Abstract
Transcription factors of the ETS family are important regulators of endothelial and hematopoietic development. We have characterized the Xenopus orthologue of the ETS transcription factor, ETV2. Expression analysis shows that etv2 is highly expressed in hematopoietic and endothelial precursor cells in the Xenopus embryo. In gain of function experiments, ETV2 is sufficient to activate ectopic expression of vascular endothelial markers. In addition, ETV2 activated expression of hematopoietic genes representing the myeloid but not the erythroid lineage. Loss of function studies indicate that ETV2 is required for expression of all endothelial markers examined. However, knockdown of ETV2 has no detectable effects on expression of either myeloid or erythroid markers. This contrasts with studies in mouse and zebrafish where ETV2 is required for development of the myeloid lineage. Our studies confirm an essential role for ETV2 in endothelial development, but also reveal important differences in hematopoietic development between organisms.
INTRODUCTION
The ETS family of transcription factors is involved in numerous biological pathways during normal development and in disease (Oikawa and Yamada, 2003). Approximately 30 ETS genes are present in vertebrate genomes and all encode proteins containing a conserved 85 amino acid winged-helix-turn-helix DNA-binding domain. This binds to the core consensus GGA(A/T) (Lelievre et al., 2001; Sharrocks et al., 2001) but additional flanking sequences appear to increase binding specificity for certain ETS proteins (Graves and Peterson, 1998).
ETS factors play a particularly important role during embryonic vascular development and many, if not most, endothelial genes are direct targets of ETS regulation (Dejana et al., 2007). For example, mouse transgenic studies have shown that ETS binding sites are essential for efficient expression of the Tie1, Tie2 and VE-cadherin genes (Gory et al., 1998; Iljin et al., 1999; Korhonen et al., 1995). Similarly, mutation of a single ETS site in the intronic enhancer of the crucial VEGF receptor gene, flk1, (also called Vegfr2 or Kdr) rendered the transgene completely inactive in endothelial cells (Kappel et al., 2000; Meadows et al., 2009). Complementing these mutation studies, transfection of ETS factors into cells in culture results in transcriptional activation of numerous endothelial genes (Birdsey et al., 2008; Hasegawa et al., 2004; Schwachtgen et al, 1997; Wakiya et al., 1996). In Xenopus embryos, over-expression of the ETS factor, ERG, is sufficient to drive ectopic expression of endothelial markers (Baltzinger et al., 1999; Meadows et al., 2009).
While an essential role for ETS factors in endothelial gene activation is well documented, it has been more difficult to determine precisely which ETS proteins are required. At least 19 of the 30 ETS transcription factors are reported to be expressed in endothelial cells, although most are present at rather low levels (Hollenhorst et al., 2004; Liu and Patient, 2008). Of the ETS genes, five (Ets1, Ets2, erg, Fli1 and etv2) show relatively high levels of expression in the embryonic vasculature (Dejana et al., 2007). The presence of multiple ETS proteins in the endothelium raises the possibility of overlapping functions during vascular development and indeed, gene-ablation studies in mice point strongly to redundant functions for different ETS proteins. Mice homozygous null for Ets1 or Ets2 are viable and exhibit no vascular defects (Bories et al., 1995; Muthusamy et al., 1995; Yamamoto et a., 1998). Mice lacking FLI1 die at E12.5 due to hemorrhaging, but initial formation of the vascular network is normal (Spyropoulos et al., 2000). Animals mutant for ERG function show severe defects in definitive hematopoiesis, but development of the embryonic vasculature was not effected (Loughran et al., 2008). Furthermore, morpholino knockdown of four vascular ETS proteins (ETS1, ERF, FLI1 and ETV2) in the zebrafish embryo resulted in a near complete loss of endothelial cells, whereas single knockdown of any of the four sequences exhibited less severe phenotypes (Pham et al., 2007).
Unlike these previous studies, which demonstrated largely redundant function for different ETS factors, knockdown of the zebrafish etv2 gene (also called Etsrp) resulted in major vascular defects (Pham et al, 2007; Sumanas and Lin, 2006). Similarly, ETV2 deficient mice essentially lack embryonic blood and vascular structures (Lee et al., 2008). Mutant mice die at approximately E9.0 to E9.5 and exhibit no detectable expression of the vascular markers FLK1 and PECAM. The etv2 null phenotype is almost identical to that of the flk1 knockout (Shalaby et al., 1995), indicating that ETV2 activity is required at the earliest stages of endothelial development. Forced expression of ETV2 in the zebrafish embryo resulted in transcriptional up-regulation of numerous hematopoietic and endothelial genes, including genes not previously known to be endothelially expressed (Gomez et al., 2009; Wong et al., 2009).
Here we report the identification and characterization of the Xenopus orthologue of ETV2. Our studies indicate that ETV2 is amongst the earliest markers of hematopoietic and endothelial precursor cells. Forced expression of ETV2 in the embryo results in ectopic activation of endothelial and hematopoietic markers. Knockdown of ETV2 function strongly reduces expression of endothelial markers, whereas markers of the myeloid and erythroid lineages appear to be unaffected.
MATERIALS AND METHODS
Preparation of in situ probes and mRNAs
The insert from a full-length Xenopus laevis etv2 clone (BC099054) was isolated using Not I and Sal I and inserted into pBluescript SK. For in situ probe synthesis etv2-pBluescript was linearized with Sal I and transcribed with T3 RNA polymerase (Megascript kit - Ambion). The coding region of ETV2 was PCR-amplified from BC099054 with Pfu polymerase, subcloned into the translation vector, pT7TS, and sequence verified. For synthesis of mRNA, etv2-pT7TS was linearized with Xba I and transcribed with T7 RNA polymerase (Message Machine kit - Ambion). The inducible etv2 construction (GR-etv2) was created by inserting the complete coding region of ETV2 into a modified pT7TS construction, downstream of the ligand binding domain of the human glucocorticoid receptor (Kolm and Sive, 1995). GR-etv2 mRNA was prepared by linearizing with Xba I and transcribing with T7 RNA polymerase. RNA concentrations were measured using a Nanodrop spectrophotometer. Synthesis of flk1, aplnr (X-msr) and erg in situ hybridization probes has been described previously (Baltzinger et al., 1999; Cleaver et al., 1997; Devic et al., 1996). Whole-mount in situ hybridization was carried out using digoxigenin-labeled probes and standard conditions.
