng et al., 2007). Since RAG1−/− mice lack CD4 and CD8 T cells, as well as NK T cells and γδ T cells, and since IL-22 is expressed in RAG1−/− mice, we hypothesized that NK cells are responsible for the IL-22 expression in the colons of the mice. We first wanted to better characterize IL-22 expression by NK cells. There are different subsets of NK cells, which in mice can be defined by CD27 expression (Hayakawa et al., 2006). Upon activation, CD27high NK cells secrete IFNγ and granzymes, and owing to high expression of chemokine receptors, they quickly traffic to sites of inflammation. On the other hand, CD27low NK cells express high levels of inhibitory receptors and upon stimulation express low levels of IFNγ and granzymes and therefore are less cytotoxic than CD27high NK cells. To investigate which subset, or both, expresses IL-22, we purified NK cells based on their level of CD27 expression and then activated the cells with different stimuli. Previously, activation by IL-12 and IL-18 has been shown to induce IL-22 in a heterogenous NK cell population (Wolk et al., 2002). However, since IL-23 is a potent stimulator of Th17 cells, has been previously shown to play a role in IL-22 expression of T cells, and is important in IBD pathogenesis, we also examined whether this cytokine is able to induce IL-22.
IL-12 and IL-18 induced IL-22 mRNA and protein in NK cells, but only in the CD27high population (Figure 4). On the other hand, IL-23 was able to induce IL-22 in both subsets, as well as in the absence of IL-18. Unlike IL-12 and IL-18, IL-23, with or without IL-18, was not able to induce IFNγ from either subset. Thus IL-22 can be differentially expressed; IL-22 can be expressed by highly activated NK cells or from what are termed more inhibitory NK cells, under the proper stimulation conditions.
Figure 4. Differential expression of IL-22 in activated NK cells by IL-23 or IL-12.
NK1.1+ TCRβ− CD27low (CD27low) or NK1.1+ TCRβ− CD27high (CD27high) NK cells were sorted from the spleen and lymph nodes of C57BL/6 mice. Cells were stimulated in vitro with IL-15 and the indicated cytokines. 18 hrs post-stimulation, IL-22 and IFNγ mRNA and protein expression was analyzed by (A) real-time RT-PCR or (B) ELISA of the supernatants, respectively. Experiment was performed three times with similar results.
NK cells can provide protection during IBD
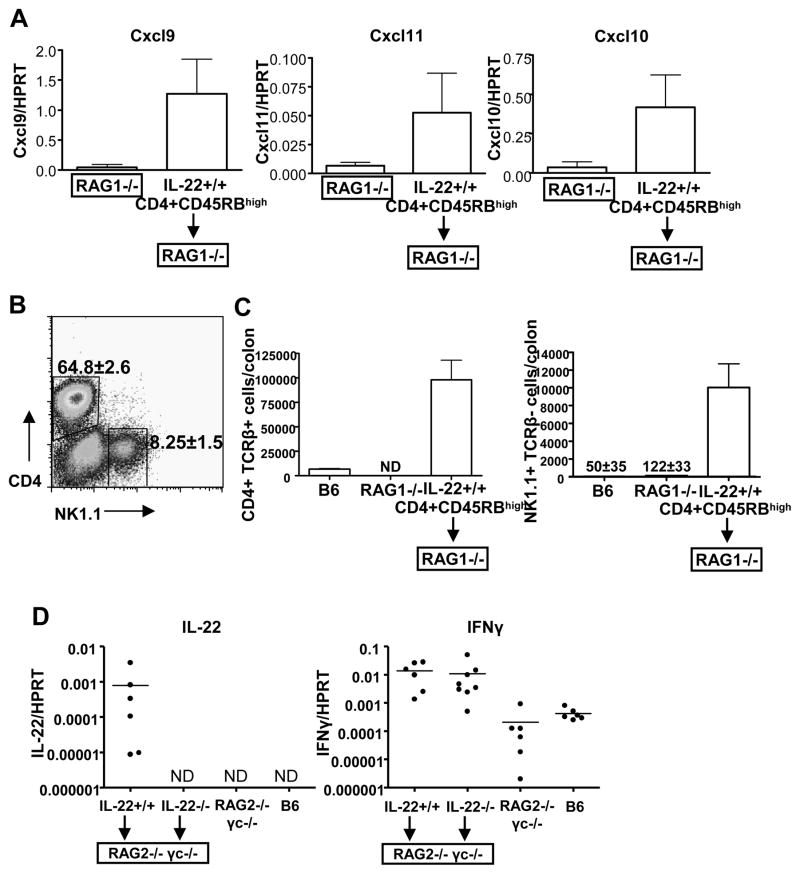
Unlike T cells, the role of NK cells during IBD has not been extensively studied. Therefore, we examined the colons of mice with CD45RBhigh-mediated colitis to see if NK cell attracting chemokines were expressed, and if so, were NK cells infiltrating the colon. By real-time RT-PCR we found in RAG1−/− mice which two weeks prior received either IL-22 wild-type CD45RBhigh CD4 T cells, that the chemokines CXCL9, CXCL10 and CXCL11 were induced approximately 5–10 fold (Figure 5A). By FACS analysis, we found a population of NK1.1+ CD4− cells in the inflamed colons of these mice (Figure 5B). On the other hand, the populations of colonic NK cells in non-diseased C57BL/6 mice or RAG1−/− mice were substantially smaller (Figure 5C). Thus, NK cells appear to infiltrate the inflamed colon and in the diseased state constitute approximately 8% of the lymphocyte population.
