Abstract
High sodium intake limits the antihypertensive and antiproteinuric effects of angiotensin-converting enzyme (ACE) inhibitors in patients with CKD; however, whether dietary sodium also associates with progression to ESRD is unknown. We conducted a post hoc analysis of the first and second Ramipril Efficacy in Nephropathy trials to evaluate the association of sodium intake with proteinuria and progression to ESRD among 500 CKD patients without diabetes who were treated with ramipril (5 mg/d) and monitored with serial 24-hour urinary sodium and creatinine measurements. Urinary sodium/creatinine excretion defined low (<100 mEq/g), medium (100 to <200 mEq/g), and high (≥200 mEq/g) sodium intake. During a follow-up of >4.25 years, 92 individuals (18.4%) developed ESRD. Among those with low, medium, and high sodium intakes, the incidence of ESRD was 6.1 (95% confidence interval [95% CI], 3.8–9.7), 7.9 (95% CI, 6.1–10.2), and 18.2 (95% CI, 11.3–29.3) per 100 patient-years, respectively (P<0.001). Patients with high dietary sodium exhibited a blunted antiproteinuric effect of ACE inhibition despite similar BP among groups. Each 100-mEq/g increase in urinary sodium/creatinine excretion associated with a 1.61-fold (95% CI, 1.15–2.24) higher risk for ESRD; adjusting for baseline proteinuria attenuated this association to 1.38-fold (95% CI, 0.95–2.00). This association was independent from BP but was lost after adjusting for changes in proteinuria. In summary, among patients with CKD but without diabetes, high dietary salt (>14 g daily) seems to blunt the antiproteinuric effect of ACE inhibitor therapy and increase the risk for ESRD, independent of BP control.
Increased urinary protein excretion is a major determinant of progressive renal function loss in participants with CKD. Studies in CKD patients with and without diabetes showed that renoprotective treatments limit GFR decline and progression to ESRD to the extent they lower proteinuria, independent of BP control.1–4 These findings imply that urinary proteins should be reduced as far as possible, ideally to <1 g/d.5
Inhibitors of the renin-angiotensin system (RAS), such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), are the antihypertensive drugs that most effectively reduce urinary proteins and slow GFR decline in patients with CKD.1–3,6 The efficacy of treatment, however, is heterogeneous and dependent on inborn7 and environmental8–12 factors. Data in experimental diabetes,13,14 adriamycin nephrosis,15 uninephrectomized rats, or in Munich Wistar rats with spontaneously reduced nephron numbers16 uniformly show that expansion of the sodium pool, with glomerular hyperfiltration and activation of the renal RAS induced by enhanced sodium intake, all contribute to blunt the BP and proteinuria lowering effect of RAS inhibitors.17 Consistently, observational studies in humans showed that increased dietary sodium intake increases proteinuria and accelerates renal disease progression.18 However, no study thus far has evaluated the associations between salt intake, proteinuria, and renal disease progression in patients receiving RAS-inhibiting treatment. Hence, in this study, we evaluated the association of sodium intake with proteinuria and progression to ESRD in 500 patients with CKD retrieved from the Ramipril Efficacy in Nephropathy (REIN)1–3 and REIN-219 trials who were receiving stable ramipril therapy. Our working hypothesis was that the blunted antiproteinuric effect of RAS inhibition therapy in patients with high salt intake might translate into less effective protection against progression to ESRD. This hypothesis was based on the experimental and human evidence discussed above and arose before expectation of outcome data in our patient population.
Results
Baseline Characteristics
The 500 included participants had a mean of 5.4±2.8 urinary sodium and creatinine measurements over a follow-up of 26.2±15.6 months. Twenty-six patients (5.2%) had only one measurement. Their baseline characteristics were similar to those of patients with more measurements (data not shown). Mean urinary sodium and sodium/creatinine excretion at baseline were 177.6±72.3 mEq/24 h and 139.0±54.9 mEq/g, respectively. On the basis of their average urinary sodium/creatinine excretion during the observation period, 111, 336, and 53 patients were categorized in the low sodium diet (LSD), medium sodium diet (MSD), and high sodium diet (HSD) groups, respectively (Table 1). Sodium intake was a relatively fixed trait because patient distribution to the three sodium intake groups did not change significantly when only baseline urinary sodium/creatinine measurements were considered (P=0.442). There were more men in the LSD group than in the MSD and HSD groups and primary glomerular diseases were more frequent in the LSD group than in the MSD group. Body mass index, BP, and creatinine clearance at baseline were similar among groups, whereas urinary protein/creatinine and urea/creatinine excretion were significantly lower in the LSD and MSD groups than in the HSD group.