Forced expression and inducible ETV2 studies
For forced expression studies, mRNA encoding ETV2 and/or EGFP was injected into 4-cell embryos into the ventral region of a single vegetal blastomere. Embryos were cultured in O.2X MMR/6% Ficoll overnight and then transferred into 0.2X MMR until they were harvested. For analysis of fold-induction, ventral posterior regions were dissected from stage 32 embryos injected with mRNAs for ETV2 plus EGFP, or EGFP control alone. Three independent biological replicates of this experiment were performed using 10 explants for each condition. Total RNA was extracted using RNAeasy columns (Qiagen) and transcript levels were assayed by qPCR in a Corbett Rotogene 6000 using standard methods.
For inducible expression studies, mRNA encoding GR-ETV2 was injected into embryos exactly as described for forced expression studies. At approximately stage 30, the medium was supplemented with dexamethasone (DEX) to a final concentration of 20 μM. Treatment with cycloheximide (10 μg/ml) was initiated 30 min prior to DEX treatment for appropriate samples. Embryos were allowed to develop in the presence of DEX for 4 hours, at which time the posterior ventral region of the embryo was excised and total RNA was isolated. Transcript levels were assayed by qPCR.
MO controls and treatment
Preparation and microinjection of Morpholino Oligomers (MOs) was performed as previously described (Garriock et al., 2005). etv2 antisense morpholino (etv2 MO1, 5′-GGCAGTAGTAGATACTGGGATCCAT-3′) is complementary to the translation start site of etv2 transcripts (GeneTools). etv2 MO1 effectively blocked translation of etv2 test transcripts containing 5′ UTR plus a portion of the coding region of ETV2, fused to the coding region of EGFP (Fig. 5A and B). For in vivo experiments, 12.5 ng, 25 ng or 50 ng of etv2 MO1 or a 5 base pair mismatch control MO (CoMO, 5′-GGTAGTAATAGATGCTGTGATCTAT-3′) was microinjected into the mediolateral region of one cell of the 2-cell embryo and then assayed at stage 32 by whole-mount in situ hybridization. etv2 knockdown results were confirmed using a second non-overlapping MO, etv2 MO2 (5′–CGCGACAGGCTCTGCTTGCACCAAA–3′).
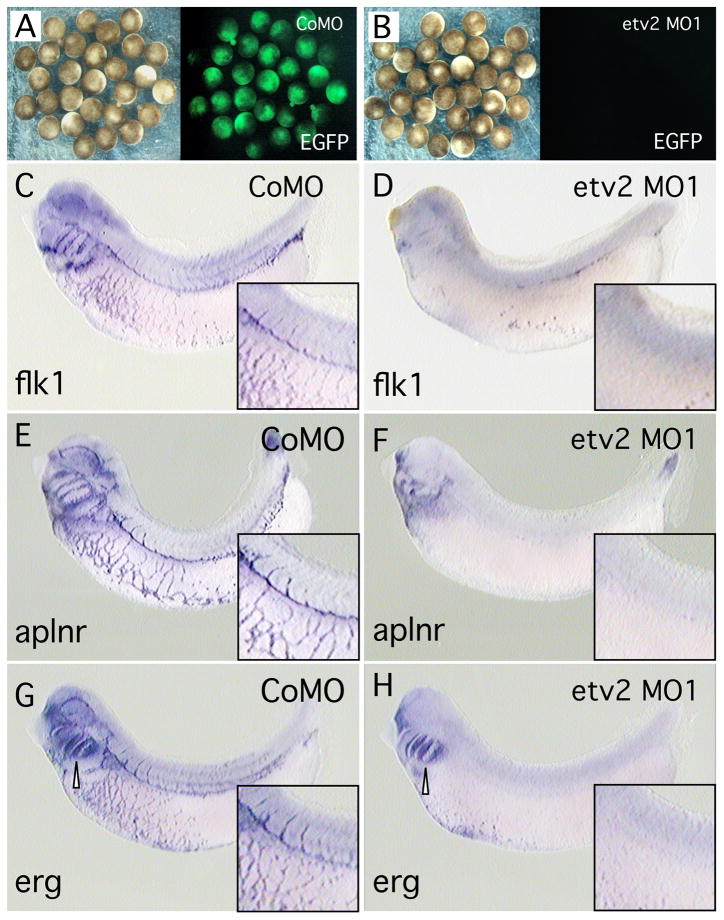
Fig. 5. Inhibition of ETV2 function strongly reduces endothelial, but not hematopoietic marker expression.
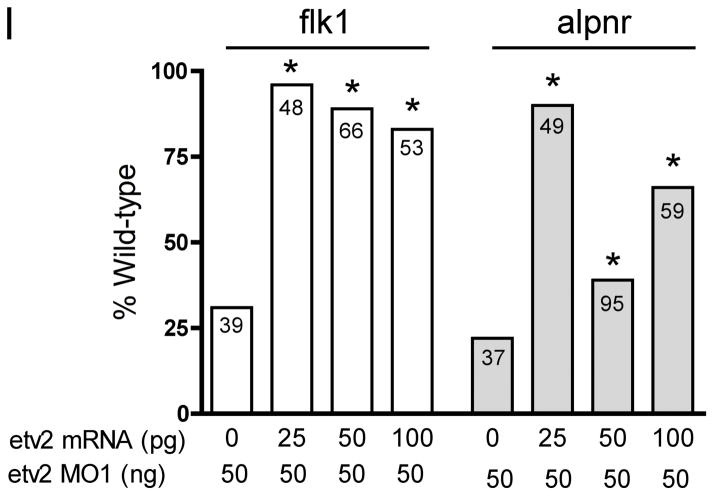
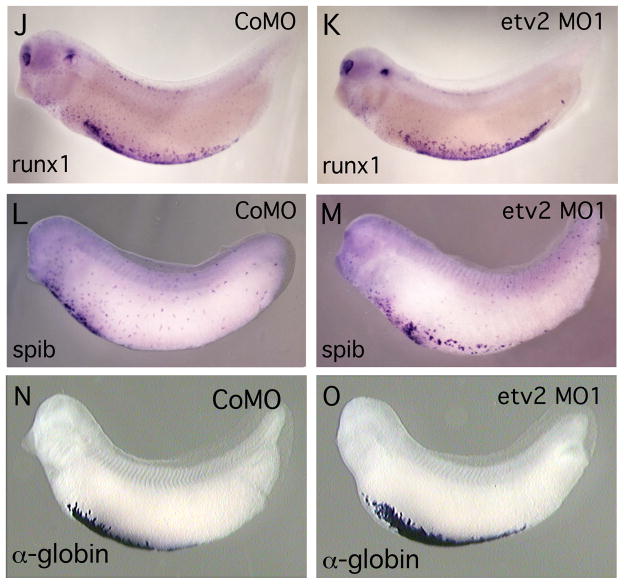
(A, B). Antisense etv2 MO1 efficiently inhibits EGFP expression from a target transcript in control experiments. (A) Brightfield and fluorescent image of embryos injected with etv2-EGFP fusion transcript and etv2 mismatch control MO (CoMO), showing strong EGFP fluorescence. (B) Brightfield and fluorescent image of embryos injected with etv2-EGFP fusion transcript and etv2 MO1. EGFP fluorescence is almost eliminated. (C–M). Embryos injected with control MO (CoMo) or etv2 MO1 and assayed for marker gene expression (as indicated) by in situ hybridization. All embryos were assayed at approximately st. 32. Inset boxes in panels C–H, show slightly enlarged and/or increased contrast views centered on the intersegmental vessels and posterior cardinal vein. (C–H) Treatment with etv2 MO1 effectively eliminated expression of the endothelial marker genes flk1, aplnr and erg. In (H), expression of erg in neural crest cells in the branchial arch region (open arrowhead) is unchanged. (I). etv2 