Figure 5. NK cells play a role in IL-22-mediated protection during IBD.
(A) NK-attracting chemokines are upregulated in the colon during IBD. Two weeks post-transfer of IL-22 +/+ CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) into RAG1−/− mice, or control RAG1−/− mice that did not receive cells, mRNA levels of the chemokines Cxcl9, Cxcl10, and Cxcl11, were examined in the ascending colon by real-time RT-PCR. mean±SD.
(B) IL-22−/− CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) cells were transferred into RAG1−/− mice and 45 days post-transfer, when mice exhibited clinical disease, spleens and colons were harvested and FACS stained for CD4 and NK1.1. Numbers indicate the mean±SD percentage of CD4+ NK1.1− (top gate) or CD4− NK1.1+ (right gate) cells out of total lymphocytes isolated from the colon. 5 mice/group.
(C) Total numbers of CD4 (CD4+ TCRβ+) and NK (NK1.1+ TCRβ−) cells in the colons of mice presented in B as determined by FACS. mean±SD. B6=C57BL/6 mouse, ND=not detected
(D) IL-22+/+ or IL-22−/− CD4+ CD45RBhigh CD25− NK1.1− T cells were transferred into RAG2−/−γc−/− mice (5×105 cells; i.p.). 42 days post-transfer cytokine induction in the ascending colon was semi-quantitated by real-time RT-PCR, as well as in untransferred RAG2−/−γc−/− mice or C57BL/6 (B6) mice. Each dot represents one mouse; bar indicates mean; ND = not detected.
To examine the relationship between NK cells and IL-22-mediated protection during IBD, we performed CD45RBhigh CD4 T cell transfers of IL-22 wild-type or IL-22 deficient cells into RAG2−/− γc−/− mice. In addition to lacking all T cell subsets, due to the lack of RAG and signaling of several cytokines (IL-2, IL-4, IL-7, IL-9, and IL-15) through the common gamma chain (γc) receptor, importantly, these mice also lack NK cells (Cao et al., 1995; DiSanto et al., 1995). As expected, transfer of IL-22 +/+ T cells into RAG2−/− γc−/− mice over the course of several weeks led to IBD with increased levels of both IFNγ and IL-22 in the colons compared to mice that did not receive transferred cells (Figure 5C). Disease course and its severity in the host RAG2−/− γc−/− mice was similar to that observed in RAG1−/− mice. When IL-22−/− T cells were transferred into RAG2−/− γc−/− mice, no IL-22 mRNA was detected in their inflamed colons, although there was an approximate 100 fold-increase in IFNγ levels compared to RAG2−/− γc−/− colons from mice that did not receive cells (Figure 5C). IFNγ levels were similar to that observed to transfers into RAG1−/− mice (Figure 2C). In addition, IL-22 secreted protein from ex vivo cultured colon tissue sections from these mice was not detected by ELISA. Similar results were obtained by performing a similar experiment using the BALB/c strain of mice (data not shown). These data strongly suggested that NK cells are a source of IL-22 in the inflamed colon.