Table 1.
Baseline characteristics based on sodium diet groups
| Sodium Diet Group | |||
|---|---|---|---|
| LSD (n=111) | MSD (n=336) | HSD (n=53) | |
| Demographics | |||
| men, n (%) | 100 (90.1) | 251 (74.7) a | 30 (56.6)a,b |
| age, yr, mean (SD) | 52.0 (14.5) | 51.2 (14.8) | 56.2 (15.3)b |
| body surface area, m2, mean (SD) | 1.81 (0.39) | 1.82 (0.24) | 1.78 (0.19)a |
| body mass index, kg/m2, mean (SD) | 25.8 (3.8) | 26.3 (4.7) | 26.1 (5.1) |
| Renal disease, n (%) | |||
| glomerular | 68 (61.8) | 161 (47.9) a | 26 (49.1) |
| interstitial, polycystic | 3 (2.7) | 13 (3.9) | 3 (5.7) |
| other, unknown | 40 (36.0) | 162 (48.2)a | 24 (45.3) |
| BP, mmHg, mean (SD) | |||
| systolic BP | 142.4 (15.5) | 144.5 (18.5) | 146.2 (18.8) |
| diastolic BP | 89.3 (10.1) | 88.8 (11.0) | 108.0 (10.7) |
| Renal parameters | |||
| creatinine clearance, ml/min, mean (SD) | 43.8 (18.6) | 43.6 (19.7) | 40.1 (22.3) |
| urinary creatinine excretion, g/d, mean (SD) | 1.4 (0.3) | 1.3 (0.4) | 1.1 (0.4)a,b |
| urinary protein excretion, g/d, median (IQR) | 3.0 (2.7) | 2.8 (2.4) | 3.1 (2.4) |
| urinary protein/creatinine excretion, g/g, median (IQR) | 2.0 (2.2) | 2.1 (1.9) | 2.6 (2.3)a,b |
| urinary urea excretion, mmol/d, mean (SD) | 19.6 (11.2) | 19.9 (7.6) | 18.2 (7.3) |
| urinary urea/creatinine excretion, mmol/g, mean (SD) | 14.4 (8.5) | 15.3 (4.9) | 17.4 (6.7)a |
| urinary sodium excretion, mEq/d, mean (SD) | 121.5 (59.6) | 185.2 (61.8)a | 242.7 (82.7)a,b |
| urinary sodium/creatinine excretion, mEq/g, mean (SD) | 87.8 (38.2) | 140.1 (31.9)a | 236.5 (64.8)a,b |
IQR, interquartile range.
P<0.05 versus LSD.
P<0.05 versus MSD.
Sodium Diet Groups
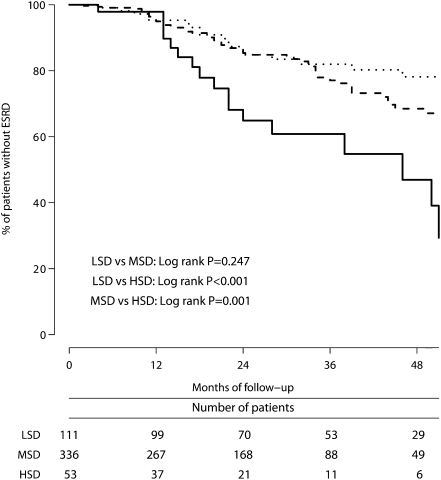
Of the 92 patients (18.4%) who progressed to ESRD, 18 (16.2%) were in the LSD group, 57 (17.0%) were in the MSD group, and 17 (32.1%) were in the HSD group (P<0.001; Figure 1). The ESRD incidence rate per 100 patient-years was 6.1 (95% confidence interval [95% CI], 3.8– 9.7) in the LSD group, 7.9 (95% CI, 6.1–10.2) in the MSD group, and 18.2 (95% CI, 11.3–29.3) in the HSD group. Patients in the HSD group had a 3.3-fold (95% CI, 1.7–6.4) and 2.4-fold (95% CI, 1.4–4.1) excess risk of progressing to ESRD compared with patients in the LSD (P<0.001) or MSD (P=0.002) groups, respectively. MSD patients compared with LSD patients had a nonsignificant 1.4-fold (95% CI, 0.8–2.4) excess risk of progressing to ESRD. Data did not change appreciably when the 26 patients with only one measurement of urinary sodium/creatinine ratio were not considered in the analyses.
