Abstract
Polycystic kidney disease (PKD) in mice can arise from defects in Nek kinases, which participate in ciliogenesis. PKD can also arise from loss of the protein TAZ, an adaptor protein in the E3 ubiquitin ligase complex that targets the ciliary protein polycystin 2 (PC2) for degradation, but whether Nek and TAZ contribute to the same biochemical pathway is unknown. Here, we report that the nimA-related protein kinase Nek1 phosphorylates TAZ at a site essential for the ubiquitination and proteasomal degradation of PC2. Loss of Nek1 leads to underphosphorylation of TAZ, thereby promoting the abnormal accumulation of PC2. Furthermore, TAZ targets Nek1 for degradation. These data suggest that TAZ and Nek1 constitute a negative feedback loop linked through phosphorylation and ubiquitination and that the interaction of Nek1 and TAZ maintain PC2 at the level needed for proper ciliogenesis.
Serine/threonine protein kinases of the Nek family are related to the kinase nimA (never in mitosis A) in Apergillus nidulans.1 Overexpression of nimA in vertebrate cells causes premature entry into mitosis; a dominant-negative mutant causes G2 arrest.2 Nek kinases function in the formation of the primary cilium in many eukaryotes.3–8 They also play roles in DNA repair9 and apoptosis.10 Spontaneous mutations in genes encoding two members of the Nek family give rise to autosomal recessive forms of polycystic kidney disease (PKD) in the mouse. Nek1 is defective in the kat and kat2J models,11–13 and Nek8 is defective in the jck model.14–16 Nek1 is associated with primary cilia and centrosomes. It plays a role in ciliogenesis and in regulating the stability of centrosomes.5,17 Overexpression of Nek1 inhibits the growth of cilia.6 Nek1 interacts with proteins involved in cell cycle control and DNA repair as well as with the kinesin protein KIF3A with implications in the development of PKD.18,19 Nek8 has not been shown thus far to be catalytically active. Nek8 nevertheless regulates the expression and localization of the polycystins4,20 and has been linked to medullary cystic kidney disease (nephronopthisis).8 Knockdown of Nek8 in zebrafish embryos results in cystic kidney.16
Independent investigations have shown that knockouts of TAZ (Wwtr1) result in the development of PKD.21–23 TAZ has multiple molecular functions with pleiotropic effects. Originally identified as a 14 to 3–3 binding protein, TAZ was described as a transcriptional co-activator with PDZ-binding domain.24 Investigations of a TAZ knockout mouse subsequently showed that TAZ also serves as an adaptor protein in a SCFβ-Trcp E3 ubiquitin ligase complex that targets polycystin 2 (PC2) for degradation.21 TAZ deficiency results in PC2 accumulation. PKD in the TAZ knockout mouse is inherited as an autosomal recessive condition and is associated with partial embryonic lethality. Knockdown of TAZ in zebrafish results in development of cystic kidney accompanied by overexpression of PC2.21 TAZ thus functions in an evolutionarily conserved pathway essential for maintaining normal levels of PC2. Its role in PC2 degradation is dependent on phosphorylation, but the kinase(s) that mediates this role has not been identified.
TAZ undergoes phosphorylation at multiple sites. Seven serines and one threonine were found to be phosphorylated on a GST-TAZ fusion protein purified from 293T cells and analyzed by mass spectrometry (Supplementary Figure 1). The sites include S306 and S309 located in the C-terminal phosphodegron essential for PC2 degradation. Although both serines are important for binding to β-Trcp in the TAZ-E3 ligase complex, phosphorylation of S309 has the major effect in mediating PC2 turnover.21 To aid identification of a kinase that phosphorylates TAZ at this site, an antiserum was raised against a phosphopeptide containing pS309 as the single phosphoamino acid: –KKGGPYHSREQSTDpSGLG– (residues 295 to 312 in mouse TAZ; phosphodegron underlined). The antiserum was affinity purified and tested on extracts of 293T cells transfected with wild-type TAZ or mutant TAZ-S309A. A TAZ antibody (nonphosphospecific) recognized multiple species of TAZ in both extracts. The anti-pTAZ antibody recognized a major and several minor bands in the wild-type extract but showed little reactivity in the mutant extract (Supplementary Figure 2A). Preadsorption with phosphopeptide removed most of the reactivity in the wild-type extract whereas preadsorption with unphosphorylated peptide still allowed recognition of several species. The affinity-purified antibody is thus highly specific for TAZ bearing pS309 (Supplementary Figure 2B).