IL-22 protects during an innate immune system mediated colitis model
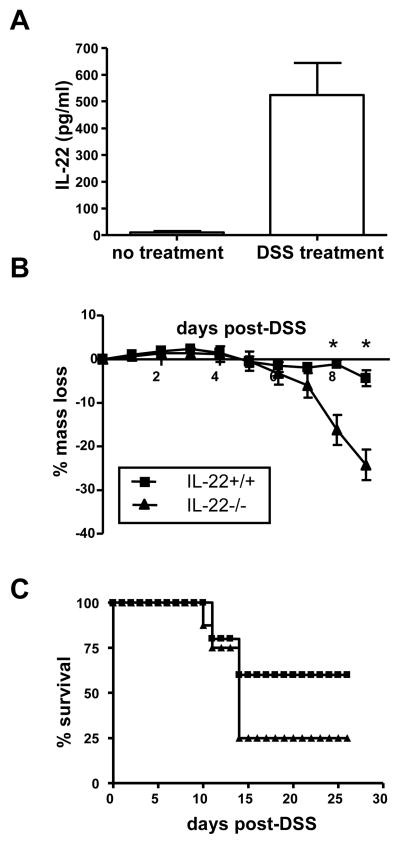
Colitis in humans is a complex interplay involving both the innate and adaptive immune systems. Since IL-22 expression is not limited to differentiated effector CD4 T cells and can also be expressed by NK cells, we wished to determine if IL-22 could also provide protection during colitis driven by the innate immune system, as well as the adaptive T cell model we presented above. One commonly employed model of innate colitis is dextran sodium sulfate (DSS)-induced colitis (Cooper et al., 1993; Mahler et al., 1998). In this model, DSS is given in the drinking water and is thought to cause disruption of the epithelial integrity of the colon, leading to inflammation and colitis within one week. Like CD45RBhigh-mediated transfer IBD, this is primarily a disease of the colon. IL-22 mRNA is upregulated in the colons of mice after DSS treatment (Brand et al., 2006; te Velde et al., 2007). We confirmed that IL-22 protein secretion is induced by DSS treatment since we detected IL-22 in excised colon cultures of mice that received 3% DSS in their drinking water for three days (Figure 6A). Since IL-22 is expressed in the colon during DSS-induced colitis, we compared the disease course between IL-22 deficient mice and wild-type control mice. IL-22 deficient mice lost significantly more mass by day 8 post-treatment than IL-22 wild-type mice (Figure 6B). In addition, IL-22 deficient mice had a higher rate of mortality compared to the wild-type mice (Figure 6C). Although T cells have previously been shown have little role in DSS-mediated colitis (Axelsson et al., 1996; Dieleman et al., 1994), we nevertheless also compared colitis between RAG1 deficient mice and IL-22/RAG1 double deficient mice to rule out IL-22 expressed by T cells. As observed for mice with T cells, RAG1 deficient mice also deficient in IL-22 had more severe disease than RAG1 deficient mice (Supplemental Figure 2). Thus, in addition to being protective during adaptive immune-mediated colitis, innate immune cell-driven IL-22 is also protective during innate immunity-mediated IBD.
Figure 6. IL-22 also provides protection during innate immune system-mediated colitis.
(A) IL-22 is secreted from the colon during DSS-mediated colitis. C57BL/6 mice were given 3% DSS ad libitum in their drinking water, or remained untreated, and three days later the mice were euthanized and their colons were excised. Colons were cultured for three days as described in the Experimental Procedures and IL-22 was detected in the supernatant by ELISA. Bars represent mean±SD of 7 mice/group.
(B) IL-22 +/+ or IL-22−/− mice were given 3% DSS ad libitum in their drinking water for seven days. Mice were massed daily and percent mass change from day 0 was calculated. Mean±SD. * indicates p value <0.05. 8–10 mice/group.
