Abstract
Psychopathy is a constellation of self-serving attitudes and antisocial behaviors with little regard to cost to self and others. Might this symptomatology arise in part from an exaggerated response of brain motivational circuitry to prospective rewards? We examined whether psychopathic tendencies are associated with increased recruitment of incentive neurocircuitry during anticipation of instrumental and conditioned rewards. Healthy controls completed the Psychopathic Personality Inventory (PPI), then were presented with response-contingent and passively-delivered rewards during functional MRI. PPI scores correlated negatively with reaction time to incentivized targets, but not with reaction time to non-incentivized targets. PPI scores also correlated positively with recruitment of ventral striatum and anterior cingulate cortex during reward instrumental anticipation. PPI scores also correlated with middle frontal cortex recruitment during anticipation of passively-received rewards. These data indicate that in psychiatrically-healthy controls, individuals with greater endorsement of psychopathic tendencies show more robust neurophysiological and behavioral signatures of incentive motivation.
Keywords: Reward, psychopathy, incentive motivation, fMRI, instrumental behavior
INTRODUCTION
Antisocial behavior, whether manifested in conduct disorder (CD), antisocial personality disorder (ASPD) or psychopathy, incurs a significant societal burden in violence and crime. Of critical research interest, therefore, is characterizing how various syndromes of impaired behavior control are instantiated in the brain. A recent series of neuroimaging experiments have suggested that proclivity for impulsivity or risk-taking correlated positively with the dynamic reactivity of the brain’s mesolimbic incentive neurocircuitry (notably the ventral striatum, VS) to cues for rewards, or deliveries of rewards (Bjork, Chen, Smith, & Hommer, 2010; Bjork, Knutson, & Hommer, 2008; Bjork, Smith, Chen, & Hommer, 2011; Buckholtz et al., 2010; Engelmann & Tamir, 2009; Hariri et al., 2006), as assessed with functional magnetic resonance imaging (fMRI).
This positive directionality may explain, for example, why subjects diagnosed with an externalizing behavior or substance use disorder show increased behavioral sensitivity to rewards relative to punishments in laboratory choice tasks (Bechara, Dolan, & Hindes, 2002; Newman & Wallace, 1993). For example, both boys selected for high levels of psychopathic traits (Blair, Colledge, & Mitchell, 2001) as well as adult psychopaths (Mitchell, Colledge, Leonard, & Blair, 2002) perseverated on selections of disadvantageous decks (larger potential rewards laden with potential for disproportionately large penalties) on the Iowa Gambling Task (IGT) (Bechara, Damasio, Tranel, & Damasio, 1997) compared to non-psychopaths, but did not show aberrant set-shifting behavior in a task devoid of rewards and punishments. Moreover, whereas adult psychopathic offenders did not show increased commission errors compared to nonpsychopathic offenders in a go-nogo discrimination task when punishment for incorrect responses was the only feedback to train associations, addition of a monetary reward contingency in the task disrupted the ability of psychopathic offenders to learn go and nogo stimuli, resulting in increased commission errors (Newman & Kosson, 1986).
How might this behavioral attunement to prospective rewards in persons with higher psychopathic tendencies be instantiated in the brain? Obtaining brain signatures of implicit valuation using fMRI may be uniquely useful if there is some disconnect between implicit valuation and overt behavioral or questionnaire measures of valuation, such as if a subject is not capable of accurately reporting incentive valuation due to deficiencies in cognition or other pathologies, or due to the coarseness and subjectivity of self-report measures. For example, in adolescents, VS responses to music clips was a better predictor of their commercial sales than the adolescents’ overt likeability ratings of the clips (Berns & Moore, In press). Similarly, correlations between VS activation by the discontinuation of painful stimuli (a negatively-framed “reward”) and self-reported pain relief differed between chronic pain patients and controls, (Baliki, Geha, Fields, & Apkarian, 2010) in effect distinguishing the subjects based on a brain signal marker.