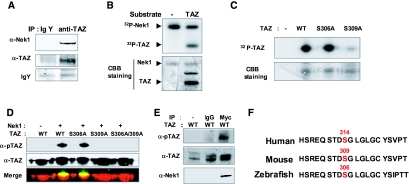
To determine whether Nek1 phosphorylates TAZ, evidence was first sought for interaction between the endogenous proteins in extracts of normal mouse kidney. Interaction was confirmed using a chicken anti-TAZ antibody (nonphosphospecific) to immunoprecipitate and anti-Nek1 antibody to blot (Figure 1A). An in vitro kinase reaction was carried out with recombinant Nek1 (GST-tagged catalytic fragment, from Invitrogen), γ-32P-ATP, and a truncated form of TAZ (residues 92 to 395 lacking the N-terminal phosphodegron, expressed as a GST fusion and purified from Escherichia coli) as a substrate. Two bands were seen corresponding to phosphorylated TAZ and autophosphorylated Nek1 (Figure 1B). Using alanine substitution mutants as substrates, phosphorylation was observed with wild-type and S306A but not with S309A, indicating S309 as the site of phosphorylation of TAZ by Nek1 (Figure 1C). The anti-pTAZ antibody was used to confirm S309 as the site of phosphorylation (Figure 1D). Evidence for phosphorylation of TAZ by full-length Nek1 was obtained using Myc-tagged Nek1 from 293T cells and purified GST-TAZ (Figure 1E). The sequence of the phosphodegron in TAZ that mediates PC2 degradation is highly conserved between human, mouse, and zebrafish (Figure 1F). A recent report has shown that S311 and S314 in human TAZ (corresponding to S306 and S309 in the mouse) are phosphorylated by kinases of the Hippo tumor suppressor pathway and that these phosphorylations mediate TAZ-βTrcp interaction and TAZ turnover.25
Figure 1.
Nek1 phosphorylates TAZ on S309. (A) Immunoprecipitation was performed on lysates from mouse kidney tissues with normal IgY and anti-chicken TAZ antibody. The western blot shows the association of TAZ with Nek1. (B and C) GST-tagged C-terminal wild-type TAZ (WT), S306A mutant (S306A), and S309A mutant (S309A) were purified from E. coli. Catalytic domain of Nek1 (Invitrogen) was incubated with purified TAZ and 10 μCi [γ-32P] ATP. The samples were resolved with SDS-PAGE, stained with Coomassie brilliant blue (CBB) solution, and detected by autoradiography. (D) Nek1 was incubated with purified C-terminal WT, S306A, S309A, and a double mutant of S306A/309A along with cold ATP. Western blot was performed with anti-pTAZ and anti-TAZ. (E) Immunoprecipitation was performed with normal IgG and anti-Myc for Myc-Nek1 introduced into 293T cells. In vitro kinase was performed with the immunoprecipitated Nek1, purified wild-type TAZ, and cold ATP. The phosphorylation of TAZ was detected by immunoblot using anti-p-TAZ. (F) Conservation of sequence in the phosphodegron motif.
To investigate whether phosphorylation of TAZ on S309 affects TAZ's role in transcription, 293T cells were transfected with expression vectors for wild-type TAZ and mutant S309A. Effects on transcription of Nek1 and Pdk2 were tested along with aldolase as a negative control and connective tissue growth factor, a direct target of TAZ,26 as a positive control. Neither wild-type nor mutant TAZ induced Pkd2, Nek1, or aldolase expression, whereas both induced connective tissue growth factor, the mutant more strongly than wild-type TAZ (Supplementary Figure 3). No significant differential effect between wild-type and mutant TAZ on these target genes was observed.