(C) Survival of IL-22+/+ or IL-22−/− mice in panel B after receiving 3% DSS in their drinking water.
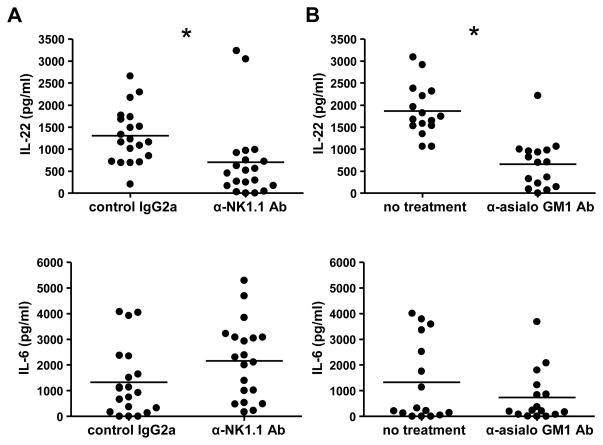
To further provide evidence that NK cells are the primary innate source of IL-22 in the colon during colitis, we used our innate model of DSS-mediated colitis in the absence of T cells. Three days after the initiation of DSS treatment, RAG1−/− mice were either given a depleting NK1.1 Ab or a control Ab. Twenty-four hours later, mice were euthanized, and IL-6 and IL-22 secretion from the colon was examined by ex vivo colon culture. Colons from both sets of mice expressed comparable levels of IL-6, indicating similar levels of inflammation (Figure 7A). However, the colons from the control Ab group secreted significantly more IL-22 than those from mice that received the NK1.1 depleting Ab (Figure 7A). Similar results were observed by depleting NK cells in RAG1−/− mice using a different depleting reagent, anti-asialo GM1 Ab (Figure 7B). These data implicate NK cells as an innate source of protective IL-22 in the inflamed colon.
Figure 7. NK cell depletion decreases IL-22 expression in the inflamed colon.
RAG1−/− mice were given 3% DSS ad libitum in their drinking water for four days. On the third day, mice were either i.p. injected with (A) 250 μg of anti-NK1.1 depleting Ab or control IgG2a or (B) 500 μg anti-asialo GM1 Ab or untreated. Twenty-four hours later, mice were euthanized, and colons were excised. Colons were cultured for three days as described in the Experimental Procedures and IL-22 or IL-6 were detected in the supernatant by ELISA. Each dot represents one colon segment, bars represent mean of 4–5 mice/group; 4 segments per mouse. * indicates p<0.05. Experiment was performed twice with similar results.
Discussion
IL-22 is a dual-natured cytokine; depending on the context of inflammation, it can have either inflammatory or protective properties. IL-22 has been shown to be an important mediator in dermal inflammation (Ma et al., 2008; Zheng et al., 2007) and, on the other hand, it provides protection to hepatocytes during liver inflammation (Radaeva et al., 2004; Zenewicz et al., 2007) and as we now show here, IBD. Th17 cells have also been termed inflammatory T cells since these cells are hypothesized to participate in, if not instigate, inflammatory responses. IL-17A, the original defining cytokine of this cell subset, is important for neutrophil recruitment and induction of antimicrobial peptides and like IL-22 is upregulated during chronic inflammatory diseases such as IBD, rheumatoid arthritis and psoriasis (Laan et al., 1999; Liang et al., 2006) (Fujino et al., 2003; Kotake et al., 1999). However, it is increasingly apparent that these cells do not solely promote inflammation, but also have protective effects. A recent study has put forward the idea based on data from many groups that the Th17 subset is not a homogenous population (McGeachy et al., 2007). In that study, based on the cytokine milieu during differentiation, IL-17 expressing cells can be inflammatory or protective depending of the co-expression of IL-10. Additionally, multiple subpopulations defined by the expression of many of the Th17 related cytokines (IL-22, IL-21, IL-17F, IL-6, TNFα) have been observed and these distinct expression patterns may represent functionally different cell subsets.