In this report, we explored whether increased brain activation by rewards would also be characteristic of psychopathic tendencies, where psychopathy is a constellation of both impulsive and premeditated antisocial “devil-may-care” behaviors and attitudes (R. D. Hare, 2006; R. D. Hare & Neumann, 2005). Greater brain sensitivity to potential rewards in persons with greater psychopathic tendencies would provide a partial account for willingness to engage in antisocial or predatory behavior for personal gain (despite deleterious effects on others). Indeed, (Buckholtz, et al., 2010) recently reported that scores on the Impulsive Antisociality factor of the Psychopathic Personality Inventory (PPI) (Lilienfeld & Andrews, 1996) correlated positively with recruitment of the ventral striatum (VS) by anticipatory cues for winning money (20¢-$5 per trial) during fMRI, as well as with VS dopaminergic responses to reward anticipation during positron emission tomography. Notably, the PPI features several items pertinent to obtaining rewards or advancing personal agendas, such as: “I quickly become very annoyed at people who do not give me what I want”, “I generally prefer to act first and think later,” “I always look out for my own interests before worrying about those of the other guy,” and “I’m good at flattering important people when it’s useful to do so.”
We investigated whether PPI scores would correlate positively with individual differences in VS recruitment during anticipation of both instrumental and passive rewards. Notably, the (Buckholtz, et al., 2010) experiment assessed mesolimbic recruitment by the anticipation of solely instrumental rewards, such that the affective components of anticipatory activation of the VS were confounded with motor-preparatory components of the instrumental behavior. We recently reported that individual differences in questionnaire impulsivity correlated positively with reward-anticipatory recruitment of the VS after the instrumental response was completed (Bjork, Smith, Chen, & Hommer, In Press), indicating a potential utility of a brain signal that tracks the anticipation of reward delivery itself. Would a tally of psychopathic behavioral tendencies and attitudes also correlate with mesolimbic anticipatory responses to cues for rewards with no response requirement at all? If so, this would suggest that greater instrumental reward-anticipatory activation of the striatum in persons with psychopathic tendencies is driven by enhanced valuation of the reward prospects themselves- cognitively “upstream” of the response mobilization.
We administered the PPI to healthy controls and administered a factorial reward anticipation (FRA) task during fMRI. The FRA task is a modified monetary incentive delay (MID) task (Knutson, Adams, Fong, & Hommer, 2001) designed to probe mesolimbic activation by anticipation of potential reward versus non-reward, under each of Pavlovian and instrumental conditions (Bjork & Hommer, 2007). We hypothesized that: 1) we would replicate the ((Buckholtz, et al., 2010) positive correlation between PPI scores and VS recruitment during anticipation of instrumental rewards, and 2) PPI scores would also correlate with recruitment of VS and anterior middle frontal cortex incentive valuation neurocircuitry (Lim, O’Doherty, & Rangel, 2011) during anticipation of passively-received rewards.
METHODS
Additional methods details are in Supplemental Methods.
Participants
All procedures were reviewed and approved by the NIAAA Institutional Review Board. Participants (n = 31; 18 males; age 22–43 (mean 31.0)) had been scanned as part of a pilot comparison of brain activation elicited by three variants of the FRA task (see below) who also completed the PPI as part of an unrelated personality study. Participants were recruited from community advertisement, and participated with written informed consent. All were deemed medically healthy in physical examination and serum chemistry panels, with no histories of significant medical illness or neurological abnormalities revealed in medical history interviews. In addition, no subject indicated a history of psychiatric disorders in a structured clinical interview for DSM-IV. Drug and alcohol abstinence was verified by urine toxicology and breath-alcohol testing.
The Factorial Reward Anticipation (FRA) task variants
Standard FRA task (FRA-standard; 17 participants)
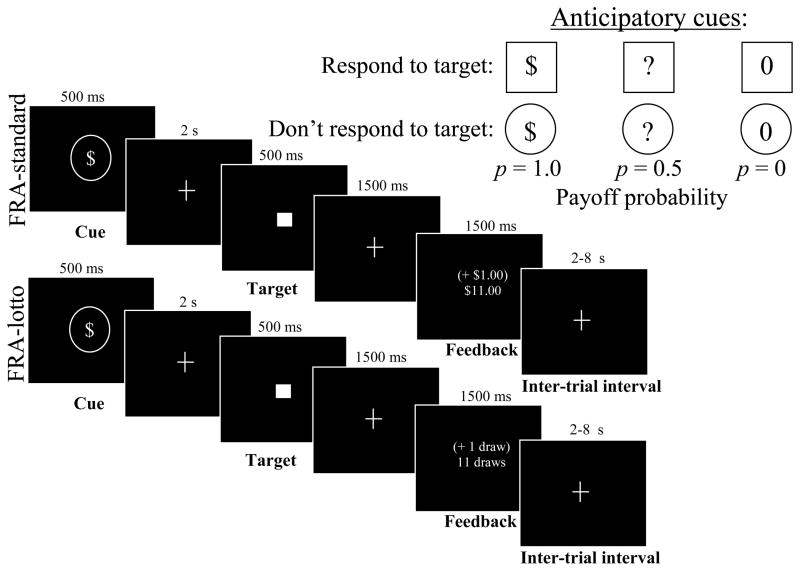