Figure 1.
In 500 patients with proteinuric chronic nephropathies, higher salt intake is associated with an increased risk of progression to ESRD. Kaplan-Meier survival curves show time to progression to ESRD in patients categorized in the LSD (dotted line), MSD (broken line), or HSD (continuous line) groups according to their urinary/creatinine ratio on follow-up.
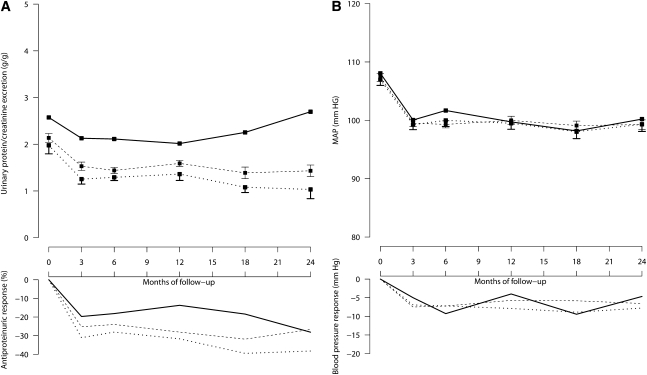
Urinary protein/creatinine excretion decreased after 3 months of treatment (Figure 2, left panel) by 31% (P<0.001), 25% (P<0.001), and 20% (P=0.036) versus baseline in the LSD, MSD, and HSD groups, respectively. Thus, the antiproteinuric efficacy of RAS inhibition was significantly higher in LSD patients compared with MSD (P=0.031) and HSD (P=0.034) patients. Consistently, there was a significant trend to less proteinuria reduction for increasing salt intake (P=0.012). After these initial changes, proteinuria declined further during follow-up at a rate of 0.66% (95% CI, 0.00%–1.29%) per month (P=0.039). However, whereas proteinuria reduction was sustained throughout the whole observation period in the LSD and MSD groups, the antiproteinuric effect of RAS inhibition waned over time and urinary protein excretion tended to increase toward baseline values in the HSD group (Figure 2, left panel). Unlike proteinuria, BP was similar in the three groups at baseline (Figure 2, right panel), shortly after RAS initiation, and on subsequent follow-up. Concomitant use of BP lowering medications was similar among groups at baseline, whereas on follow-up there were fewer patients taking diuretic therapy in the LSD group than in the MSD or HSD groups (Table 2).
Figure 2.
In 500 patients with proteinuric chronic nephropathies taking ramipril therapy, higher salt intake is associated with more proteinuria at baseline and less proteinuria reduction on follow-up, but does not appear to appreciably affect BP control. A and B, respectively, show 24-hour urinary protein excretion and mean arterial pressure during follow-up in patients categorized in LSD (dotted lines), MSD (broken lines), or HSD (continuous lines) groups according to their urinary sodium/creatinine ratio on follow-up. The two upper panels show mean and SEM values, whereas the two lower panels show median changes from baseline.
Table 2.
Concomitant antihypertensive treatments at baseline and throughout follow-up in patient groups categorized as having been on an LSD, MSD, or HSD
| Baseline, n (%) | Follow-up, n (%) | |||||
|---|---|---|---|---|---|---|
| LSD | MSD | HSD | LSD | MSD | HSD | |
| α-Adrenergic agents | 33 (29.7) | 92 (27.4) | 12 (22.6) | 31 (27.9) | 68 (20.2)a | 7 (13.2) |
| β blockers | 24 (21.6) | 81 (24.1) | 14 (26.4) | 29 (26.1) | 78 (23.2) | 13 (24.5) |
| Calcium channel antagonists | 25 (22.5) | 92 (27.4) | 20 (37.7) | 63 (56.8)a | 184 (54.8)a | 30 (56.6)a |
| Diuretics | 40 (36.0) | 132 (39.3) | 23 (43.4) | 35 (31.5) | 154 (45.8)a,b | 25 (47.2)b |
Note that patients in the intensified BP control arm of the REIN-2 (which was achieved with felodipine) were classified for this study as receiving calcium channel antagonists as concomitant treatment.