CD4 T cells, as well as NK T cells and γδ T cells, all contribute to IBD pathogenesis (Saubermann et al., 2000; Simpson et al., 1997). On the other hand, the role of another lymphocyte subset, NK cells, has not been as extensively studied in this context. NK cells provide an innate immune defense mechanism that can be rapidly activated to secrete IFNγ and granzymes to quickly kill pathogen-infected cells or cancerous cells. Since IFNγ is essential for IBD development, NK cells could be hypothesized to contribute to this pathogenesis. However, although we have not excluded the possibility that they contribute to disease under other circumstances, in the present study we have shown that NK cells serve a protective role during IBD. We observed no difference in colitis in RAG1−/− mice that received either IL-22 wild-type or IL-22 deficient T cells. Moreover, RAG1−/− mice that received IL-22 deficient T cells surprisingly had high levels of IL-22 in their inflamed colons, indicating an innate immune source of IL-22. Importantly, this innate IL-22 was protective since when IL-22−/− RAG1−/− double deficient mice were used as hosts for IBD experiments, mice that received IL-22 deficient T cells had significantly greater disease than mice that received IL-22 wild-type T cells. Thus, the innate IL-22 in RAG1−/− mice is able to confer protection. As NK cell expression of IL-22 had been previously reported, we examined IBD using RAG2−/− γc−/− mice as hosts, which differ from RAG1−/− only in their absence of NK cells. Upon transfer of IL-22 deficient T cells, these mice did not express IL-22 in the inflamed colon unlike RAG1−/− mice, which did; this provided compelling evidence that NK cells are a source of IL-22. Further experiments depleting NK cells during innate-immune mediated colitis, in the complete absence of T cells, showed that this depletion significantly reduced levels of IL-22 in the colon. Thus, NK cells are an important source of IL-22 which protects the colon during IBD.
A previous report showed that NK cells protect during a T cell-mediated mouse model of IBD by performing NK-depletion experiments during colitis; however their data suggested this was due to direct effects of the NK cells on perforin production by the activated T cells that mediated disease (Fort et al., 1998). We now provide data that NK cells are an innate source of IL-22 in the inflamed colon and that these cells contribute to the IL-22-mediated protection of the host tissues during IBD. This effect is independent of effects on T cells as shown in the innate DSS colitis model. Further, our study is the first report of a functional role attributed to IL-22 expression by NK cells, instead of Th17 cells.
NK cell expression of IL-22 is differentially regulated between subsets. Mature NK cells can be defined by surface expression of CD27, a member of the TNFR super family. CD27high cells have a lower threshold for activation and express high levels of cytokines and granzymes making them highly cytotoxic (Hayakawa et al., 2006). On the other hand, CD27low cells have higher expression of inhibitory receptors and therefore their activation is more strongly regulated. We show that IL-12 and IL-18 stimulation can only induce IL-22 expression in CD27high NK cells, but not CD27low cells. On the other hand, IL-23 is able to induce IL-22 expression in both subsets, independently of IL-18, and is unable to induce IFNγ. These distinct cytokine expression patterns may allow for optimal immune responses while limiting tissue damage during inflammation.
The cytokines of the IL-10-related cytokine family, with the exception of IL-10 itself, affect tissue responses and not the immune system. IL-22 appears to be the most relevant cytokine for the tissues comprising the gastrointestinal tract. IL-22R expression is greatest in the colon and small intestine when compared to other tissues, such as the skin, where the predominant IL-10 family member receptors are the IL-20Rα and IL-20Rβ chains. We also found that during IBD, IL-19 mRNA was not significantly induced in the colon and we were unable to detect IL-20 transcripts. In addition, we only observed Stat3 activation in colon epithelial cells upon IL-22 stimulation, but not with IL-10, IL-19 nor IL-20. This is in agreement with previous studies showing that IL-10 effects are limited to immune cells and IL-19 and IL-20 primarily function in the skin (Blumberg et al., 2001; Wolk et al., 2004; Wolk et al., 2005).
