Abstract
BACKGROUND
Offspring of women with gestational diabetes (OGD) have greater risk for obesity and impaired metabolic health. Whether impaired metabolic health occurs in the absence of obesity is not clear. The purpose of this study was to investigate the independent and interactive effects of intrauterine exposure to gestational diabetes and of children's current weight status on their metabolic health.
METHODS
Children aged 5–10 years (N=51) with and without intrauterine exposure to gestational diabetes (OGD vs CTRL) were grouped into normal weight (BMI<85th %) and overweight (BMI>85th %) according to Centers for Disease Control growth curves. Lipid profile was obtained by fasting blood draw, insulin sensitivity (SI) and secretion by liquid meal tolerance test, and body composition by dual-energy X-ray absorptiometry.
RESULTS
Despite similar average BMI percentiles among normal weight OGD vs CTRL, and overweight OGD vs CTRL, OGD had greater total %fat and trunk fat adjusted for leg fat compared to CTRL (P<0.05). Overweight children had lower SI (P<0.05) and greater basal, static, and total insulin secretion independent of SI (P<0.05). OGD was independently associated with greater static insulin secretion (P<0.05) and the interaction between OGD and overweight was associated with greater basal insulin secretion independent of SI (P<0.01). OGD and overweight were each associated with lower HDL-C (P<0.05).
CONCLUSION
Intrauterine exposure to gestational diabetes was associated with greater central adiposity and insulin secretion, and lower HDL-C, irrespective of current weight status. Future research should examine respective contributions of the intrauterine environment and of underlying genotype on children's metabolic health.
Keywords: body composition, glucose, insulin, lipid, obesity, pediatric, pregnancy, prenatal programming
INTRODUCTION
Gestational diabetes mellitus (GDM) occurs in approximately 2–10% of pregnant women in the United States (1), and this incidence is increasing (2). African American women have 1.8 times the risk of GDM as compared to Caucasians (3). Following a diagnosis of GDM, both the mother and child are at risk for future health problems.
Children of GDM mothers are more likely to be overweight or obese (4–9), and to have greater central adiposity as suggested by measurements of waist circumference (10–13). Lower insulin sensitivity and/or impaired glucose tolerance (13–15), high blood pressure (10; 14), and dsylipidemia (14) are also more common among children born to women with GDM. Given the comorbidity between obesity and components of the metabolic syndrome, it is not clear whether intrauterine exposure to GDM even in the absence of current obesity increases the risk for impaired metabolic health.
The purpose of this study was to investigate the independent and interactive effects of intrauterine exposure to GDM and current overweight status on indices of metabolic health among prepubertal children. We hypothesized that children exposed to GDM in utero would have a less favorable metabolic profile, even if they were of normal body weight, compared to children from a non-GDM prenatal environment.
METHODS
Participants
Children aged 5–10 years were recruited to fill two groups: offspring of women with gestational diabetes (OGD) and offspring of non-diabetic women (CTRL). Mothers self-reported their GDM status during the target pregnancy, and this status was verified by review of prenatal medical records when available (~80% of the sample). Mothers also reported whether the children were breast-fed as infants (coded as yes/no). Children were eligible if they were singletons and were born at ≥37 weeks gestation. Children who were growth restricted in utero (<2500g at birth), had congenital defects, type 1 diabetes, or a current weight of <11kg (precluding adequate blood sampling), were excluded.
Procedure
Children and their mothers attended two study-related visits. At the first visit, informed consent/assent was obtained and children underwent a physical examination by a certified nurse practitioner during which pubertal status was evaluated using the Marshall and Tanner (16) criteria. During the second visit, mothers brought their children to the Clinical Research Unit (CRU) of the Center for Clinical and Translational Science (CCTS) at 6.30am following an overnight fast. Dual-energy X-ray absorptiometry (DXA) was used to obtain body composition, fasting blood draws were obtained, and a liquid meal tolerance test was used to measure insulin sensitivity and secretion. Maternal educational attainment and household socioeconomic status (SES) was assessed with the Hollingshead 4-factor index of social class (17). The Institutional Review Board for Human Use at the University of Alabama at Birmingham approved all procedures.