P<0.05 versus baseline use in the same diet group.
P<0.05 versus LSD in the same time period.
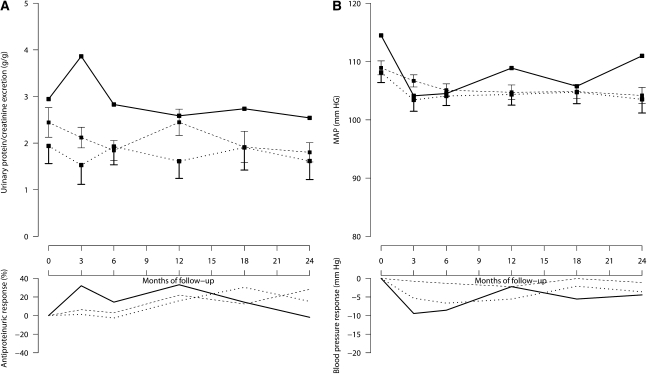
As observed in the 500 patients receiving ramipril therapy, as well as in the cohort of 172 controls taking non–RAS inhibitor therapy, participants in the HSD group tended to have more proteinuria at baseline and on follow-up than those in the LSD and MSD groups, respectively. In controls, however, there were no appreciable differences in follow-up changes in proteinuria among salt intake groups. Again, BP was similar among groups throughout the whole observation period (Figure 3, left and right panels, respectively).
Figure 3.
In 172 patients with proteinuric chronic nephropathies taking non-RAS inhibitor therapy, higher salt intake tends to be associated with more proteinuria, but does not appear to appreciably affect proteinuria reduction on follow-up or BP control. A and B, respectively, show 24-hour urinary protein excretion and mean arterial pressure during follow-up in patients categorized in LSD (dotted lines), MSD (broken lines), or HSD (continuous lines) groups according to their urinary sodium/creatinine ratio on follow-up. The two upper panels show mean and SEM values, whereas the two lower panels show median changes from baseline.
Sodium Excretion as a Continuum
An 100 mEq/g increase in urinary sodium/creatinine ratio was associated with a 1.61-fold (95% CI, 1.15–2.24) increase in ESRD occurrence. This association was independent of age, sex, underlying renal disease, previous inclusion in the REIN or REIN-2 trials, and baseline BP. The significance of the association, however, was partially lost (hazard ratio [HR], 1.38; 95% CI, 0.95–2.00) after adjusting for baseline proteinuria (Table 3).
Table 3.