This task presented the subject with 18 instances of each of six trial types, where trial types were defined by the factorial combination of the probability of winning money in the trial (three levels: no chance, 50% chance, or 100% chance), with the presence or absence of a requirement to emit a motor response for trial success (two levels: instrumental and passive). Trials were pseudorandomized in order or presentation, and were separated by a jittered inter-trial interval. Each trial featured an anticipatory cue, a target, and trial feedback (Figure 1, top). Response trials (square cues) required the subject to respond on a button box while the subsequent target was presented. Squares enclosing a “$” (square-$ trials), a “?” (square-? trials), or a “0” (square-0 trials) signaled 1.0, 0.5, and 0 probabilities, respectively, of winning $1 for hitting the target. Non-response trials (circle cue series) instructed the subject to withhold a response to the target. Circles enclosing a “$” (circle$ trials), a “?” (circle-? trials), or a “0”(circle-0 trials) indicated 1.0, 0.5, and 0 probabilities, respectively, of automatic receipt of $1 after the target was presented. The lengthy (500 ms) target presentations enabled a successful response if subjects were attending to the task. During feedback, current trial and cumulative winnings were presented. A perfect performance would result in $54 in winnings. Prior to entry into the scanning room, each subject was read an instruction script about the response requirements and reward probabilities signaled by the six anticipatory cues. The subject was also shown an envelope stuffed with the cash he or she could win. This was followed by a 4-minute practice session.
Figure 1.
Factorial Reward Anticipation (FRA) task variants. Trials lasted 6 s, and were separated by jittered intervals of 2–8 s. Subjects first saw one of six cues indicating whether the subject needed to respond (squares) or to not respond (circles) to the target that followed, and what the consequences of the trial could be. In response trials, subjects were required to respond to the target during its 500 ms presentation. In the standard task variant (top panel series), the cue signaled a certain gain of $1 (square-$), a 50% chance of a $1 gain (square-?), or no gain (square-0) for hitting the target. In non-response trials, subjects were not to respond to the targets, but anticipated the identical range of signaled gain probabilities. Each trial concluded with 1.5 s of feedback of gain or non-gain. The FRA-UP variant of this task was identical, except that subjects were not told the exact probability of the uncertain (?-cue) payoffs. In the FRA-lotto variant (bottom panel series), the fixed $1 reward was replaced by a random marble draw worth a mean value of ~$1.
FRA with uninstructed probabilities (FRA-UP; 9 participants)
This variant was identical to the FRA-standard in all respects, except that subjects were not instructed about the exact payoff probability signaled by the square-? and circle-? cues in the instruction script. The participant could deduce over time (across square-? and circle-? trials) that their payoff chance was 50-50.
FRA with skewed distribution of trial payoffs (FRA-lotto; 5 participants)
In this variant, the uniform $1 reward was replaced with a promised marble “draw,” (Figure 1, bottom) which signified the eligibility to draw a colored marble out of a bag for winnings after the scan, where different colored marbles signified payoff amounts from 25¢ to $50. The skewed distribution of marble-colors was such that the mean payoff of a marble draw was approximately $1 to maintain the same expected value as with the other FRA variants, but with exceptionally high-magnitude potential payoffs. Subjects were read a similar instruction script as with FRA-standard before scanning, but with “a draw” replacing “a dollar.” Subjects also were shown the envelope with cash, and practiced drawing a marble from the bag. After scanning, the subject drew his or her marbles from the bag and was paid accordingly.
Psychopathic Personality Inventory (PPI)
The PPI was administered during the participant mental and physical screening visit (prior to fMRI scanning). The PPI was designed to assess psychopathic traits in non-forensic, community samples (Lilienfeld & Andrews, 1996), but also detected high scorers on the Psychopathy Checklist-Revised (R. D. Hare, 1991) (i.e. “true” psychopaths) with 86% accuracy in an incarcerated sample (Polythress, Edens, & Lilienfeld, 1998). The PPI subscales have been factor analyzed into two subscales (Benning, Patrick, Hicks, Blonigen, & Krueger, 2003): Impulsive Antisociality (PPI-IA) with item content pertaining to impulsive acts and attitudes, and Fearless Dominance (PPI-FD) with item content pertaining to (lack of) empathy or emotionality.
fMRI data collection and analysis
fMRI acquisition
We used a 3 T scanner (General Electric, Milwaukee, WI) and a quadrature head coil. Each subject’s head was immobilized by a deflatable head restraint. We collected 24 3.8-mm-thick axial slices with a 1 mm interslice gap. In-plane resolution was 3.75 × 3.75 mm. Functional scans were acquired using a T2*-sensitive echoplanar sequence with a repetition time (TR) = 2000 msec, echo time (TE) =40 msec, flip = 90°. After the task, high resolution structural scan were acquired using a T1-weighted MP-RAGE sequence (TR, 100 msec; TE, 7 msec; flip, 90°) for co-registration of functional data to anatomical brain landmarks.