Stat3 is the main signaling pathway activated in responsive cells upon stimulation with IL-10 related cytokines. IL-22 activates Stat3 in a wide variety of tissues, including keratinocytes, hepatocytes, and as shown by us and other laboratories, colon epithelial cells (Dumoutier et al., 2000; Wolk et al., 2004; Zenewicz et al., 2007). IL-22 has several different effects on the gastrointestinal epithelium. IL-22 can induce IL-6 and IL-8 secretion from human colonocytes, as well as activate NF-κB and inducible nitric oxide synthase (Andoh et al., 2005; Ziesche et al., 2007). However, these pro-inflammatory effects on the gastrointestinal tract appear to be of limited effect in vivo. Overexpression of IL-22 in the colon leads to induction of mucus-associated molecules, such as MUC1, MUC3, and MUC13, leading to enhanced mucus production due to the restitution of goblet cells (Sugimoto et al., 2008). This may provide protection to the colonic epithelium during inflammation by reducing the translocation of commensal bacteria across the barrier. In addition, IL-22 induces defensin expression in colonocytes and these antimicrobial peptides may also provide protection (Brand et al., 2006).
One of the pitfalls with current therapies for chronic inflammation, such as TNFα inhibitors, is that they disrupt and weaken the normal immune response, leading to increased disease susceptibility. The benefit of an IL-22 mediated therapy may be that the cytokine only signals to tissues and has no direct effects on the immune response. This specific targeting should allow modulation of tissue responses to alleviate tissue destruction during inflammation while having limited effects on the immune response itself. IL-22 colon-targeted gene expression performed by Sugimoto et al. has already shown that IL-22 holds promise for IBD therapy (Sugimoto et al., 2008). Since IL-22 appears to be both pro-inflammatory and protective, caution must be used in developing treatments based on this cytokine or blocking its function with antibodies. In addition, IL-22 has proliferative effects on cells (Radaeva et al., 2004), warranting further studies to see if continuous IL-22 stimulation allows for tumor progression. Gaining a better understanding of both the short-term and long-term effects of IL-22 on different tissues is needed to enable the development of IL-22-related therapeutics for chronic inflammatory diseases such as IBD.
Experimental Procedures
Mice
IL-22 deficient mice were as previously described (Zenewicz et al., 2007). Mice used were at generation 10 of backcross to the C57BL/6 strain. Mice within experiments were sex- and age- matched. RAG1−/− mice (The Jackson Laboratory; Bar Harbor, ME) and RAG2−/− γc−/ mice (Taconic Farms; Hudson, NY) were bred in house. The progeny of a cross of IL-22−/− mice to RAG1−/− mice were intercrossed to generate IL-22−/− RAG1−/− double knockout mice that upon genetic screening, were then breed as IL-22−/− RAG1−/− × IL-22−/− RAG1−/−. All mice were cared for in accordance with institutional animal care and use committee-approved protocols at the Yale University animal facility.
CD45RBhigh transfers
Splenocytes and inguinal and axillary lymph nodes from IL-22 +/+ or IL-22 −/− mice were CD4 MACS (Miltenyi Biotech; Auburn, CA) purified and then naïve CD4 T cells were further purified by FACS-sorting to collect a population of cells that were CD4+ CD45RBhigh CD25− NK1.1−. 5×105 cells were transferred i.p. into the indicated recipient mice (RAG1−/−, IL-22−/−RAG1−/− or RAG2−/−γc−/−). Mice were massed twice a week and euthanized when they reached −30% of their initial mass.
DSS-induced colitis
Mice were given 3% dextran sodium sulfate (DSS) (molecular weight: 36,000–50,000) (MP Biomedicals, Inc.; Solon, OH) ad libitum in their drinking water for seven days. Body mass was measured every 24 hrs and mice were euthanized when they reached −30% of their initial mass. Where indicated, mice were given i.p. 250 μg of anti-NK1.1 (PK136), 250 μg of control IgG2a, or 500 μg of anti-asialo GM1 (SH-34) (all from BioXCell, West Lebanon, NH).