MO1 knockdown of endothelial marker expression is efficiently rescued with etv2 mRNA. All embryos were injected with 50 ng of MO1 plus varying amounts of etv2 mRNA as indicated. Marker gene expression was assayed by in situ hybridization and embryos showing mostly normal endothelial pattern were scored as wild-type. The number in each bar indicates the number of embryos scored for each treatment. Asterisks show statistically significant differences relative to value of MO1 alone, analyzed using Chi-squared test. (J–O) Treatment with etv2 MO1 had no detectable effect on expression of myeloid (runx1 and spib) or erythroid (tadpole α-globin) markers.
RESULTS
The Xenopus etv2 orthologue is expressed in precursor cells of the blood and vascular lineages
Searches of the Xenopus tropicalis genome and EST databases identified a previously uncharacterized member of the Xenopus ETS gene family. Comparison of the primary sequence of the Xenopus protein to mouse and zebrafish ETS proteins showed that sequence identity was greatest with ETV2 (also called ER71 or Etsrp). Within the ETS DNA-binding domain Xenopus ETV2 shares 66% and 84% sequence identity to the mouse and zebrafish ETV2 proteins respectively (Fig. 1). Outside of the ETS DNA-binding domain the sequence similarity is much lower.
Fig. 1. Sequence alignment of vertebrate ETV2 proteins.
Clustal alignment of ETV2 protein sequences from human, mouse, Xenopus and zebrafish. Dark shading indicates identical residues and light shading shows conservative substitutions. The conserved ETS family DNA-binding domain is underlined. The ETS domain is moderately conserved between different vertebrates, whereas other regions of the protein are highly divergent. ETV2 protein sequence sources are as follows - mouse (NCBI reference NP_031985), Human (NP_055024) zebrafish (NP_001032452), Xenopus laevis (BC099054).
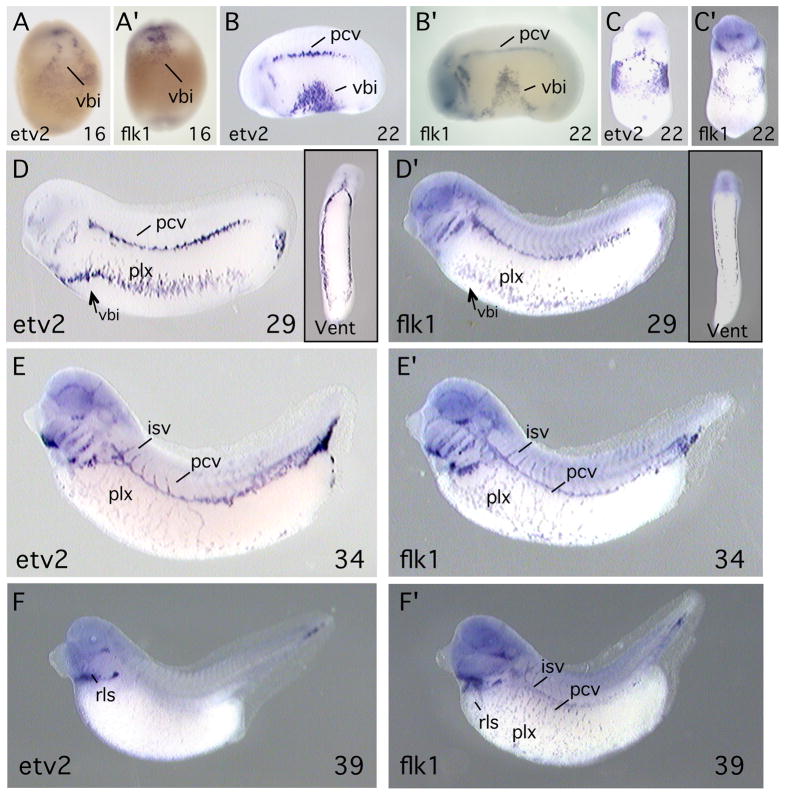
Whole-mount in situ hybridization was carried out to determine the expression pattern of etv2 in Xenopus embryos. At all developmental stages, expression of etv2 was compared to that of flk1, which is a marker of the endothelial/hematopoietic lineage in the embryo (Cleaver et al., 1997). etv2 transcripts were first detected in the region of the ventral blood island that is the location of blood precursor cells (Fig. 2A). As expected, this region is also positive for flk1 transcripts (Fig. 2A′). In the neurula embryo (stage 22), expression of etv2 persisted in the blood islands, but was also visible in lateral regions, in angioblasts that will contribute to the posterior cardinal vein (Figs. 2C). As development proceeded, etv2 became strongly expressed throughout all structures of the primitive vascular network, including the posterior cardinal veins, aortic arches, vascular plexus and intersomitic vessels (Figs. 2D, E). At these stages the pattern of etv2 expression continues to appear identical to that of the hematopoietic/endothelial marker flk1 (Fig. 2D′,E′). However, by tadpole stages (stage 39, Fig. 2F), etv2 transcripts decreased to almost undetectable levels in vascular structures and the blood islands, and became restricted to a small region near the head corresponding to the rostral lymphatic sac (Ny et al., 2005). In contrast, flk1 expression continued throughout the maturing vascular network of the tadpole stage embryo (Fig. 2F′).