Body composition
Body composition was measured by DXA (Lunar iDXA, GE Healthcare, General Electric Company, Madison, WI). Scans were analyzed for total and regional fat and lean mass using the encore software package (version 1.33; GE Healthcare, General Electric Company, Madison, WI).
Liquid meal tolerance test
Blood samples were obtained via intravenous catheter prior to and following consumption of Carnation Instant Breakfast (Nestlé USA, Inc., Glendale, CA) mixed with whole milk. The meal was individually prepared to provide ~1.75 g of carbohydrate per kg of lean body mass and children were required to consume the entire meal within 5 minutes. Blood samples were obtained at −15 and −5 minute (averaged to provide fasting value), and at 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 210, and 240 minutes. Sera were separated and stored at −85°C until assayed for glucose, insulin, and C-peptide concentrations.
Serum assays
Blood samples were obtained following an overnight fast. Sera were separated and stored at −85°C until assay. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) concentrations were measured using enzymatic/ colorimetric methods on the SIRRUS analyzer. The inter-assay CV for each was 4.87%, 5.30%, and 4.28%, respectively. Intra-assay CVs were 1.33%, 1.11%, and 1.08%, respectively. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald method (18).
Glucose, insulin, and C-peptide were analyzed by the Core laboratory of the UAB Nutrition Obesity Research Center, Diabetes Research Training Center, and CCTS. Fasting glucose was measured with the SIRRUS analyzer (Stanbio Laboratory, Boerne, TX). This analysis had a mean intra-assay coefficient of variation (CV) of 1.28% and an inter-assay CV of 3.10%. Insulin and C-peptide were assayed using the TOSOH AIA-600 II automated analyzer (TOSOH Bioscience, Inc., San Francisco, CA). The analyses for insulin had mean intra- and inter-assay CV of 4.42% and 1.49%, respectively. For C-peptide, the intra- and inter-assay CV were 1.67% and 2.59%, respectively.
Insulin sensitivity and secretion
Glucose, insulin, and C-peptide concentrations throughout the 4-hour test were used to derive insulin sensitivity and indices of β-cell response using the “Oral Glucose Minimal Model” (19). Indices of β-cell response included total β-cell response (i.e. overall secretion; PhiTOT), basal β-cell response (i.e. β-cell sensitivity to glucose under fasting conditions; PhiB), dynamic β-cell response (i.e. β-cell response to the rate of increase in glucose; PhiD), and static β-cell response (i.e. β-cell response to glucose concentrations above basal across the test period; PhiS).
Impaired fasting glucose
Fasting glucose concentration was averaged across two blood draws obtained prior to the liquid meal tolerance test. Children with fasting glucose ≥100 mg/dL were categorized as having impaired fasting glucose (IFG; (20)) while others were categorized as normal glucose tolerant (NGT). No child had a fasting glucose concentration of ≥110 mg/dL.
Statistical analysis
Children were grouped into normal weight (NW; BMI<85th percentile) and overweight (OW; BMI>85th percentile) according to Centers for Disease Control and Prevention growth curves (21). Two- way analyses of variance (ANOVA) were used to examine the main and interactive effects of weight status (NW vs OW) and of intrauterine exposure to GDM (OGD vs CTRL) on the outcomes of interest (i.e. body composition, glucose, insulin sensitivity, lipids). Analyses of covariance (ANCOVA) were used to further explore group differences in trunk fat and the indexes of insulin secretion. Leg fat mass was included as a covariate in the trunk fat model to examine whether group differences in trunk fat reflected greater relative fat mass in the trunk versus periphery. Insulin sensitivity was added to the models for insulin secretion indexes in order to examine whether differences in insulin secretion were attributable to differences in insulin sensitivity. All blood-derived variables were log10 transformed prior to analysis. Kruskal-Wallis tests were used to test for significant differences in non-parametric variables (i.e. ethnicity, gender, breast-feeding status, and prevalence of impaired fasting glucose). Alpha was set at 0.05 for statistical significance, and all analyses were performed using the Statistical Package for the Social Sciences, version 18 (SPSS; SPSS Inc., Chicago, IL).