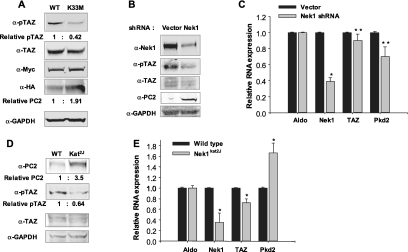
The effect of TAZ phosphorylation by Nek1 on levels of PC2 was investigated. Expression of a kinase-inactive Nek1 mutant (K33M) in 293T cells led to reduction in the level of TAZ phosphorylated on S309 with little or no effect on the overall level of TAZ compared with cells expressing wild-type Nek1 (Figure 2A). Importantly, PC2 levels were significantly lower in cells expressing catalytically active compared with inactive Nek1 (Figure 2A). Infection of normal primary mouse kidney epithelial cells with a small hairpin RNA lentivirus targeting Nek1 led to reductions in Nek1 RNA and protein. This was accompanied by decreased levels of pTAZ. PC2 protein accumulated in cells with reduced Nek1 despite reduced levels of Pkd2 RNA (Figure 2, B and C). To further confirm Nek1 as a TAZ kinase and regulator of PC2 turnover, levels of pTAZ and PC2 were compared in established renal tubular epithelial cells from wild-type and Nek1 mutant (kat2J) mice.9 Kat2J mice carry a frameshift mutation in the catalytic domain of Nek1, giving rise to a truncated inactive form of the enzyme.13 The level of pTAZ was clearly reduced and that of PC2 elevated in the Nek1 mutant compared with normal cells (Figure 2D). RNA levels for Nek1 and TAZ were lower and Pkd2 higher in the kat2J mutant cells (Figure 2E). Taken together, these results indicate that phosphorylation of TAZ on S309 by Nek1 leads to PC2 degradation and implicate the previously identified ubiquitin ligase complex containing pTAZ as mediating the destruction.21
Figure 2.
Lack of Nek1-mediated phosphorylation of TAZ leads to increased levels of PC2. (A) HA-tagged PC2 and Myc-tagged wild-type Nek1 (WT) or kinase-defective mutant of Nek1 (K33M) were expressed in 293T cells. Western blots were done with anti-HA, anti-pTAZ, anti-TAZ, anti-Myc, and anti-GAPDH antibodies. (B and C) Normal primary mouse kidney epithelial cells were infected with lentivirus targeting mouse Nek1 and nontargeting lentivirus (Vector) and selected with puromycin. (B) Five days after infection, cells were prepared for immunoblot. Nek1 depletion resulted in reduced phosphorylation of TAZ and accumulation of PC2. (C) Quantitative reverse transcription PCR (qRT-PCR) was performed for Nek1, TAZ, Pkd2, and Aldolase. *P < 0.001; **P < 0.01. (D and E) Established renal tubular epithelial cells from wild-type and Nek1 mutant (Kat2J) mice were used for immnuoblotting. (D) Western blots showed accumulation of PC2 and reduced phosphorylation of TAZ in Nek1 mutant Kat2J renal tubular epithelial cells. (E) qRT-PCR was performed for Nek1, TAZ, Pkd2, and Aldolase in wild-type and Kat2J renal tubular cells. *P < 0.005.
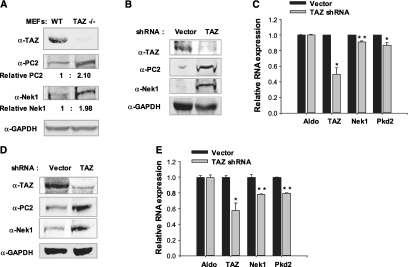
TAZ may regulate PC2 levels directly and indirectly, the latter through targeting Nek1 for degradation. This possibility was investigated first by comparing endogenous levels of Nek1 and PC2 in fibroblasts from wild-type and TAZ knockout mice. Nek1 and PC2 were present at roughly 2-fold higher levels in TAZ-deficient compared with normal fibroblasts (Figure 3A). Small hairpin RNA was used to downregulate TAZ RNA and protein in primary mouse kidney epithelial cells. This resulted in increased levels of Nek1 and PC2 protein (Figure 3B). Nek1 and PC2 mRNA levels were not significantly altered in TAZ-depleted cells (Figure 3C). Similar results were seen in wild-type embryo fibroblasts (Figure 3, D and E). These results suggest that Nek1 is targeted for degradation by TAZ.