Time-dependent Cox model, HRs per 100 mEq/d of urinary sodium excretion and per 100 mEq/g of urinary sodium/creatinine excretion
| Urinary Sodium Excretion | Urinary Sodium/Creatinine Excretion | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Unadjusted | 1.35 (0.96–1.89) | 0.089 | 1.61 (1.15–2.24) | 0.005 |
| Univariable adjusted | ||||
| age | 1.35 (0.96–1.90) | 0.082 | 1.60 (1.14–2.24) | 0.006 |
| sex | 1.33 (0.95–1.88) | 0.099 | 1.77 (1.26–2.50) | 0.001 |
| REIN/REIN 2 cohort | 1.37 (0.97–1.93) | 0.074 | 1.77 (1.28–2.54) | 0.001 |
| diagnosis | 1.34 (0.95–1.88) | 0.094 | 1.61 (1.15–2.25) | 0.005 |
| BP | 1.35 (0.95–1.91) | 0.096 | 1.69 (1.19–2.40) | 0.003 |
| proteinuria | 1.11 (0.77–1.60) | 0.592 | 1.38 (0.95–2.00) | 0.086 |
| Multivariable adjusteda | ||||
| without proteinuria | 1.67 (1.16–2.39) | 0.006 | 1.67 (1.07–2.60) | 0.025 |
| including proteinuria | 1.36 (0.89–2.06) | 0.150 | 1.37 (0.84–2.22) | 0.202 |
| Adjusted for changes during follow-up (time-dependent) | ||||
| BP | 1.67 (1.16–2.42) | 0.006 | 1.59 (1.01–2.50) | 0.047 |
| proteinuria | 1.28 (0.86–1.92) | 0.223 | 1.14 (0.72–1.80) | 0.573 |
The multivariable model was adjusted for age, sex, mean arterial BP at baseline, antihypertensive co-medication at baseline, urinary urea excretion during follow-up, and baseline creatinine clearance
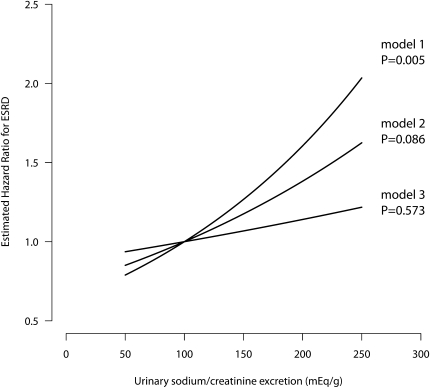
In the multivariable model adjusted for age, sex, BP, creatinine clearance, and concomitant antihypertensive treatment at baseline and 24-hour urea excretion throughout the whole study period, an 100 mEq/g increase in urinary sodium/creatinine ratio was associated with 1.67-fold (95% CI, 1.07–2.60) excess risk of progression to ESRD (Table 3). Exploratory analyses showed that the association was independent of changes in BP and antihypertensive co-medication on follow-up. Conversely, the significance of the association (HR, 1.14; 95% CI, 0.72–1.80) was fully lost after adjustment for baseline and follow-up 24-hour urinary protein excretion (Table 3). Similar findings were obtained when urinary sodium/creatinine excretion was considered as a continuous variable (Figure 4). With this approach, unadjusted analyses showed a strong association between urinary sodium excretion and progression to ESRD (model 1, Figure 4). The significance of the association was attenuated when analyses were adjusted for baseline proteinuria (model 2) and was fully lost when adjustments included changes in proteinuria during the follow-up (model 3).
Figure 4.
In 500 patients with proteinuric chronic nephropathies taking ramipril therapy, the association between salt intake and risk of progression to ESRD is lost when analyses were adjusted for changes in proteinuria on follow-up. The three curves show the association between urinary sodium/creatinine excretion on a continuous scale and ESRD according to three Cox proportional hazards models: unadjusted (model 1), adjusted for baseline proteinuria (model 2), and adjusted for baseline proteinuria and for changes in proteinuria during follow-up (model 3).
Although similar findings were obtained when urinary sodium excretion was not normalized for concomitant urinary creatinine excretion (Table 3), the creatinine normalized model provided a better fit according to the Bayesian information criteria (1012 versus 1016 for the non-normalized model, using the same patients and measurements in both models).
Relationships between Sodium, Proteinuria, and ESRD
Urinary sodium/creatinine excretion was significantly and positively correlated with urinary protein/creatinine excretion at baseline (R=0.134, P=0.013) and follow-up (R=0.182, P<0.001), whereas no correlation was found with BP at baseline (R=0.005, P=0.927) or during follow-up (R=0.031, P=0.556). In turn, urinary protein/creatinine ratio at baseline (HR, 1.30; 95% CI, 1.19–1.41) and follow-up (HR, 1.38; 95% CI, 1.30–1.47) predicted ESRD progression, independent of sex, age, creatinine clearance, and BP.