RESULTS
Fifty-five children enrolled in the study. Four of the OW-OGD girls were found to be at Tanner stage 2 of pubertal development and so were excluded from the final analyses of the body composition outcomes (N=51). A further eight children were excluded from the blood-derived outcomes because no blood was obtained from these children. Characteristics of the children are shown in Table 1. Overall, 75% of the cohort was African American (AA), and 51% was female. There were no statistical differences in the ethnic or gender distribution across the groups. There was also not a statistical difference in the prevalence of breastfeeding across groups. Maternal education ranged from less than seventh grade to completion of graduate professional training with most women being high school graduates or having a partial college education. There were no group differences in maternal education or in total household SES (data not shown). Average BMI percentile of the OGD vs CTRL children within each weight class did not differ and among the OW children specifically, the frequency of obese children (i.e. BMI ≥ 95th percentile) in the OGD vs CTRL groups did not differ significantly (% obese in OGD vs CTRL = 54% vs 73%, respectively).
Table 1.
Characteristics of the study population (mean ± SEM unless noted).
| CTRL | OGD | ANOVA1 | |||
|---|---|---|---|---|---|
| NW | OW | NW | OW | ||
| N | 19 | 8 | 11 | 13 | |
| Ethnicity %AA (n) | 84% (16) | 100% (8) | 64% (7) | 54% (7) | |
| Gender %male (n) | 58% (11) | 63% (5) | 55% (6) | 23% (3) | |
| Breastfed %Yes (n) | 63% (12) | 25% (2) | 73% (8) | 69% (9) | |
| Age (yr) | 7.0 ± 0.3 | 7.8 ± 0.5 | 8.1 ± 0.5 | 7.2 ± 0.4 | I |
| Weight (kg) | 24.7 ± 1.8 | 37.8 ± 2.7 | 27.1 ± 2.3 | 35.2 ± 2.2 | W |
| Height (cm) | 125.5 ± 2.5 | 133.4 ± 3.8 | 129.1 ± 3.2 | 125.4 ± 3.0 | |
| BMI percentile | 49.1 ± 4.0 | 94.2 ± 6.2 | 55.3 ± 5.3 | 96.1 ±4.9 | W |
| Fasting glucose (mg/dL)2 | 94.2 ± 1.9 | 92.2 ± 2.8 | 95.1 ± 2.5 | 93.2 ± 2.3 | |
| Fasting insulin (uIU)2 | 3.4 ± 0.6 | 6.2 ± 1.0 | 3.2 ± 0.9 | 9.0 ± 0.8 | W |
| Impaired fasting glucose % (n)2 | 19% (3) | 14% (1) | 33% (3) | 55% (6) | |
Significant ANOVA results are coded as follows: W = significant effect of children's weight category (P<0.05); I = interaction between weight status and intrauterine exposure to GDM (P<0.05).
Blood-derived variables are from a reduced sample size (16 NW CTRL; 7 OW CTRL; 9 NW OGD; 11 OW OGD).
Results of the ANOVA revealed main effects of both weight status (P<0.001) and intrauterine exposure to GDM (P<0.05) on total %fat (Figure 1A). With respect to the relative distribution of body fat in the trunk versus periphery, there was an interactive effect of weight status and intrauterine exposure to GDM such that OW-OGD had more trunk fat mass adjusted for leg fat mass than any other group (P<0.05; Figure 1B). This effect was evident regardless of whether total body fat mass or leg fat mass was included in the model as a covariate.
Figure 1.

(A) Total body % fat was greater among OW children (P<0.001), and those exposed to GDM in utero (P<0.05); (B) Trunk fat, relative to leg fat, was greater among OGD versus CTRL (P<0.01), and an interactive effect with weight status was noted whereby OW-OGD had more trunk fat adjusted for leg fat compared to children with just one risk factor (P<0.05). *P<0.05; **P<0.01; ***P<0.001.