Figure 3.
Lack of TAZ results in the accumulation of Nek1 protein but not RNA. (A) Cell lysates from wild-type or TAZ−/− mouse embryo fibroblasts were used for immnuoblotting. Western blots showed that TAZ−/− resulted in the accumulation of PC2 and Nek1. (B) Normal mouse kidney epithelial cells were infected with lentivirus targeting mouse TAZ and nontargeting lentivirus (Vector). Five days after infection, cells were prepared for western blot. (C) qRT-PCR for RNA levels of Aldolase, TAZ, Nek1, and Pkd2 in TAZ-depleted mouse kidney cells. TAZ depletion did not affect the RNA levels of Nek1 and Pkd2. *P < 0.001; **P < 0.0001. (D) Mouse embryo fibroblasts were infected with lentivirus targeting mouse TAZ and nontargeting lentivirus. Four days after infection, western blot was performed for PC2 and Nek1. (E) qRT-PCR for RNA levels of Aldolase, TAZ, Nek1, and Pkd2 in TAZ-depleted mouse embryo fibroblasts. *P < 0.001; **P < 0.01.
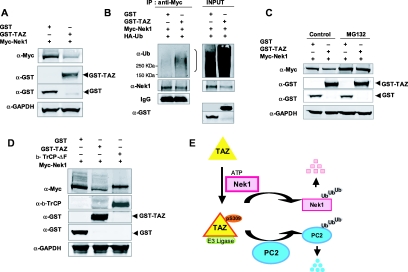
To confirm Nek1 as a target of TAZ-mediated degradation, 293T cells were transfected with Myc-tagged Nek1 along with GST-TAZ (full length) or empty GST vector. Expression of exogenous TAZ resulted in reduced levels of Nek1 (Figure 4A). Immunoprecipitation was carried out with anti-Myc antibody and blotted with anti-ubiquitin antibody using cells coexpressing HA-ubiquitin. Reduction of Nek1 in cells expressing GST-TAZ was accompanied by polyubiquitination of Nek1 (Figure 4B). Addition of the proteasome inhibitor MG132 prevented the loss of Nek1 in TAZ-transfected cells (Figure 4C). Nek1 degradation was also inhibited by coexpression of the F-box deletion mutant β-TrcpΔF (Figure 4D), previously shown to inhibit TAZ-mediated degradation of PC2.21 These results provide further evidence that TAZ targets Nek1 and PC2 in a β-Trcp-mediated degradation pathway.
Figure 4.
Nek1 and TAZ form a negative feedback loop in the regulation of PC2 levels. (A) 293T cells were transfected with Myc-tagged Nek1 along with GST or GST-TAZ. Western blot was performed for GST, Myc, and GAPDH. (B) Immunoprecipitation was performed with anti-Myc and immunoblotted for Nek1 with the cell extracts as described in Figure 4A. (C) 293T cells were cotransfected with Myc-Nek1 and GST or GST-TAZ. Forty hours after transfection, cells were treated with 10 mM MG132 for 4 hours. Western blots for Myc, GST, and GAPDH were done. (D) Myc-Nek1 was expressed along with GST, GST-tagged wild-type TAZ, or F-box deletion mutant of β-Trcp (β-Trcp-ΔF). Western blot shows that expression of β-Trcp-ΔF prevents Nek1 degradation. (E) Model of the feedback loop between Nek1 and TAZ in the regulation of PC2 (see text).
Loss of function of Nek1 or TAZ results in similar outcomes with respect to the development of PKD accompanied by abnormal accumulation of PC2. This suggests the importance of maintaining PC2 levels within a normal physiologic range. Nek1 and TAZ form a negative feedback loop that provides a mechanism for increasing or decreasing levels of PC2 (Figure 4E). Nek1 phosphorylates TAZ, activating its function in the E3 ligase leading to PC2 ubiquitination and degradation. Phosphorylated TAZ also directs ubiquitination and degradation of Nek1, resulting in less activated TAZ and allowing PC2 levels to increase. Regulation of the levels of pTAZ as a direct mediator of PC2 degradation is achieved through this loop. Linkages between phosphorylation and ubiquitination have increasingly been recognized in the control of many biologic processes.27 Results presented here showing that PC2 levels are regulated through these posttranslational modifications may offer possibilities for developing therapeutic approaches targeting these pathways in the mouse models.