Fig. 2. etv2 is expressed in hematopoietic and endothelial precursor cells in the Xenopus embryo.
Transcripts of etv2 (A–F) and flk1 (A′-F′) were detected by in situ hybridization. flk1 is an established marker of the hematopoietic and endothelial lineages. Developmental stage of the embryos is indicated at the lower right of each panel. Embryos shown in A, A′, C, C′ and insets in D, D′ are ventral views. All other views are lateral with anterior to the left. Abbreviations: vbi, ventral blood island; pcv, posterior cardinal vein; plx, vascular plexus; isv, intersegmental vessel; rls, rostral lymphatic sac.
Xenopus ETV2 is sufficient to initiate ectopic transcription of endothelial and myeloid marker genes
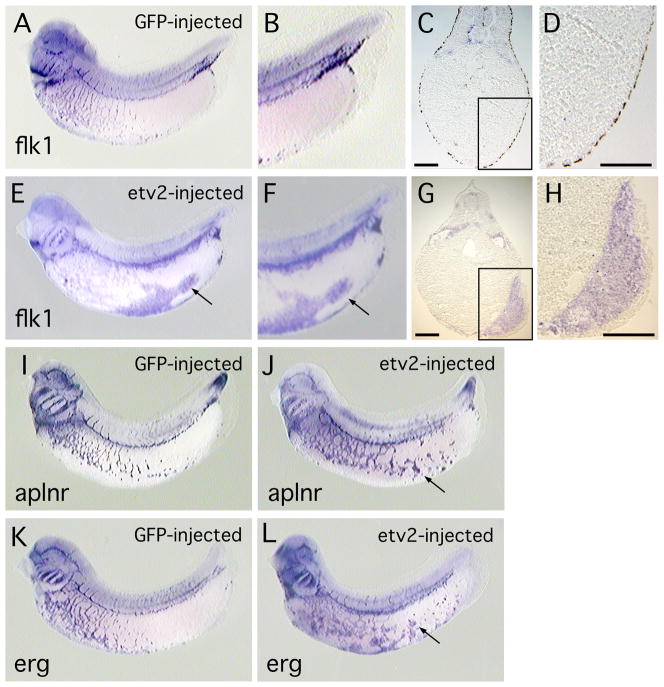
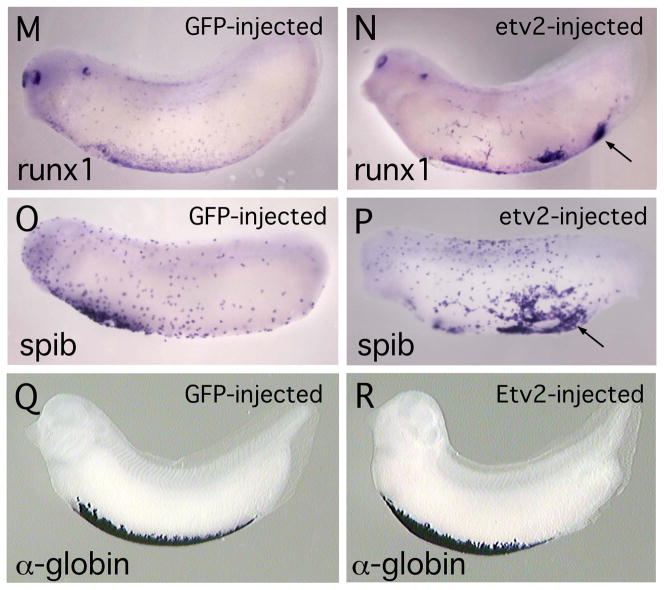
It has previously been demonstrated that the ETS family protein, ERG, is capable of activating ectopic expression of endothelial markers in the Xenopus embryo (Baltzinger et al., 1999; Meadows et al., 2009). Similarly, the zebrafish ETV2 orthologue is sufficient to activate ectopic expression of endothelial genes (Gomez et al, 2009; Sumanas and Lin, 2006; Wong et al, 2009). To determine whether Xenopus ETV2 is able to induce endothelial markers we used microinjection to target ETV2 protein to posterior ventral regions of the embryo, an area that is relatively free of endogenous endothelial precursor cells (see Fig. 2E′). In all cases GFP mRNA was co-injected with etv2 mRNA and served as a tracer. As shown in Figure 3, forced expression of ETV2 resulted in strong ectopic activation of the endothelial marker genes flk1, aplnr (also called X-msr) and erg. flk1 was used as a positive control in these experiments because it is directly regulated by ETV2 in studies using mouse stem cells (Lee et al., 2008) and is activated by ETV2 in zebrafish embryos (Sumanas and Lin, 2006). In some extreme cases, large regions of tissue exhibited ectopic marker expression, as shown in histological sections through an embryo stained for flk1 transcripts (Fig. 3E, F), however, it was more common to observe isolated patches of ectopic marker expression (Fig. 3J, L). Scoring of multiple embryos showed that overexpression of ETV2 resulted in ectopic expression of endothelial markers in a dose dependent manner and, at the highest levels of injected mRNA, a large majority of embryos showed ectopic marker expression (Table 1). Quantitation of transcript levels by qPCR showed greater than 90X activation of flk1 expression in ectopic regions (Table 1). As illustrated in Fig. 2, etv2 is expressed at high levels in the ventral blood island throughout early development (Fig. 2A-D). To determine whether ETV2 might be able to induce transcription of hematopoietic markers, we assayed for activation of the myeloid markers runx1 and spib (Liu and Patient, 2008) and the erythroid marker, tadpole α-globin (hba3). We observed that transcription of runx1 and spib was indeed activated at ectopic locations in ETV2 over-expressing embryos (Fig 3M, P and Table 1) but we never observed ectopic expression of α-globin (compare Figs. 3Q, R). Overall, the gain-of-function studies demonstrate that ETV2 is sufficient to activate transcription of representative endothelial and hematopoietic marker genes.
Fig. 3. Forced expression of ETV2 in the Xenopus embryos results in ectopic expression of endothelial and myeloid marker genes.