FMRI pre-processing
Blood Oxygen-Level Dependent (BOLD) signal was analyzed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Time-series datasets were time-shifted to approximate simultaneous slice acquisition, warped into Talairach space as 3.75mm isotropic voxels, corrected for head motion, and spatially smoothed to a uniform 8mm full-width half maximum in brain voxels. Processed time series were then modeled with canonical gammavariate hemodynamic responses time-locked to anticipatory cues of the FRA task to assess anticipatory activation. These idealized hemodynamic responses were scaled so that observed beta weights (partial correlations) would reflect percent-signal-change. The drifting effect in the signal was fitted with extended polynomials for each run.
fMRI statistical analysis
In addition to the modeled anticipatory hemodynamic responses, outcome notifications and residual head motion following volume correction were also entered into the model as events/covariates of no interest. To calculate contrast-based activation akin to that used in (Buckholtz, et al., 2010), we performed a linear contrast (hereafter “contrast”) between anticipation of responding for a positive expected value (EV; i.e. potentially-rewarding square-$ and square-? trials) versus zero EV (square-0 trials) in the response trials. We also calculated this same contrast (circle-$ and circle-? trials versus circle-0 trials) in the non-response trial series. We also calculated a higher-order contrast: activation during anticipation of responding for potential reward versus for no reward, where this contrast was then subtracted by the contrast of anticipating passive receipt of potential reward versus nonreward.
Maps of the correlation between PPI scores and task contrast activation were calculated using ANFI module 3dMEMA (http://afni.nimh.nih.gov/sscc/gangc/MEMA.html), which uses a linear mixed-effects multilevel model that incorporates both within-subject and cross-subjects variability. This analysis thus controlled for any differences in brain activation as a function of which FRA task variant was used. Voxelwise correlations between PPI and contrast activations are reported only after survival of family-wise error (FWE) rate correction to a type 1 error P < .05.
Volume of interest (VOI) analysis
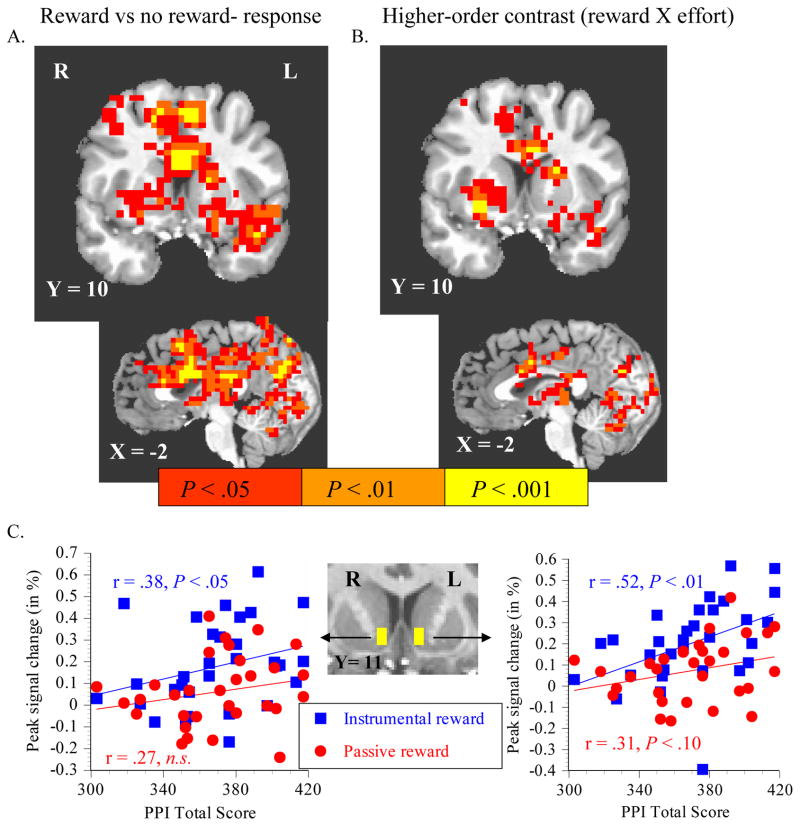
We characterized whether activation by FRA anticipatory cue types singly correlated with psychopathy. We extracted peak modeled signal change (~6 s lag) in the nucleus accumbens (NAcc), using masks anatomically localized a priori at the ventromesial junction of caudate and putamen (Haber & Knutson, 2010) (Figure 2, part C, inset). Each subject’s hemodynamic responses were averaged by anticipatory cue type, modeled and corrected for low-frequency baseline drifts, and passed through the NAcc masks.