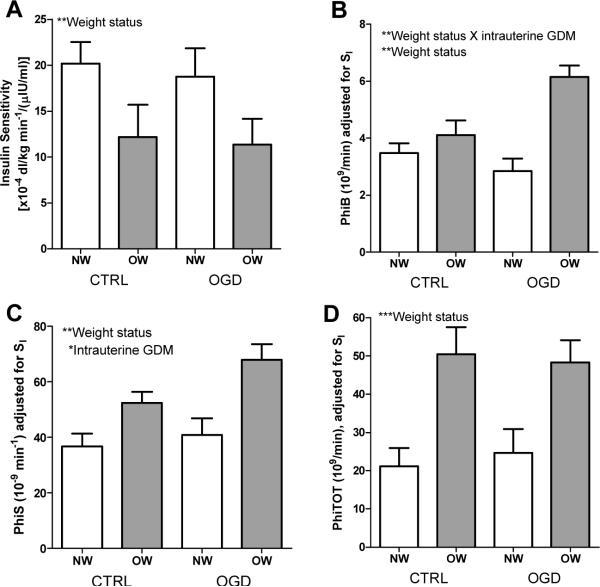
Although average fasting glucose concentration did not differ by group, there was a tendency for more of the children with intrauterine exposure to GDM to have impaired fasting glucose (Table 1). A main effect of weight status on fasting insulin concentration was found, whereby, irrespective of intrauterine exposure to GDM, OW children had higher insulin compared to NW children (P<0.001; Table 1). This effect was mirrored in the derived SI measure (P<0.01, Figure 2A). There was an interactive effect of weight status and intrauterine exposure to GDM on PhiB such that OW-OGD had the highest PhiB (P<0.01); an effect that remained significant after adjustment for SI (P<0.01, Figure 2B). There was no effect of weight status or intrauterine exposure to GDM on PhiD (not shown), but main effects of each were found for PhiS, even after adjusting for SI (P<0.05, Figure 2C). A main effect of weight status on PhiTOT was found, with greater PhiTOT among OW versus NW children, irrespective of intrauterine exposure to maternal GDM (P<0.001). This effect remained significant after adjustment for SI (P<0.001, Figure 2D). These results for the insulin secretion indexes remained statistically significant even after children's total %fat was added to the models (P<0.05; not shown), with the exception of the effect of intrauterine exposure to GDM on PhiS, which weakened to a trend (P=0.054)
Figure 2.
(A) Insulin sensitivity (SI) was lower among OW versus NW (P<0.01); (B) PhiB adjusted for SI was higher among OW versus CTRL (P<0.01), and an interactive effect with intrauterine exposure to GDM implied higher PhiB among children with both risk factors as compared to those with only one risk factor (P<0.01); (C) PhiS, adjusted for SI, was higher among OW versus NW (P<0.01) and among OGD versus CTRL (P<0.05); and (D) PhiTOT, adjusted for SI, was higher among OW versus NW, irrespective of intrauterine exposure to GDM (P<0.001). *P<0.05; **P<0.01; ***P<0.001.
Average TC for the whole cohort was 161.8 ± 32.0 mg/dL and did not differ by group. Similarly, average LDL-C concentration was 93.9 ± 29.9 mg/dL for the whole cohort and also did not differ by group. There was a main effect of weight status on TG concentrations, with higher TG among OW versus NW (P<0.05; Figure 3A). Main effects of both intrauterine exposure to GDM and weight status were found for HDL-C concentrations, such that each of these risk factors was associated with lower HDL-C (P<0.05; Figure 3B).
Figure 3.