(A–D). Control embryo, injected with EGFP mRNA and assayed for flk1 transcripts. (A) View of whole embryo, and (B) enlarged view of ventral posterior region of embryo showing near absence of endothelial (flk1-expressing) cells. (C) Transverse section through embryo and (D) enlarged view, showing absence of flk1 marker expression in posterior of embryo. (E–H). Embryo injected with etv2 mRNA and assayed for flk1 expression. (E) View of whole embryo and (F) enlarged view, showing high levels of ectopic flk1 transcript. (G) Transverse section through embryo and (H) enlarged view, showing large domains of ectopic flk1 transcripts. Scale bar in both G and H is 100 microns. (I–J). Control embryo, and embryo injected with etv2 mRNA, assayed for aplnr expression. (I) View of embryo injected with EGFP mRNA. (J) View of embryo expressing ETV2 and showing ectopic aplnr transcripts. (K–L). Control embryo and embryo injected with etv2 mRNA, assayed for erg expression. (K) View of embryo injected with EGFP mRNA. (L) View of embryo expressing ETV2 and showing ectopic erg transcripts. For etv2 mRNA-injected embryos shown in (E, J, L), in addition to ectopic marker expression, note the disruption of the normal patterning of endothelial structures, relative to controls. (M, N). Control embryo and embryo injected with etv2 mRNA, assayed for runx1 expression. (M) View of embryo injected with EGFP mRNA. (N). View of embryo expressing ETV2 and showing ectopic expression of runx1. In N, in addition to ectopic expression, note reduced expression and altered distribution of endogenous runx1. (O,P). Control embryo and embryo injected with etv2 mRNA, assayed for spib expression. (O) View of embryo injected with EGFP mRNA. (P). View of embryo expressing ETV2 and showing ectopic expression of spib. (Q, R). Control embryo and embryo injected with etv2 mRNA, assayed for α-globin expression. (Q). Control embryo injected with EGFP mRNA. (R). Whole embryo expressing ETV2, showing no appreciable difference in expression of α-globin transcripts. In all panels, arrows indicate regions of ectopic marker expression.
Table 1.
Ectopic transcription of endothelial and hematopoietic markers in response to forced expression of ETV2
| Marker | mRNA | Normal gene expression % | Ectopic gene expression % | Number of embryos | Fold activated |
|---|---|---|---|---|---|
| flk1 | GFP (500 pg) | 98 | 2 | 52 | |
| etv2 (400 pg) | 9 | 91 | 32 | ||
| etv2 (200 pg) | 6 | 94 | 34 | ||
| etv2 (100 pg) | 10 | 90 | 21 | 97 +/− 28 | |
| etv2 (40 pg) | 53 | 47 | 72 | ||
| aplnr | GFP (500 pg) | 92 | 8 | 52 | |
| etv2 (100 pg) | 0 | 100 | 33 | 73 +/−25 | |
| erg | GFP (500 pg) | 100 | 0 | 20 | |
| etv2 (100 pg) | 16 | 84 | 25 | 8.5 +/−2.2 | |
| runx1 | GFP (500 pg) | 100 | 0 | 34 | |
| etv2 (200 pg) | 29 | 71 | 24 | 11 +/−4.4 | |
| spib | GFP (500 pg) | 96 | 4 | 24 | |
| etv2 (200 pg) | 24 | 76 | 21 | 12 +/−2.8 | |
| α-Globin | GFP (500 pg) | 100 | 0 | 26 | |
| etv2 (100 pg) | 100 | 0 | 24 | ND | |
| etv2 (400 pg) | 93 | 7 | 14 |
For assays of fold activation, samples of tissue over-expressing ETV2 were dissected from embryos and three biological replicates were assayed using qPCR (see Methods).
ETV2 directly activates transcription of flk1, aplnr, runx1 and spib
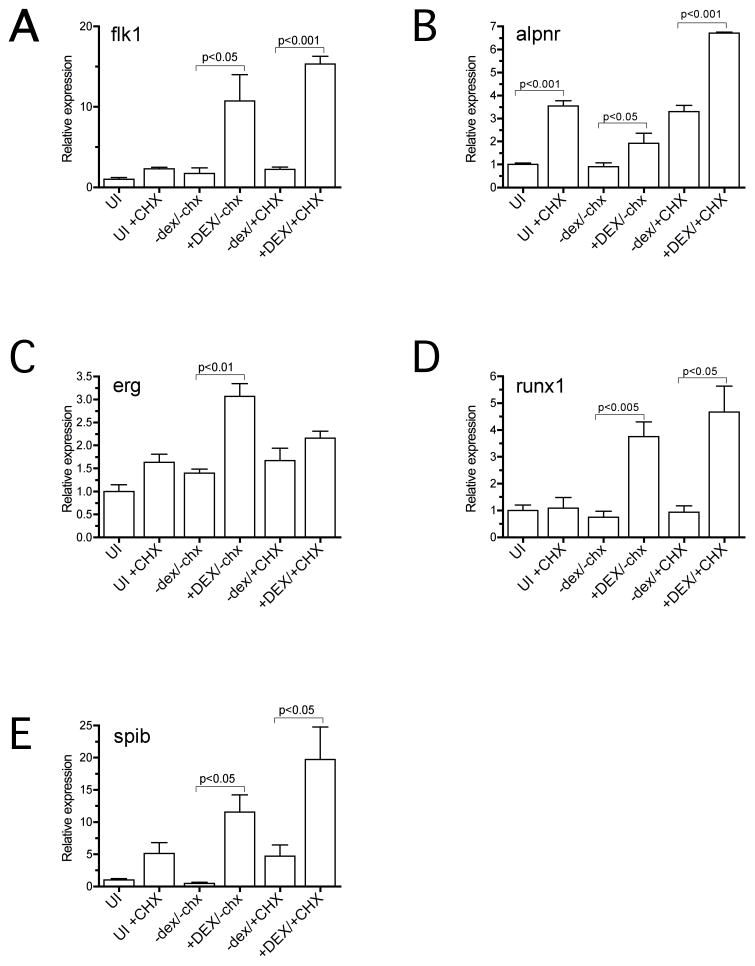
Forced expression studies alone do not allow us to determine which of the endothelial and hematopoietic genes, if any, are direct targets of ETV2 regulation. To address this question we made use of a dexamethasone (DEX) inducible form of ETV2, GR-ETV2. Messenger RNA encoding the GR-ETV2 protein plus EGFP lineage tracer was targeted to the ventral posterior region of the embryo as described previously. At the tailbud stage (st 32) DEX induction was carried out for 4 hours in the presence of the protein synthesis inhibitor cycloheximide (CHX) and then ventral posterior regions of the embryo were excised and RNA was harvested. With protein synthesis blocked, only genes that can be directly activated by ETV2 should be transcribed. Previous studies have indicated that some genes are transcriptionally activated by CHX treatment alone (Afouda et al., 2008; Yasuo and Lemaire, 1999) and so a CHX-only control was included in the experiments. Induction of target genes was analyzed using qPCR and the results are presented in Fig. 4. First, we observed that flk1 was a direct target of ETV2 regulation (Fig. 4A). This result was predicted based on previous studies showing that ETS proteins directly bind to flk1 regulatory elements (Kappel et al., 2000; Lee et al., 2008; Meadows et al, 2009). Second, we observed that, of the marker genes upregulated following forced expression (Fig. 3), aplnr, runx1 and spib were directly regulated by ETV2. Transcripts for each of these factors were induced by ETV2 at least 4-fold over control levels and significantly more than induced by CHX alone (Fig. 4B, D, E). On the other hand, erg did not show convincing up-regulation relative to control treatments following ETV2 induction, suggesting that ETV2 alone is not sufficient to activate transcription.