CONCISE METHODS
Lentivirus-Based RNA Interference Plasmid Preparation, Virus Production, and Infection
The lentivirus-based RNA interference transfer plasmids targeting mouse Nek1 (gene access number NM_00175089) at positions 2078 to 2098 (pLKO-Puro1.-Nek1) or mouse TAZ (gene access number NM_133784) at positions 1048 to 1068 (pLKO-Puro1.-TAZ) and control plasmid (pLKO-Puro.1) were prepared and lentivirus was generated and infected as described previously.28
Immunoblot Analysis
Cells were collected and extracted in lysis buffer (0.5% Triton X-100, 20 mM Tris, pH 7.5, 2 mM magnesium chloride, 1 mM dithiothreitol, 1 mM EGTA, 50 mM β-glycerophosphate, 25 mM sodium fluoride, 1 mM sodium vanadate, 100 μg/ml phenylmethanesulfonylfluoride, and protease inhibitor cocktail [Roche, Indianapolis, IN]). After adjusting proteins quantities, proteins were separated by SDS-PAGE and blotted to nitrocellulose membranes. Anti-Nek1 (Abnova), anti-Myc (Santa Cruz), anti-GAPDH (Calbiochem), and anti-GST (Santa Cruz) were used for immunoblotting. Anti-PC2 and anti-TAZ antibodies were generated as described previously.21
Kinase Assay
For Nek1 kinase assays, the catalytic domain of Nek1 with amino acids 1 to 505 fused to a GST tag at the C-terminus was used (Invitrogen, catalog no. PV4202, lot no. 374733B; the sequence is available at http://tools.invitrogen.com/content/sfs/coapdfs/kinase/PV4202%20374733B.pdf). Myc-tagged full-length Nek1 was immunoprecipitated from 293T cells with anti-Myc (Santa Cruz) and incubated with purified GST-tagged C-terminal fragment of TAZ (amino acids 92 to 395) and 10 μCi [γ-32P] ATP or cold ATP in Kinase Buffer A (Invitrogen) at 30°C for 20 minutes. The samples were resolved by SDS-PAGE, stained with Coomassie brilliant blue, and the dried gel was subjected to autoradiography. Affinity-purified polyclonal pS309-TAZ antibody was used for immunoblotting to detect the specifically phosphorylated TAZ species.
Real-Time PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and cDNA generated from 1 μg of total RNA per sample using the QuantiTect Reverse Transcription Kit (Qiagen). Samples in triplicate were amplified using SYBR green I dye in a LightCycler 480 detection system (Roche Applied Science). The data were analyzed by the comparative CT (ΔΔCT) method and quantitated relative to the Aldolase A gene and normalized to the control. Primers used for amplification are 5′-AGCAGAATGGCATTGTACCC-3′ and 5′-TAGACATGGTGGTCGCTCAG-3′ for mouse Aldolase A, 5′-GGCCAGGGAACAAGGATGGAGGA-3′ and 5′-TGCCTTTCTGGGAGCCATCGAC-3′ for mouse Nek1, 5′-CCACGTCCGATGAGCAGCAGAAGT-3′ and 5′-ACCCAGTCGCGCATCCTCAGC-3′ for mouse Pdk2, and 5′-TGCTGGGAATCCGCTCGGGA-3′ and 5′-TGGATTGGCGCGAGTGCGAG-3′ for mouse TAZ. Error bars represent the mean ± 1 SD. The significance of differences between the experimental groups was calculated using the t test.
Immunoprecipitation Assay
TAZ or Myc-tagged Nek1 was immunoprecipitated from mouse kidney cell extracts or Myc-Nek1 expressed 293T cells with anti-TAZ chicken antibody or anti-Myc (Santa Cruz) in buffer (0.5% Triton X-100, 20 mM Tris pH 7.5, 2 mM magnesium chloride, 1 mM dithiothreitol, 1 mM EGTA, 50 mM β-glycerophosphate, 25 mM sodium fluoride, 1 mM sodium vanadate, 2 μg ml−1 leupeptin, 2 μg ml−1 pepstatin A, 100 μg ml−1 phenylmethanesulfonylfluoride, 1 μg ml−1 antipain) followed by incubation with protein A/G agarose at 4°C for 2 hours. The samples were combined with protein A/G agarose, washed with lysis buffer, and resolved by SDS-PAGE.