Figure 2.
Correlation between PPI total scores and brain activation. Across all FRA task variants, there was a positive voxelwise correlation between PPI-total scores and activation by anticipation of potential reward (p = 1.0 and p = 0.5) versus nonreward (p = 0) (A). PPI-total scores also correlated with activation by the higher-order combination of instrumental reward versus nonreward anticipation, where passive reward versus nonreward anticipation was masked out (B). Peak modeled signal change (~6 s lag) during anticipation of certain (p =1.0) reward for a successful instrumental response directly correlated with PPI-scores in the nucleus accumbens (NAcc) (C), with a trend for a correlation during passive anticipation of certain reward in left NAcc. All statistical maps are overlaid on a T1-weighted image at the indicated Taliarach coordinate.
Results
Task behavior
Reaction time (RT) data were not available from one FRA-lotto subject. In response trials, subjects responded significantly more slowly to zero-probability targets (0) compared to $ and ? targets (main effect (F(2,56) = 6.251, P < .01). There were no significant main or interactive effects of FRA task variant or of scanning run on RT. Omission errors in response trials and commission errors in non-response trials were too low (< 3 %) for meaningful analysis.
Correlation of PPI scores with task behavior and brain activation
Because there was a strong within-subject correlation between PPI-IA and PPI-FD factor scores (n = 31; Spearman r = .782, P < .0001), our analysis simply focused on PPI total scores. The distributions of PPI total scores between participants assigned the different task variants essentially overlapped (ANOVA P > .9) (Fig S1). In a brain-wide 3dMEMA analysis, task activation did not significantly differ between FRA task variants1, nor did signal change in the VOI differ by task variant (see supplemental Figure S1). These similarities, coupled with how 3dMEMA accounts for any task-based variance when calculating the omnibus correlation between PPI scores and task contrast activation, enabled admixing data from the three FRA task variants.
Across all task variants, PPI total scores correlated negatively with RT to the square-$ (reward P = 1.0) targets (Spearman r = −.363, P < .05), with a trend toward a negative correlation with RT to the square-? (reward P = .5) targets (Spearman r = −.308, P < .10). PPI total scores did not correlate with RT to nonincentive (square-0) targets (Spearman r = −.116, n.s.).
As hypothesized, there was a significant positive voxelwise correlation (covariate effect) between PPI total scores and activation by anticipation of responding for potential reward (square-$ plus square-? trials) versus responding for non-reward (square-0) in several large voxel clusters (Figure 2, part A). Total PPI scores also correlated with activation in large clusters by the higher-order contrast between the instrumental reward anticipation contrast masked by the passive reward anticipation contrast (Figure 2, part B). These correlation maps were roughly similar when PPI-IA factor scores (but not PPI-FD) were substituted for PPI-total scores (supplemental Figure 2). In nonresponse trials, PPI scores correlated positively with reward-versus-nonreward activation of the mesial frontal cortex (Figure 3, part A). These PPI-correlated activations spatially exceeded the 60 shared-face voxels required to yield an adjusted type 1 error rate of P < .05 for the observed voxelwise P < .025 threshold. For illustration purposes, all clustered voxels meeting an omnibus P < .05 threshold are illuminated in figures
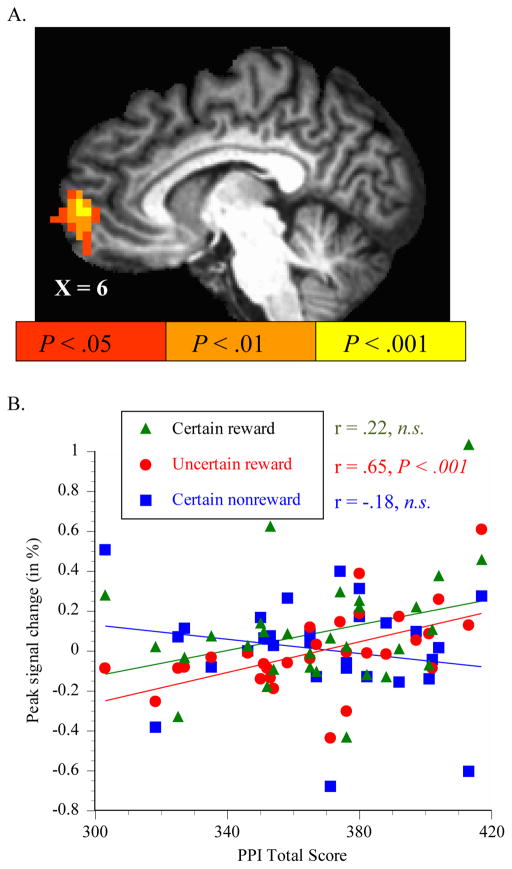
Figure 3.