Fig. 4. ETV2 directly activates transcription of a subset of endothelial and hematopoietic markers.
Xenopus embryos were injected with mRNA encoding a dexamethasone (DEX) inducible version of ETV2 and cultured in the presence of DEX and/or the protein synthesis inhibitor, cycloheximide (CHX). Transcript levels for marker genes were quantitated for the various treatments using real-time qPCR. In all cases, transcript levels were normalized against transcripts of RNA polymerase 2. Graphs show results of triplicate biological replicates for each treatment. Presence of absence of DEX or CHX in a treatment is indicated by upper or lower-case type respectively. Statistical significance of differences between indicated samples is shown (P value measured by Student’s T test). The most important values are presented in the last two columns, which show transcript levels of putative target genes with uninduced ETV2 (−dex) and induced ETV2 (+DEX), both in the presence of CHX. The marker genes, flk1, aplnr, runx1 and spib appear to be direct targets of ETV2 regulation, whereas erg is probably not.
Loss of function studies demonstrate that ETV2 is required for expression of endothelial but not hematopoietic marker genes
To explore the requirement of ETV2 for endothelial and hematopoietic development, we used antisense morpholino oligomers (MOs) to specifically inhibit ETV2 expression in the embryo. First, we demonstrated that the etv2 MO1 effectively blocked translation of a target transcript in the Xenopus embryo (Fig. 5A, B). Second, expression of endothelial marker genes was examined in etv2 MO-treated embryos. The results show a dramatic reduction in expression of the endothelial markers flk1, aplnr and erg (Fig. 5C-H, Table 2). In the case of erg, note that expression in vascular tissues was strongly reduced, while expression in neural crest cells in the branchial arch region was unaffected (Fig. 5H). This observation clearly demonstrates the specificity of MO1 for ETV2 targets and suggests that MO treatment is not producing vascular defects through an off-target mechanism. To confirm the specificity of the MO knockdown we have carried out a standard rescue experiment. Embryos were injected with a fixed high dose of etv2 MO (50 ng) together with increasing amounts of etv2 mRNA (0–100 pg). The levels of etv2 mRNA were kept fairly low to avoid ectopic expression of markers that might interfere with analysis of the rescue phenotype. As shown in Fig. 5I, injection of etv2 mRNA very effectively rescued the MO knockdown phenotype. Previous studies have shown that etv2 knockout mice exhibit a complete absence of hematopoietic cells (Lee et al., 2008) and zebrafish lacking ETV2 function show absence of myeloid marker expression, whereas erythroid marker expression is normal (Sumanas et al., 2008). Somewhat surprisingly, inhibition of ETV2 function in Xenopus embryos produced no detectable alteration in expression of hematopoietic markers. For example, the myeloid markers runx1 and spib were expressed normally in all MO-injected embryos (Fig. 5J–M). At this stage of development, spib and runx1 are markers for migratory myeloid cells (Tracey et al., 1998; Costa et al., 2008). Although the migratory nature of the cells makes direct comparisons somewhat difficult, inspection of multiple etv2 MO-treated embryos (>20 for each gene) showed no consistent differences in the number of cells detected, the intensity of the in situ signal or the distribution of cells compared to controls (compare Fig. 5I, J and 5K, L). This surprising result indicates that, whereas ETV2 is sufficient to activate transcription of runx1 and spib (Figs. 3, 4, Table 1), it is not required for normal expression of these genes in hematopoietic cells. Similarly, we observed no effect on expression of α-globin in etv2 MO1 treated Xenopus embryos (Fig. 5N,O, Table 2). From these preliminary studies, we conclude that ETV2 is important for regulation of endothelial genes, but apparently is not required for development of the hematopoietic lineage. To confirm the requirement of ETV2 for endothelial development we have shown that a different non-overlapping MO, etv2 MO2, also results in elimination of endothelial marker gene expression in the Xenopus embryo (Table 2 and data not shown). Once again, etv2 MO2 had no detectable effect on myeloid or erythroid marker expression (Table 2).
Table 2.