DISCLOSURES
None.
Acknowledgments
This work was funded by grants from the National Institutes of Health (RO1 CA-092520) and the Polycystic Kidney Disease Foundation of America (171G08b). We thank Dr. Mark C. White (Simon Fraser University, Canada) and Dr. Yumay Chen (University of California–Irvine) for the generous gifts of Nek1 plasmid and Nek1 kat2J cells, respectively. The authors acknowledge Dr. John Asara at the Beth Israel Deaconess Medical Center Mass Spectrometry Core for mass spectrometry analyses. We also acknowledge helpful discussions with Dr. David Beier and Dr. Jing Zhou at Harvard Medical School.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Tying TAZ and Nek1 into Polycystin 2 Levels,” on pages 791–793.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Osmani SA, Pu RT, Morris NR: Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell 53: 237–244, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Lu KP, Hunter T. Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell 81: 413–424, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Parker JD, Bradley BA, Mooers AO, Quarmby LM: Phylogenetic analysis of the Neks reveals early diversification of ciliary-cell cycle kinases. PLoS One 2: e1076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J: Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol 19: 469–476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahjoub MR, Trapp ML, Quarmby LM: NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol 16: 3485–3489, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Shalom O, Shalva N, Altschuler Y, Motro B: The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett 582: 1465–1470, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Quarmby LM, Mahjoub MR: Caught Nek-ing: Cilia and centrioles. J Cell Sci 118: 5161–5169, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F: NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol 19: 587–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Chen PL, Chen CF, Jiang X, Riley DJ: Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control. Cell Cycle 7: 3194–3201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Craigen WJ, Riley DJ: Nek1 regulates cell death and mitochondrial membrane permeability through phosphorylation of VDAC1. Cell Cycle 8: 257–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janaswami PM, Birkenmeier EH, Cook SA, Rowe LB, Bronson RT, Davisson MT: Identification and genetic mapping of a new polycystic kidney disease on mouse chromosome 8. Genomics 40: 101–107, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Vogler C, Homan S, Pung A, Thorpe C, Barker J, Birkenmeier EH, Upadhya P: Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J Am Soc Nephrol 10: 2534–2539, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE: Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci U S A 97: 217–221, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atala A, Freeman MR, Mandell J, Beier DR. Juvenile cystic kidneys (jck): A new mouse mutation which causes polycystic kidneys. Kidney Int 43: 1081–1085, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Iakoubova OA, Dushkin H, Beier DR: Localization of a murine recessive polycystic kidney disease mutation and modifying loci that affect disease severity. Genomics 26: 107–114, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR: A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development 129: 5839–5846, 2002 [DOI] [PubMed] [Google Scholar]
- 17. White MC, Quarmby LM: The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 9: 29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Surpili MJ, Delben TM, Kobarg J: Identification of proteins that interact with the central coiled-coil region of the human protein kinase NEK1. Biochemistry 42: 15369–15376, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LSB, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trapp ML, Galtseva A, Manning DK, Beier DR, Rosenblum ND, Quarmby LM: Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr Nephrol 23: 377–387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, Bronson R, Yaffe MB, Zhou J, Benjamin T: TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol 27: 6383–6395, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W: Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A 104: 1631–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H: Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB: TAZ: A novel transcriptional co-activator regulated by interactions with 14–3-3 and PDZ domain proteins. EMBO J 19: 6778–6791, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL: The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. J Biol Chem 285: 37159–37169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL: TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem 284: 13355–13362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunter T: The age of crosstalk: Phosphorylation, ubiquitination, and beyond. Mol Cell 28: 730–738, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Yim H, Erikson RL: Polo-like kinase 1 depletion induces DNA damage in early S prior to caspase activation. Mol Cell Biol 29: 2609–2621, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]