Discussion
This study has two key findings. First, in humans with nondiabetic CKD who received ACE inhibitor therapy, high salt intake is associated with increased risk of progression to ESRD. Second, the excess risk associated with increased salt exposure seems to be mediated by blunted antiproteinuric effects of ACE inhibitor therapy in this population. Among the 500 study participants, those who had a urinary sodium excretion >200 mEq/g of urinary creatinine had a 2.4- and 3.3-fold higher incidence of ESRD compared with those with a urinary sodium/creatinine excretion between 100 and 200 mEq/g or <100 mEq/g, respectively. Despite a similar BP control, urinary proteins decreased more in patients in the LSD and MSD groups than in those in HSD group. More importantly, in the HSD group, the antiproteinuric effect of ramipril therapy waned over time and urinary protein excretion tended to increase toward baseline values. These findings are consistent with well established evidence that the renoprotective effect of ACE inhibitors or ARBs is largely explained by their effect of reducing urinary proteins,1–4 an effect that is limited or even blunted by excess sodium intake.8–12 Increased sodium exposure could also explain the “escape phenomenon” observed in previous studies and why it was more frequent in participants who were not taking concomitant diuretic therapy.20–23
On average, a 100-mEq increase in daily sodium excretion per gram of creatinine (equivalent to an incremental intake of 125 mEq of sodium or 7 g of salt) increased the risk of ESRD by 61%. This excess risk was independent of age, sex, underlying renal disease, creatinine clearance at inclusion, protein intake, and BP control throughout the observation period, but was no longer significant when the analyses were adjusted for 24-hour urinary protein excretion at inclusion and on follow-up. Conversely, urinary sodium excretion was positively correlated with baseline and follow-up proteinuria that, in turn, independently predicted the risk of ESRD progression. Although the numbers of events and patients were too small to formally test the possibility of a significant interaction between urinary sodium excretion, proteinuria, and risk of progression to ESRD, the above findings converge to indicate that the association between salt intake and outcome was largely mediated by the effects of salt exposure on proteinuria. The finding that high sodium intake was associated with more proteinuria already at inclusion was consistent with previous data showing that daily sodium intake >200 mEq enhanced proteinuria in participants not receiving RAS inhibitor therapy.18 Thus, our data suggest that participants with high salt intake had more proteinuria at inclusion because of the association of sodium overload with urinary proteins.8–11,24–28 This interpretation is consistent with evidence that, among the 172 controls on non-RAS inhibitor therapy, proteinuria was also more severe in those with high salt intake, while patient characteristics and BP control were similar among salt intake groups.
The finding that BP control was independent of daily sodium intake can be explained by the fact that antihypertensive therapy was titrated to predefined BP targets. Indeed, participants with higher sodium intake more frequently required combined treatment with a diuretic, which was the first-line therapy in both the REIN and REIN-2 studies.1–3,19 Addressing why the antiproteinuric and renoprotective effects of ACE inhibitor therapy in participants with high sodium intake were not restored by concomitant diuretic therapy is beyond the purposes of this study. A reasonable speculation is that sodium overload was not fully corrected by diuretic therapy. To note, sodium overload increases ACE activity in renal and vascular tissues, which enhances vascular conversion of AngI to AngII and blunts the effects of ACE inhibition in rats and humans with high sodium intake.29 Independent of BP control, enhanced intrarenal ACE activity has been associated with accelerated renal damage in several experimental models of chronic renal disease30 and might explain at least part of the excess proteinuria and renal risk associated with high sodium intake observed in this study.
Average sodium intake approximated 10 g per day, more than two-fold the intake recommended by current guidelines for renal patients.31 This is of concern because our data show that even a small increase in salt intake is associated with an incremental risk of ESRD. A daily salt intake >14 g (equivalent to >200 mEq/g creatinine) was associated with an ESRD rate of 18.2% per 100 patient-years, compared with 7.9% in participants with less salt intake. Previous studies consistently showed the benefits of a low-sodium diet on BP and proteinuria, but provided no information on the harmful consequences of high salt intake on hard clinical end points.8–11 These novel findings are relevant to health care providers because prevention strategies aimed to avoid extreme excess in sodium intake—even without dietary restrictions that might affect patient compliance32—would be extremely important to substantially limit the risk of renal disease progression in clinical practice.