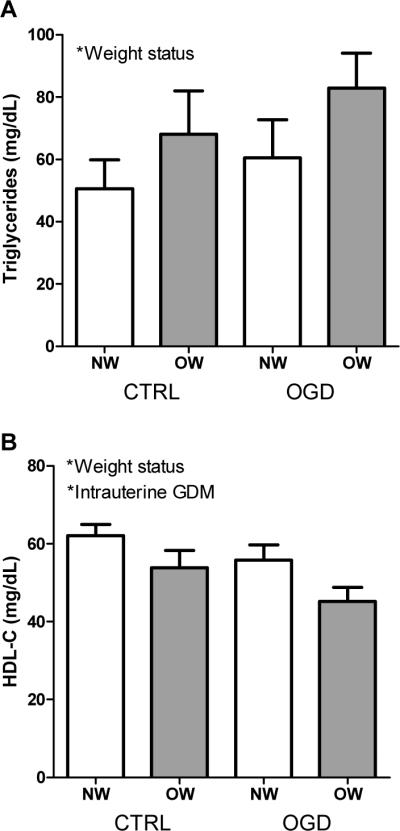
(A) Triglyceride concentrations were higher among OW versus NW children (P<0.05); and (B) HDL-C concentrations were lower among both OW versus NW (P<0.05) and OGD versus CTRL (P<0.05). *P<0.05; **P<0.01; ***P<0.001.
DISCUSSION
The purpose of this study was to investigate the independent and interactive effects of intrauterine exposure to GDM and current weight status on the metabolic health of prepubertal children. Results in this cohort showed main effects of weight status on a number of variables; overweight children had lower insulin sensitivity, higher basal, static, and total insulin secretion, higher triglyceride and lower HDL-C concentrations compared to normal weight children. Main effects of intrauterine exposure to GDM were also found, with offspring of GDM mothers having greater total and trunk fat, higher static insulin secretion, and lower HDL-C concentrations. Furthermore, interactive effects of intrauterine exposure to GDM and current weight status were found for trunk fat and basal insulin secretion such that overweight children of GDM mothers had greater trunk fat and basal insulin secretion as compared to children with just one of these risk factors. These findings suggest that intrauterine exposure to GDM may be associated with impaired metabolic health, irrespective of current weight status, but when combined with current overweight status, children exposure to GDM in utero have a worse metabolic profile than those with only one of these risk factors.
In this cohort, main effects of intrauterine exposure to GDM on children's total body fat and trunk fat were found, suggesting that greater adiposity may be present even among children with a BMI in the normal weight range who were delivered from mothers with GDM. Although other studies have shown greater adiposity among children from mothers with GDM (4–9), to our knowledge, this is the first time that evidence for proportionately greater body fat has been shown among normal weight children who were exposed to maternal GDM in utero. Greater trunk fat, relative to peripheral fat, among children with intrauterine exposure to GDM also extends previous findings of larger waist circumferences in this population (10–13). Furthermore, the interaction of overweight status with intrauterine exposure to GDM suggests that when children of GDM mothers gain weight, they preferentially deposit fat in the abdomen. Although the mechanism that underlies this pattern of excess adiposity is not clear, it may be related to an underlying genotype that is common to mother and child, or to the hyperinsulinemia found in these children. Previous research has shown that hyperinsulinemia is associated with subsequent weight gain in children and adolescents (22–24), and is associated with greater central adiposity in prepubertal children (25; 26). Furthermore, we and others have previously shown that maternal glucose concentrations during pregnancy are positively associated with both adiposity (7; 27) and hyperinsulinemia (28; 29) among offspring. Consequently, these data support the hypothesis that abnormalities in glucose metabolism in a pregnancy complicated by GDM may contribute to excess insulin-mediated adipose deposition among offspring (30; 31).