Inhibition of ETV2 function results in reduced expression of endothelial markers but not myeloid or erythroid markers
| Marker | Treatment | Normal gene expression % | Reduced gene expression % | Number of embryos |
|---|---|---|---|---|
| flk1 | etv2 control MO (50 ng) | 96 | 4 | 48 |
| etv2 control MO (25 ng) | 100 | 0 | 26 | |
| etv2 control MO (12.5 ng) | 100 | 0 | 21 | |
| etv2 MO1 (50 ng) | 34 | 66 | 29 | |
| etv2 MO1 (25 ng) | 23 | 77 | 40 | |
| etv2 MO1 (12.5 ng) | 24 | 76 | 82 | |
| etv2 MO2 (50 ng) | 19 | 81 | 29 | |
| aplnr | etv2 control MO (25 ng) | 96 | 4 | 46 |
| etv2 MO1 (25 ng) | 20 | 80 | 30 | |
| etv2 MO2 (50 ng) | 22 | 78 | 40 | |
| erg | etv2 control MO (25 ng) | 100 | 0 | 42 |
| etv2 MO1 (25 ng) | 8 | 92 | 25 | |
| etv2 MO1 (50 ng) | 0 | 100 | 29 | |
| etv2 MO2 (50 ng) | 11 | 89 | 37 | |
| runx1 | etv2 control MO (50 ng) | 100 | 0 | 44 |
| etv2 MO1 (50 ng) | 100 | 0 | 35 | |
| etv2 MO2 (50 ng) | 92 | 8 | 25 | |
| spib | etv2 control MO (50 ng) | 98 | 2 | 44 |
| etv2 MO1 (50 ng) | 97 | 3 | 32 | |
| etv2 MO2 (50 ng) | 100 | 0 | 22 | |
| α-globin | etv2 control MO (50 ng) | 94 | 6 | 17 |
| etv2 MO1 (50 ng) | 96 | 4 | 72 | |
| etv2 MO2 (50 ng) | 78 | 22 | 18 |
DISCUSSION
etv2 is expressed in developing hematopoietic and endothelial cells
Studies of mouse and zebrafish have reported expression of the ETS family gene, etv2, in embryonic angioblasts and in cells of the hematopoietic lineage (Lee et al., 2008; Sumanas and Lin, 2006). In this report we describe the identification of the Xenopus orthologue of etv2 (Fig. 1) and show that it is expressed in embryonic angioblasts and hematopoietic cells (Fig. 2). Expression of etv2 is first observed in hematopoietic precursor cells in the ventral blood island and later in embryonic angioblasts throughout the developing vascular network. At all early stages of development, the etv2 expression pattern is indistinguishable from that of the hematopoietic/endothelial marker, flk1 (Fig. 2). etv2 transcripts are present throughout tissues of the developing vascular system during the time that angioblasts migrate, form into vascular cords and undergo tubulogenesis to form the primitive vascular network. However, at later developmental stages, expression of etv2 is dramatically down-regulated and becomes almost undetectable by the tadpole stage (st 39).
ETV2 is sufficient to activate ectopic expression of endothelial and myeloid markers
The ETS family protein, ERG, has the remarkable ability to induce ectopic expression of endothelial markers in the frog embryo (Baltzinger et al, 1999; Meadows et al., 2009). This property is shared by the zebrafish ETV2 orthologue, which is able to activate expression of numerous endothelial genes in zebrafish embryos (Gomez et al., 2009; Sumanas and Lin, 2006; Wong et al., 2009). Our studies show that forced expression of ETV2 in Xenopus also activates transcription of representative endothelial marker genes, including flk1, aplnr and erg (Fig. 3). Concomitant with ectopic marker activation, overexpression of ETV2 in the Xenopus embryo resulted in interference with normal vascular patterning. This is most clearly illustrated by the absence, or altered structure, of intersegmental vessels or overgrowth of the vascular plexus in anterior ventral regions of the embryo (Fig. 3E–N). These observations imply that levels of ETV2 must be regulated within narrow limits for normal vascular development. Because etv2 is expressed prominently in the ventral blood islands of the Xenopus embryo (Fig. 2A, B, C), it seemed possible that overexpression might result in ectopic activation of hematopoietic markers. Indeed forced expression of ETV2 strongly activated transcription of the myeloid markers, runx1 and spib (Fig. 3N, P). This ectopic activation was not shared by the erythroid marker, tadpole α-globin, which exhibited no evidence of ectopic activation (Fig. 3R), even at the highest levels of ETV2 forced expression (Table 1). Similar to observations of endothelial markers, over-expression of ETV2 appeared to reduce expression of the endogenous runx1 transcript (Fig. 3N), although endogenous spib and α-globin expression appeared largely normal (Fig. 3P, R). Our observations are consistent with previous studies of ETV2 overexpression in the zebrafish embryo that also reported reduced expression of a subset of endogenous genes (Sumanas et al., 2008).
We used an inducible protein construction in the presence of a protein synthesis inhibitor to determine which of the endothelial and hematopoietic markers might be direct targets of ETV2 (Fig. 4). These experiments confirmed that flk1 is a direct target of ETV2 regulation (Lee et al., 2008) and also showed that ETV2 is capable of directly activating transcription of aplnr, runx1 and spib1 (Fig 4). As with the forced expression studies described above, the results of induced activation experiments must be interpreted with caution. Whereas a positive result in the induction assay suggests that ETV2 is capable of directly activating the gene in question, the failure to observe activation does not exclude ETV2 from a direct role in gene regulation. ETS family transcription factors are widely understood to function in cooperation with other transcription regulatory proteins that act to increase the stability and specificity of ETS interaction with its DNA recognition sequence and increase efficiency of transcriptional activation (Crepieux et al., 1994; Sharrocks et al., 2001). Therefore, it is possible that ETV2 does indeed function to regulate transcription of the erg gene (Fig. 4C), but requires additional factors to stabilize binding or to promote efficient transcription. If these additional partner proteins are themselves targets of ETV2 transcriptional activation, then they will not be synthesized in the presence of protein inhibitor and so downstream gene expression will not be observed. On the other hand, the high levels ETV2 protein present in the forced expression and induction experiments may also lead to inappropriate activation of genes. While it seems likely that induced genes would normally be responsive to ETS factors, it is not clear that they would all be regulated specifically by ETV2 in vivo.
ETV2 is required for transcription of endothelial markers but is dispensable for the myeloid and erythroid lineages
Studies of inhibition of ETV2 function in the Xenopus embryo revealed important similarities but also some significant differences from previous studies in zebrafish and mouse. First we will consider markers of the endothelial lineage. Reduction of ETV2 function in the Xenopus embryo strongly inhibited expression of the endothelial markers flk1, aplnr and erg. This inhibition was specific to the endothelial lineage since expression of erg in non-endothelial tissues, for example neural crest cells, was unaltered relative to controls (Fig. 5G, H). These observations confirm previous studies in zebrafish (Liu and Patient, 2008; Sumanas and Lin, 2006) and mouse (Lee et al., 2008), which also demonstrate that development of the endothelial lineage is completely dependent on ETV2 function. Second, our studies of ETV2 function during hematopoietic development revealed interesting differences compared to mouse and zebrafish. As shown in Fig. 5, expression of the myeloid markers, runx1 and spib, was not altered following ETV2 knockdown. Similarly, the erythroid marker, α-globin was expressed normally (Fig. 5N, O). These results contrast with observations in the mouse, where no hematopoietic cells were detected in the absence of ETV2 activity (Lee et al., 2008) and zebrafish where myeloid, but not erythroid, development was eliminated in ETV2 knockdown embryos (Sumanas et al., 2008). Based on these results, it seems likely that some other transcription factor, perhaps another member of the ETS family can substitute for ETV2 function in the hematopoietic lineage of the Xenopus embryo, but not in zebrafish or mouse.