Monitoring Salt Intake
We categorized our patients according to three ranges of sodium intake that were defined on the basis of cut-off levels similar to those used in previous studies.10,11,18,33 In contrast to previous studies that used a single baseline measurement of urinary salt excretion or the average of the measurements on follow-up,34 in our time-dependent Cox model we used, for the first time in this clinical setting, a cumulative average of sodium excretion. This is a gold-standard approach to model the relationship between longitudinally measured covariates and a given event35 that has been extensively applied in cardiovascular studies to assess the risk of events associated with the consumption of certain foods or nutrients. This approach allowed us to reduce within-person data variability and more reliably quantify long-term sodium exposure.36 In this patient cohort, sodium intake was a relatively fixed trait and few patients appreciably changed their dietary sodium intake during follow-up. Normalizing urinary sodium excretion to concomitant creatinine excretion allowed us to account for erroneous urine collections, but also resulted in an excess of women and older patients in the HSD group that was likely explained by the reduced urinary creatinine excretion in these two populations. Because sodium and protein intake are often correlated, we also adjusted the Cox model for urinary urea excretion as a marker of dietary protein intake. Thus, the association between urinary sodium excretion and ESRD reflected a genuine predictive value of sodium intake unaffected by a confounding effect of concomitant protein intake.
Strengths and Limitations
In addition to the use of gold-standard measures to monitor salt intake, this study had two major strengths. First, our analyses considered a hard end point, such as ESRD. Second, the data were obtained from a large and homogenous population prospectively followed and treated according to standardized guidelines in the setting of controlled clinical studies. This enhanced the clinical relevance of the study findings and limited the confounding effect of random fluctuations due to heterogeneous patient characteristics and treatments. This enhanced the reliability of the analysis and the robustness of the findings, despite the relatively small number of patients and events. The findings were further strengthened by evidence that a similar association between sodium exposure and outcomes was observed when urinary sodium excretion was considered as a categorical or a continuous variable. Moreover, the finding that average sodium excretion in our study population was similar to that reported in other observational studies in renal patients37 or in general population samples38 enhanced the generalizability of the results. The major limitation of this study is that this was a post hoc analysis of trials originally designed for other purposes. Because of the observational nature of our study, a direct causal relationship between higher salt intake and worse outcome while taking ACE inhibitor therapy cannot be definitely proven. Such an association, however, was not appreciable in controls taking non-RAS inhibitor therapy. Independent of the above, the pathogenic role of excess sodium exposure could be definitely addressed by intervention trials prospectively testing the association of diets with different salt intake on renal disease progression.
Our present observational analysis suggests that in CKD patients receiving ACE inhibitor therapy, high sodium intake is associated with accelerated progression to ESRD, mediated by increased proteinuria but independent of underlying renal disease, BP control, and urea excretion, taken as a marker of dietary protein intake. Avoiding excess sodium exposure may be important to slow renal disease progression and limitations in salt intake are expected to achieve major clinical benefits in this population that will largely offset the small inconveniences of minimal dietary restrictions. Optimal salt intake to optimize renoprotection in the setting of a multimodal approach titrated to urinary proteins and other determinants of renal disease progression5 needs to be identified in the setting of prospective clinical trials.
Concise Methods
Patients
Of the 177 patients with proteinuric CKD included between 1992 and 1995 in the REIN trial1–3 and randomized to ramipril therapy and the 335 patients included between 1999 and 2003 in the REIN-2 trial all treated with ramipril19 but not already included in the REIN trial, 500 (97.7%) had at least one measurement of 24-hour urinary sodium excretion and were considered in this analysis. Both trials included participants 18–70 years of age with CKD and persistent proteinuria (urinary protein excretion ≥1 g/24 h for at least 3 months without urinary tract infection or overt heart failure). Full study characteristics and inclusion and exclusion criteria are detailed elsewhere.1–3,19 The primary outcome analyzed in both studies was the incidence of doubling of serum creatinine or ESRD. Patients from both studies were recommended a low-sodium diet and a daily protein intake of approximately 0.8 g/kg. No change to diet was introduced during the observation period. Thus, all 500 patients included in this study fulfilled the same selection criteria, had the same recommended diet, and were receiving stable ACE inhibitor therapy with ramipril at the same daily dose (5 mg). The control group was composed of 172 patients from the placebo arm of the REIN study who fulfilled the same selection criteria and had been managed according to the same treatment and monitoring guidelines, but had not received RAS inhibitor therapy. Patients in the REIN and REIN-2 trials provided written informed consent to participate, according to the Declaration of Helsinki guidelines. The study protocols were approved by the ethics committee and institutional review board of each of the participating centers.