In this cohort, both overweight status and intrauterine exposure to GDM were associated with perturbations in insulin action. Specifically, overweight children had lower insulin sensitivity and higher basal, static phase, and total insulin secretion following a liquid meal challenge. These findings are consistent with previously published studies showing that overweight children have lower insulin sensitivity and greater insulin secretion (25; 32; 33). Intrauterine exposure to GDM was independently associated with greater static insulin secretion, and interacted with current overweight status to increase the rate of basal or fasting insulin secretion. These effects did not appear to be attributable to greater adiposity or to lower insulin sensitivity. These findings extend the existing literature by showing that the high insulin secretion present in utero or at birth among offspring of GDM or hyperglycemic mothers (29; 30) persists until middle-childhood even among children who remain at a normal body weight. The trend for a higher prevalence of impaired fasting glucose shown among children in this cohort who were exposed to GDM in utero is also consistent with existing literature (13; 15), and may provide an early marker of impaired glucose tolerance. More work is needed, however, to investigate whether abnormalities in glucose metabolism persist across childhood or whether they resolve during infancy and reappear in subsequent years.
Results of this study also showed higher triglyceride concentrations and lower HDL-C among overweight children, and an independent association of intrauterine exposure to GDM with lower HDL-C. Although other studies have shown similar patterns of dyslipidemia among overweight compared to lean children (32; 34; 35), few studies have reported lipid profiles among children with and without intrauterine exposure to GDM. Of those that have, results have shown either no difference in lipids (36), or, consistent with the current study, lower HDL-C among children exposed to GDM (14). Similar to greater adiposity, lower concentrations of HDL-C might also be secondary to hyperinsulinemia. The association of hyperinsulinemia with low HDL-C has long been recognized (37; 38). The mechanisms underlying this effect are complex and may include a direct effect of insulin to inhibit the biosynthesis of HDL-C (39), and an indirect effect of reduced insulin sensitivity to increase hepatic production of very-low-density-lipoprotein triglycerides, in turn leading to lipoprotein remodeling which presents as low HDL-C concentrations (for review see (40; 41)). Consequently, even in the absence of excess weight, lower HDL-C in children exposed to GDM in utero suggests that they may be predisposed to experience a sequelae of events that will ultimately lead to a clustering of symptoms associated with the metabolic syndrome.
Strengths of this study included robust measures of body composition and metabolic health, and the dichotomy of children into normal weight and overweight groups which enabled the effect of intrauterine exposure to GDM to be evaluated independent of children's weight status. This study was limited by the small sample size, particularly for blood-derived variables, and a lack of detailed information regarding maternal glycemic control and weight status during pregnancy which may have influenced the children's outcomes.
To conclude, results of this study support the hypothesis that intrauterine exposure to GDM is associated with excess trunk fat and insulin secretion, and low HDL-C concentrations, independent of children's current weight status. Furthermore, excess weight gain among children of GDM mothers may contribute to preferential deposition of trunk fat and a higher rate of fasting insulin secretion. Although more work is needed to confirm these findings and to understand the underlying mechanisms, these results raise concern that intrauterine exposure to maternal diabetes may be a clinically important predictor of metabolic health even among normal weight individuals.
ACKNOWLEDGEMENTS
This work was supported by the Thrasher Research Fund (NR-0025) and the National Institutes of Health (F32 DK-082028, UL-1RR025777, P30 DK-056336, P60 DK-079626). The authors thank Mead Johnson Nutritionals for supporting the development of the liquid meal tolerance test, in children. The authors thank Rachel Copper and Mickey Parks from the University of Alabama (UAB) Center for Women's Reproductive Health for administrative, nursing, and data collection support. The authors also thank Maryellen Williams and Cindy Zeng from the Core laboratory of the UAB Diabetes Research and Training Center, the Nutrition and Obesity Research Center, and the Center for Clinical and Translational Science, for conducting the laboratory analyses.