Correlation between PPI total scores and anterior mesofrontal cortex (mFC) recruitment during anticipation of passively-delivered rewards. PPI-total scores correlated positively with anterior mFC recruitment by the contrast between anticipation of certain (p = 1.0) and uncertain (p = 0.5) delivery of rewards with no response requirement (A). A post hoc investigation of peak modeled hemodynamic responses to each nonresponse trial type singly indicated that this contrast was primarily driven by higher mFC responses to uncertain potential rewards in high PPI scorers (B).
In the VOI analysis, peak signal change elicited by cues to respond for certain (P = 1.0) rewards (Square-$) correlated with PPI total scores in both left and right NAcc (Figure 2, part C), with a trend toward a correlation between PPI total scores and cues for passive receipt of certain reward in the left NAcc. PPI total scores also correlated with activation by cues to respond for uncertain (P = .5) reward (Square-?) in the right NAcc (Spearman r = .433, P < .05), with a trend in left NAcc (Spearman r = .331, P < .10). Finally, PPI scores also correlated with activation by cues to respond for no (P = 0) reward (square-0) in left NAcc (Spearman r = .356, P < .05), but not right NAcc (Spearman r = .071, n.s.).
A post hoc VOI analysis of signal change in nonresponse trials characterized which trial types(s) drove the correlation between mFC contrast activation and PPI scores. This indicated that the positive correlation in mFC resulted from a combination of both higher signal increase anticipating rewards (notably anticipation of uncertain payoffs) in subjects with higher PPI scores, and greater deactivation anticipating nonrewards in subjects with higher PPI scores (Figure 3, part B).
DISCUSSION
We replicated the positive correlation (in control participants) between psychopathic tendencies and VS recruitment by cues to respond for instrumental rewards recently reported by (Buckholtz, et al., 2010). In addition, we also detected PPI-correlated activation in posterior mesofrontal and mesial occipital cortices-- regions consistently recruited by visual signals of prospective instrumental rewards in several paradigms. Conversely, there were no voxel clusters that showed a negative correlation with PPI, even at a relaxed threshold. In the NAcc, PPI scores correlated positively with activation during anticipation of responding to both incentivized and non-incentivized targets, also suggesting increased motivation with increasing PPI. Finally, PPI total scores correlated inversely with reaction time to incentivized, but not non-incentivized targets, providing a behavioral indicator of increased specific motivation to respond for reward in high PPI scorers. These findings are in accord with other reports of a positive correlation between mesolimbic incentive neurocircuitry recruitment by rewards during fMRI and questionnaire or behavioral measures of impulsivity or risk-taking (Bjork, Chen, et al., 2010; Bjork, et al., 2008; Bjork, et al., 2011; Engelmann & Tamir, 2009; Hariri, et al., 2006; Tobler, O’Doherty, Dolan, & Schultz, 2007).
A novel finding here was a relation between psychopathic tendencies and mesolimbic recruitment by passively-received rewards. Critically, the region of anterior mFC showing the correlation spatially overlaps with mFC regions activated in previous experiments on the integration of the expected or experienced value of several kinds of rewards- such as foods (T. A. Hare, Camerer, & Rangel, 2009), odors (Rolls, Grabenhorst, & Parris, 2010), small-immediate versus larger-delayed rewards (Kable & Glimcher, 2007), and perceived probability of winning money (Knutson & Cooper, 2005). In the VS, we found a positive correlation between PPI scores and activation during anticipation of uncertain passive rewards and PPI scores in right NAcc, and a trend toward a correlation in the left NAcc. These correlations collectively suggest that higher levels of psychopathic tendencies are characterized by greater implicit valuation of potential rewards, where this valuation is either irrespective of instrumental effort requirement, or may in fact promote instrumental effort.