Taken together, these studies indicate that an essential role for ETV2 in development of the endothelial lineage has been conserved in all vertebrates, but that its function for hematopoiesis differs between organisms. Further studies will be required to determine the molecular mechanisms underlying these differences.
Acknowledgments
Thanks to Roger Patient and Aldo Ciau-Uitz for providing probes and sharing data prior to publication. Thanks also to Aldo for identifying the rostral lymphatic sac. MCS is supported by an American Heart Association predoctoral grant, #09PRE2250806. CTM acknowledges support from the Bellows Foundation and National Institutes of Health Grant T3208659 (GraduateTraining in Biochemistry and Molecular Biology). PAK is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine and is supported by the Sarver Heart Center and by the NHLBI of the NIH, grants #HL74184 and HL093694.
References
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- Baltzinger M, Mager-Heckel AM, Remy P. Xl-erg: expression pattern and overexpression during development plead for a role in endothelial cell differentiation. Dev Dyn. 1999;216:420–433. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<420::AID-DVDY10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC, Randi AM. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bories JC, Willerford DM, Grévin D, Davidson L, Camus A, Martin P, Stéhelin D, Alt FW. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature. 1995;377:635–638. doi: 10.1038/377635a0. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Tonissen KF, Saha MS, Krieg PA. Neovascularization of the Xenopus embryo. Dev Dyn. 1997;210:66–77. doi: 10.1002/(SICI)1097-0177(199709)210:1<66::AID-AJA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Costa RM, Soto X, Chen Y, Zorn AM, Amaya E. spib is required for primitive myeloid development in Xenopus. Blood. 2008;112:2287–2296. doi: 10.1182/blood-2008-04-150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepieux P, Coll J, Stehelin D. The Ets family of proteins: weak modulators of gene expression in quest for transcriptional partners. Crit Rev Oncog. 1994;5:615–638. [PubMed] [Google Scholar]
- Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996;59:129–140. doi: 10.1016/0925-4773(96)00585-0. [DOI] [PubMed] [Google Scholar]
- Garriock RJ, D’Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev Biol. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Gomez GA, Veldman MB, Zhao Y, Burgess S, Lin S. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PLoS One. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gory S, Dalmon J, Prandini MH, Kortulewski T, de Launoit Y, Huber P. Requirement of a GT box (Sp1 site) and two Ets binding sites for vascular endothelial cadherin gene transcription. J Biol Chem. 1998;273:6750–6755. doi: 10.1074/jbc.273.12.6750. [DOI] [PubMed] [Google Scholar]
- Graves BJ, Petersen JM. Specificity within the ets family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Abe M, Yamazaki T, Niizeki O, Shiiba K, Sasaki I, Sato Y. Transcriptional regulation of human angiopoietin-2 by transcription factor Ets-1. Biochem Biophys Res Commun. 2004;316:52–58. doi: 10.1016/j.bbrc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iljin K, Dube A, Kontusaari S, Korhonen J, Lahtinen I, Oettgen P, Alitalo K. Role of ets factors in the activity and endothelial cell specificity of the mouse Tie gene promoter. FASEB J. 1999;13:377–386. doi: 10.1096/fasebj.13.2.377. [DOI] [PubMed] [Google Scholar]
- Kappel A, Schlaeger TM, lame I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–3085. [PubMed] [Google Scholar]
- Kolm PJ, Sive HL. Efficient hormone-inducible protein function in Xenopus laevis. Dev Biol. 1995;171:267–272. doi: 10.1006/dbio.1995.1279. [DOI] [PubMed] [Google Scholar]
- Korhonen J, Lahtinen I, Halmekytö M, Alhonen L, Jänne J, Dumont D, Alitalo K. Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood. 1995;86:1828–1835. [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelièvre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol. 2001;33:391–407. doi: 10.1016/s1357-2725(01)00025-5. [DOI] [PubMed] [Google Scholar]
- Liu F, Patient R. Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ Res. 2008;103:1147–1154. doi: 10.1161/CIRCRESAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- Loughran SJ, Kruse EA, Hacking DF, de Graaf CA, Hyland CD, Willson TA, Henley KJ, Ellis S, Voss AK, Metcalf D, Hilton DJ, Alexander WS, Kile BT. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol. 2008;9:810–819. doi: 10.1038/ni.1617. [DOI] [PubMed] [Google Scholar]
- Meadows SM, Salanga MC, Krieg PA. Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate flk1 expression during vascular development. Development. 2009;136:1115–1125. doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy N, Barton K, Leiden JM. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature. 1995;377:639–642. doi: 10.1038/377639a0. [DOI] [PubMed] [Google Scholar]
- Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Héligon C, Terclavers S, Ciesiolka M, Kälin R, Man WY, Senn I, Wyns S, Lupu F, Brändli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtgen JL, Janel N, Barek L, Duterque-Coquillaud M, Ghysdael J, Meyer D, Kerbiriou-Nabias D. Ets transcription factors bind and transactivate the core promoter of the von Willebrand factor gene. Oncogene. 1997;15:3091–3102. doi: 10.1038/sj.onc.1201502. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation in vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sharrocks D. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5562. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD, Jr, Pepling ME, Horb ME, Thomsen GH, Gergen JP. A Xenopus homologue of aml-1 reveals unexpected patterning mechanisms leading to the formation of embryonic blood. Development. 1998;125:1371–80. doi: 10.1242/dev.125.8.1371. [DOI] [PubMed] [Google Scholar]
- Wakiya K, Begue A, Stehelin D, Shibuya M. A cAMP response element and an Ets motif are involved in the transcriptional regulation of flt-1 tyrosine kinase (vascular endothelial growth factor receptor 1) gene. J Biol Chem. 1996;271:30823–30828. doi: 10.1074/jbc.271.48.30823. [DOI] [PubMed] [Google Scholar]
- Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- Yasuo H, Lemaire P. A two-step model for the fate determination of presumptive endodermal blastomeres in Xenopus embryos. Curr Biol. 1999;9:869–879. doi: 10.1016/s0960-9822(99)80391-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, Henkel GW, Maki RA, Werb Z, Oshima RG. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]