REFERENCES
- 1.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services; Atlanta, GA: 2011. [Google Scholar]
- 2.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 3.Dooley SL, Metzger BE, Cho N, Liu K. The influence of demographic and phenotypic heterogeneity on the prevalence of gestational diabetes mellitus. Int J Gynaecol Obstet. 1991;35:13–18. doi: 10.1016/0020-7292(91)90057-c. [DOI] [PubMed] [Google Scholar]
- 4.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 5.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 6.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21:149–157. doi: 10.1080/14767050801929430. [DOI] [PubMed] [Google Scholar]
- 7.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 8.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50:972–979. doi: 10.1097/GRF.0b013e31815a61d6. [DOI] [PubMed] [Google Scholar]
- 9.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22:215–220. doi: 10.1038/ajh.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54:504–507. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirkola J, Pouta A, Bloigu A, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010;33:1115–1121. doi: 10.2337/dc09-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87–92. doi: 10.1007/s00125-010-1925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egeland GM, Meltzer SJ. Following in mother's footsteps? Mother-daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet Med. 2010;27:257–265. doi: 10.1111/j.1464-5491.2010.02944.x. [DOI] [PubMed] [Google Scholar]
- 14.Tam WH, Ma RC, Yang X, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics. 2008;122:1229–1234. doi: 10.1542/peds.2008-0158. [DOI] [PubMed] [Google Scholar]
- 15.Vääräsmäki M, Pouta A, Elliot P, et al. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol. 2009;169:1209–1215. doi: 10.1093/aje/kwp020. [DOI] [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Clinical Growth Charts. 2009 [Google Scholar]
- 22.Adam T, Toledo-Corral C, Lane C, et al. Insulin sensitivity as an independent predictor of fat mass gain in Hispanic adolescents. Diabetes Care. 2009;32:2114–2115. doi: 10.2337/dc09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Suppl 2):B142–149. [PubMed] [Google Scholar]
- 24.Odeleye OE, de Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes. 1997;46:1341–1345. doi: 10.2337/diab.46.8.1341. [DOI] [PubMed] [Google Scholar]
- 25.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 26.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res. 2001;9:423–431. doi: 10.1038/oby.2001.56. [DOI] [PubMed] [Google Scholar]
- 27.Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care. 2011;34:741–745. doi: 10.2337/dc10-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush NC, Chandler-Laney PC, Rouse DJ, Granger WM, Oster RA, Gower BA. Higher Maternal Gestational Glucose Concentration Is Associated with Lower Offspring Insulin Sensitivity and Altered {beta}-Cell Function. J Clin Endocrinol Metab. 2011;96:E803–9. doi: 10.1210/jc.2010-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzger BE, Silverman BL, Freinkel N, Dooley SL, Ogata ES, Green OC. Amniotic fluid insulin concentration as a predictor of obesity. Arch Dis Child. 1990;65:1050–1052. doi: 10.1136/adc.65.10_spec_no.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 32.Higgins PB, Gower BA, Hunter GR, Goran MI. Defining health-related obesity in prepubertal children. Obes Res. 2001;9:233–240. doi: 10.1038/oby.2001.27. [DOI] [PubMed] [Google Scholar]
- 33.Chandler-Laney PC, Phadke R, Granger W, et al. Adiposity and β-cell function: relationships differ with ethnicity and age. Obesity. 2010;18:2086–92. doi: 10.1038/oby.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quijada Z, Paoli M, Zerpa Y, et al. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes. 2008;9:464–471. doi: 10.1111/j.1399-5448.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 35.Calcaterra V, Klersy C, Muratori T, et al. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clin Endocrinol (Oxf) 2008;68:868–872. doi: 10.1111/j.1365-2265.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- 36.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karhapää P, Malkki M, Laakso M. Isolated low HDL cholesterol. An insulin-resistant state. Diabetes. 1994;43:411–417. doi: 10.2337/diab.43.3.411. [DOI] [PubMed] [Google Scholar]
- 38.Zavaroni I, Bonini L, Fantuzzi M, Dall'Aglio E, Passeri M, Reaven GM. Hyperinsulinaemia, obesity, and syndrome X. J Intern Med. 1994;235:51–56. doi: 10.1111/j.1365-2796.1994.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 39.Nonomura K, Arai Y, Mitani H, Abe-Dohmae S, Yokoyama S. Insulin down-regulates specific activity of ATP-binding cassette transporter A1 for high density lipoprotein biogenesis through its specific phosphorylation. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundy SM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. 1998;81:18B–25B. doi: 10.1016/s0002-9149(98)00033-2. [DOI] [PubMed] [Google Scholar]