A controversial issue, however, is whether milder degrees of psychopathic traits lie on the same dimensional and mechanistic spectrum as full-blown psychopathy, especially n community controls. Some theorists have argued that clinical psychopaths may be organically and psychologically distinct (from the ostensibly milder forms suggested by questionnaires) (Harris, Skilling, & Rice, 2001). Indeed, whereas Ted Bundy and Hannibal Lecter define real-world and cinematic extremes of psychopathy, our subjects were not psychopaths in the forensic psychiatric sense, but were healthy controls, who had PPI scores ranging from 303–417, with a mean of 368, less than the mean PPI score of 385 in a mixed accumulated sample of offenders with and without psychopathy (Scott Lilienfeld, personal communication).
Other evidence from forensic populations, however, indicates that psychopathic tendencies are indeed dimensional (Edens, Marcus, Lilienfeld, & Poythress, 2006). We note too that a “psychopath” as defined by the Hare Psychopathy Checklist (R. D. Hare, 1991) is defined as a respondent scoring at or above a cutpoint on a dimensional tally of symptom items. Moreover, in a student sample, basal cortisol itself (where reduced physiological arousal is ostensibly a biomarker of psychopathy) inversely correlated with selection of disadvantageous card choices in the IGT (van Honk, Schutter, Hermans, & Putman, 2003), suggesting a gradient of bias toward reward-approach versus punishment avoidance. Finally, we contend also that psychopathic tendencies must be dimensional because the brain is dimensional. Brain function results from gradients of synaptic connections, neurotransmitter release and degradation in its connections between regions that integrate incentive valuation, theory of mind (i.e. what another person is thinking or feeling), mental representation of future self with reward or with punishment, as well as connections that infuse emotional meaning and somatic markers of stimuli.
Further research could ultimately resolve whether endorsement of behaviors and attitudes suggestive of psychopathic tendencies in community samples represent a milder form of “true” psychopathy. First, expanded cross-sectional studies that include forensic samples could determine whether the relationship between psychopathic traits and mesolimbic recruitment by rewards remains linear and positive across the whole range of psychopathy. An inverted-U-shaped relationship between reward-elicited mesolimbic activity and psychopathy across a fuller range would argue against (or at least complicate) the dimensional approach. Second, collection of physiological markers of arousal during incentive processing could concurrently capture psychophysiological responses to evocative stimuli, where deficient recruitment of these responses is ostensibly characteristic of psychopaths. For example, if increased physiological arousal as a function of increasing reward prospects was detected in tandem with increasing elicited brain activation in community samples but not in forensic psychopaths, this would also argue against a unitary dimensionality. Accordingly, blunted physiological activation by increasing reward prospects in tandem with greater mesolimbic activation could actually serve as a combined biomarker of true psychopathy, where psychopaths retain computation-based (Knutson, Taylor, Kaufman, Peterson, & Glover, 2005) activation of mFC and VS with increasing reward prospects, yet remain devoid of emotional components of reward processing.
Regardless of whether high scorers or the PPI other measures of psychopath-like tendencies lie on the same dimension as the forensic psychopath, or whether their increased brain activation with reward results from some other mechanism, we find it interesting that individual differences in these self-interested behaviors correlate with how the motivational neurocircuitry of the brain reacts to prospective rewards. These data may hint at why neurobiological features conferring risk for psychopathy have been evolutionarily conserved and not eliminated. Notably, some psychopathic traits in moderate degrees may be beneficial. For example, Benning et al (Benning, Patrick, Salekin, & Leistico, 2005) reported that scores on the Fearless Dominance factor of the Psychopathic Personality Inventory (PPI) (Lilienfeld & Andrews, 1996) correlated negatively with personality disorder symptoms and neuroticism. Moreover, in some contexts such as business (Babiak, Neumann, & Hare, 2010) or politics, willingness to take risks, indifference to withering criticism, or skill in manipulating others may yield tremendous benefit.