Lymphocyte preparation and flow cytometry
Colons were removed from euthanized mice, placed into Bruff’s media and passed through a wire-mesh screen. Briefly, colon homogenate was incubated with 100 U/ml collagenase (Sigma; St. Louis, MO) and 20 μg/ml DNase I (Sigma) for 40 min at 37°C. To remove colonic debris, homogenates were centrifuged at 300 rpm for 3 min, and then supernatants were centrifuged at 1500 rpm for 10 min. The cells were resuspended in 1 ml complete media and 4 ml of 30% OptiPrep (Axis-Shield; Oslo, Norway) in a sodium phosphate buffer and 1 ml of media was carefully layered on top. Cells were centrifuged at 2700 rpm for 20 min. The top layer and interface were harvested as the lymphocyte population. Cells were stained with fluorescently-conjugated Abs in 1% BSA in PBS and fixed in 2% PFA. Cells were analyzed using a FACSCalibur (BD Biosciences; San Jose, CA) and data were analyzed by FlowJo v. 6.1 (TreeStar, Inc., Ashland, OR).
Real-time RT-PCR
RNA from cells or organs was isolated using Trizol reagent (Invitrogen; Carlsbad, CA). RNA was subjected to reverse transcriptase using Superscript II (Invitrogen) with oligo dT primer. cDNA was semi-quantitated using commercially available primer/probe sets (Applied Biosystems; Foster City, CA) and the ΔΔCT method. HPRT was included as an internal control.
Ex vivo colon culture and ELISAs
1 cm sections of the ascending colon were excised, removed of feces, washed three times with sterile PBS, and then longitudally halved. The colon sections were then placed into culture in complete Bruff’s media (10% FBS, L-glutamine, penicillin, streptomycin and tetracycline) and cultured at 37°C with 5% CO2. Supernatants were harvested after three days, and the concentration of cytokine was determined by ELISA. IFNγ and IL-6 ELISA (BD Pharmingen; San Diego, CA) or IL-22 ELISA (Antigenix America, Inc.; Huntington Station, NY) were performed according to the manufacturer’s protocols.
NK cell stimulation
Spleens and lymph nodes were isolated from C57BL/6 mice and prepared as described previously (Zenewicz et al., 2007) and NK1.1+ TCRβ− CD27 low or high cells were sorted on a FACS Aria. The cells were stimulated in vitro with the indicated combination of the following cytokines: 50 ng/ml IL-15 (Peprotech; Rocky Hill, NJ), 50 ng/ml IL-12 (BD Pharmingen), 50 ng/ml IL-23 (eBioscience; San Diego, CA) and 20 ng/ml IL-18 (MPL; Naka-ku Nagoya, Japan) for 18 hrs or remained untreated. mRNA was harvested and cytokine expression was semi-quantitated by real-time RT-PCR as described above.
Detection of activated Stat3
Caco-2 cells (ATCC HTB-37) were grown in DMEM media supplemented with 20% FBS, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were stimulated for 20 min with the indicated concentration of recombinant human IL-10, IL-19, IL-20, or IL-22 (all from Peprotech). Cell lysates were separated under reducing conditions on 4% to 12% gradient gel using the NuPAGE electrophoresis system (Invitrogen). Gels were transferred to Immobolin P membrane (Millipore; Billerica, MA), blocked with 5% dry milk in PBS with 0.01% Tween. Blots were then incubated overnight at 4°C with one of the following primary Ab: anti-phospho-Stat3 Tyr705 (polyclonal) or anti-Stat3 Ab (polyclonal) (both from Cell Signaling Technology; Danvers, MA). Blots were washed, incubated with appropriate secondary antibodies conjugated to horse radish peroxidase, and then developed using chemiluminescent substrate (Pierce, Rockford, IL) and film.
Histology
Organs were removed and fixed in 4% paraformaldehyde overnight at 4°C, then embedded in paraffin, sectioned, and stained with H&E. Slides were prepared at the Yale University Program for Critical Technologies in Molecular Medicine, Department of Pathology.
Statistics
Prism 4.03 software (Graphpad Software; San Diego, CA) was used for statistical analyses. p values of less than 0.05 were considered statistically significant.
Supplementary Material
Supplemental Figure 1 IL-22R is expressed highly in the gastrointestinal tract
(A) IL-10 family receptor mRNA expression by real-time RT-PCR in the indicated mouse tissues. Bars represent the mean expression of the cytokine gene to HPRT using the ΔΔCT method. Experiment was performed two times with similar results.