Finally, we note that a key limitation of this preliminary study was the admixture of different FRA task variants to derive the dataset for correlation with PPI scores. We suggest, however, that the absence of significant differences in mesolimbic recruitment, reaction time and PPI scores between the three FRA task variants, coupled with statistical control in MEMA of any extant task-based activation differences, supported task admixture for assessment of PPI correlations. In conclusion, these data and other data (Buckholtz, et al., 2010) linking psychopathic tendencies to increased brain reward functioning extend previous reports linking brain reward responsiveness to other manifestations of disinhibition, including risk-taking personality (Bjork, et al., 2008), laboratory impulsivity (Hariri, et al., 2006), or externalizing disorders (Bjork, Chen, et al., 2010), and provides a starting point for more comprehensive cross-sectional studies that include more severe psychopathic populations, as well as for longitudinal studies linking brain reward function at baseline to real-life psychosocial outcomes.
Supplementary Material
Highlights.
Previous research indicates that impulsive persons have greater brain activation by rewards
We scanned controls with an fMRI task that probes mesolimbic activation by instrumental and passive rewards
Psychopathic tendencies correlated negatively with reaction time to targets to win rewards
Psychopathic tendencies correlated positively with mesolimbic recruitment by both instrumental and passive rewards
Psychopathy as a psychological construct may feature increased valuation of rewards
Acknowledgments
This research was sponsored by intramural research funds of the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Activation by FRA task contrasts themselves were originally presented in Bjork, J. M., & Hommer, D. W. (2007). Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behav Brain Res, 177(1), 165-170. and are not re-presented here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babiak P, Neumann CS, Hare RD. Corporate psychopathy: Talking the walk. Behav Sci Law. 2010;28(2):174–193. doi: 10.1002/bsl.925. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40(10):1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the psychopathic personality inventory: validity and implications for clinical assessment. Psychol Assess. 2003;15(3):340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Salekin RT, Leistico AM. Convergent and discriminant validity of psychopathy factors assessed via self-report: a comparison of three instruments. Assessment. 2005;12(3):270–289. doi: 10.1177/1073191105277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Moore SE. A neural predictor of cultural popularity. Jounal of Consumer Psychology (In press) [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry. 2010;51(7):827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behav Brain Res. 2007;177(1):165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103(8):1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Psychosocial problems and recruitment of incentive neurocircuitry: Exploring individual differences in healthy adolescents. Developmental Cognitive Neuroscience. 2011;1(4):570–577. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: Effort anticipation, reward anticipation, and reward delivery. Hum Brain Mapp. doi: 10.1002/hbm.21351. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol. 2001;29(6):499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13(4):419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Molko N, Cohen L, Wilson AJ. Arithmetic and the brain. Curr Opin Neurobiol. 2004;14(2):218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Lilienfeld SO, Poythress NG., Jr Psychopathic, not psychopath: taxometric evidence for the dimensional structure of psychopathy. J Abnorm Psychol. 2006;115(1):131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, William Deakin JF. Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. Neuroimage. 2004;21(3):984–990. doi: 10.1016/j.neuroimage.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Tamir D. Individual differences in risk preference predict neural responses during financial decision-making. Brain Res. 2009;1290:28–51. doi: 10.1016/j.brainres.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised Manual. Toronto: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD. Psychopathy: a clinical and forensic overview. Psychiatr Clin North Am. 2006;29(3):709–724. doi: 10.1016/j.psc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hare RD, Neumann CS. Structural models of psychopathy. Curr Psychiatry Rep. 2005;7(1):57–64. doi: 10.1007/s11920-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26(51):13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GT, Skilling T, Rice ME. The construct of psychopathy. Crime and Justice. 2001;28:197–264. [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. J Pers Assess. 1996;66(3):488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lim SL, O’Doherty JP, Rangel A. The Decision Value Computations in the vmPFC and Striatum Use a Relative Value Code That is Guided by Visual Attention. J Neurosci. 2011;31(37):13214–13223. doi: 10.1523/JNEUROSCI.1246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Colledge E, Leonard A, Blair RJ. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40(12):2013–2022. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol. 1986;95(3):252–256. [PubMed] [Google Scholar]
- Newman JP, Wallace JF. Diverse pathways to deficient self-regulation: Implications for disinhibitory psychopathology in children. Clin Psychol Rev. 1993;13:699–720. [Google Scholar]
- Polythress NG, Edens JF, Lilienfeld SO. Criterion-related validity of the psychopathic personality inventory in a prison sample. Psychol Assess. 1998;10(4):426–430. [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Neural systems underlying decisions about affective odors. J Cogn Neurosci. 2010;22(5):1069–1082. doi: 10.1162/jocn.2009.21231. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97(2):1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14:1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pasher H. Puzzingly High Correlations in fMRI studies of Emotion, Personality, and Social Cognitition. Perspectives on Psychological Science. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.