(B) Human colonic epithelial cells are responsive to IL-22. Caco-2 cells were stimulated for 20 min with 100 μg/ml recombinant human IL-10, IL-19, IL-20, IL-22 or remained unstimulated (Ø). Phosphorylation or total Stat3 in the cell lysates was determined by immunoblotting with the respective antibodies. Experiment was performed three times with similar results.
Supplemental Figure 2 Kinetics of IBD at later time points
IL-22 +/+ or IL-22 −/− CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. into IL-22−/− RAG1−/− double deficient mice. (10 mice per group.)
(A) Percent survival of the mice. Mice were euthanized according to protocol when they reached −30% mass loss.
(B) Mice were massed weekly and percent change from week 0 was calculated. Each dot represents one mouse, bar indicates mean. * indicates p<0.05.
Supplemental Figure 3 Exacerbated colitis in IL-22 deficient mice is independent of T cells
RAG1−/− or IL-22−/− RAG−/− mice were given 3% DSS ad libitum in their drinking water for seven days. Mice were massed daily and percent mass change from day 0 was calculated. Mean±SD. 10 mice/group.
Acknowledgments
LAZ was supported by a NRSA Training Grant (2 T32 AI07019-29) and post-doctoral fellowships from the American Liver Foundation and the American Cancer Society. RAF is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used in this manuscript
- IBD
inflammatory bowel disease
- DSS
dextran sodium sulfate
Footnotes
Conflict of Interest
LAZ and RAF declare no conflict of interest. GDY, DMV, AJM and SS were employees of Regeneron Pharmaceuticals at the time this work was performed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+) -cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181–191. doi: 10.1007/BF02285159. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Boniface K, Lecron JC, Bernard FX, Dagregorio G, Guillet G, Nau F, Morel F. Keratinocytes as targets for interleukin-10-related cytokines: a putative role in the pathogenesis of psoriasis. Eur Cytokine Netw. 2005;16:309–319. [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A. 2000;97:10144–10149. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Leach MW, Rennick DM. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J Immunol. 1998;161:3256–3261. [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, Sundberg JP. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998;274:G544–551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994a;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994b;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, Exley M, Snapper S, Balk SP, Hagen SJ, et al. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119–128. doi: 10.1053/gast.2000.9114. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Hollander GA, Mizoguchi E, Allen D, Bhan AK, Wang B, Terhorst C. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol. 1997;27:17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:325–330. doi: 10.1002/ibd.20079. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Reineke U, Witte K, Friedrich M, Sterry W, Asadullah K, Volk HD, Sabat R. Is there an interaction between interleukin-10 and interleukin-22? Genes Immun. 2005;6:8–18. doi: 10.1038/sj.gene.6364144. [DOI] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but Not Interleukin-17 Provides Protection to Hepatocytes during Acute Liver Inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Muhl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 IL-22R is expressed highly in the gastrointestinal tract
(A) IL-10 family receptor mRNA expression by real-time RT-PCR in the indicated mouse tissues. Bars represent the mean expression of the cytokine gene to HPRT using the ΔΔCT method. Experiment was performed two times with similar results.
(B) Human colonic epithelial cells are responsive to IL-22. Caco-2 cells were stimulated for 20 min with 100 μg/ml recombinant human IL-10, IL-19, IL-20, IL-22 or remained unstimulated (Ø). Phosphorylation or total Stat3 in the cell lysates was determined by immunoblotting with the respective antibodies. Experiment was performed three times with similar results.
Supplemental Figure 2 Kinetics of IBD at later time points
IL-22 +/+ or IL-22 −/− CD4+ CD45RBhigh CD25− NK1.1− T cells (5×105) were transferred i.p. into IL-22−/− RAG1−/− double deficient mice. (10 mice per group.)
(A) Percent survival of the mice. Mice were euthanized according to protocol when they reached −30% mass loss.
(B) Mice were massed weekly and percent change from week 0 was calculated. Each dot represents one mouse, bar indicates mean. * indicates p<0.05.
Supplemental Figure 3 Exacerbated colitis in IL-22 deficient mice is independent of T cells
RAG1−/− or IL-22−/− RAG−/− mice were given 3% DSS ad libitum in their drinking water for seven days. Mice were massed daily and percent mass change from day 0 was calculated. Mean±SD. 10 mice/group.