Measurements
The exposure of interest, daily sodium intake, was estimated by measuring 24-hour urinary sodium excretion. To correct for body size and possible collection errors, urinary sodium excretion was normalized to urinary creatinine excretion by calculating the sodium/creatinine ratio from 24-hour urine samples (sodium/creatinine ratio, mEq/g).39 Urinary urea and protein excretion were normalized to urinary creatinine excretion, as well. BP was measured at randomization and every 3 months thereafter. Creatinine clearance, 24-hour urinary protein, and sodium and urea excretion were measured at randomization, at 3 and 6 months after randomization, and every 6 months thereafter. Baseline data were taken when all participants had completed the 6-week wash-out period from previous ACE inhibitor therapy, that is, at randomization for patients from the REIN trial and at the inclusion visit for those from the REIN-2 trial. Thus, all baseline data were without ACE inhibition and all outcome data were with ramipril (5 mg/d) therapy.
Statistical Analyses
As described in previous similar studies,11,18 we identified patients with a LSD, MSD, or HSD based on urinary sodium/creatinine excretion averaged throughout the study <100 mEq/g, between 100 mEq/g and 200 mEq/g, and >200 mEq/g (these cut-off levels of 100 mEq/g and 200 mEq/g approximated 125 and 250 mEq/d, equivalent to 7 and 14 g of salt/d, respectively). Consistency of sodium intake was assessed using the Stuart–Maxwell test. Differences in baseline characteristics were determined using the Wilcoxon rank-sum test and Fisher’s exact test, as appropriate. Differences in ESRD incidence rates were tested using the chi-squared test. Differences in short-term changes in proteinuria (percentage values6,10) and BP (absolute values) were tested with Wilcoxon rank-sum tests; subsequent changes were analyzed using a joint modeling approach incorporating survival outcomes40 to account for survivor bias. Antihypertensive co-medication was described; Fisher’s exact test and McNemar’s test were performed for comparisons among groups and time periods, respectively. Survival curves were drawn using the Kaplan–Meier method; the log-rank test was used to assess differences in survival among groups and Cox proportional hazards analysis was used to calculate hazard rates. Nonlinearity was tested by plotting the Martingale residuals.
To reduce within-person data variability and reliably quantify individual sodium exposure, sodium intake was also modeled continuously using time-dependent Cox models, with cumulative average of urinary sodium/creatinine excretion as the independent variable.35,41 The hazard ratio for ESRD was determined per 100 mEq/g increase in sodium/creatinine ratio. Potential confounders included in the Cox models were sex, age, baseline mean arterial BP, use of antihypertensive co-medication at baseline, 24-hour urinary urea excretion during follow-up, creatinine clearance at baseline, and log-transformed 24-hour proteinuria at baseline. For exploratory purposes, we adjusted for changes in mean BP and antihypertensive co-medication during follow-up, and log-transformed 24-hour proteinuria during follow-up in separate Cox models. Correlations between urinary sodium excretion and proteinuria or BP at baseline and during follow-up were analyzed using linear regression; at least two measurements per patient were required. Urinary sodium and urea excretion at the last visit were not considered to avoid an undesirable adjustment for sequelae,35 related to an anorectic decrease in nutritional intake just before the start of dialysis in patients progressing to ESRD.42 All analyses were also performed using non-normalized sodium excretion as an independent variable and the two sodium metrics were compared through Bayesian information criteria.43
All statistical analyses were performed using R software (version 2.5.1). All data are presented as mean ± SD unless indicated otherwise. P<0.05 was considered to be statistically significant.
Disclosures
None.
Acknowledgments
We thank Paul van Dijk for epidemiologic advice and Petros Pechlivanoglou for statistical assistance. We also thank all of the investigators and patients involved in the REIN and REIN-2 studies that provided essential information to perform these analyses. Manuela Passera helped to prepare the manuscript.
The REIN study was supported by a grant from Hoechst Marion Roussel Clinical Research Institute, Frankfurt am Main, Germany. The REIN-2 study was supported in part by a grant from Aventis Pharma SA, Antony, France. This work was supported by the Applied Genomic Strategies for Treatment and Prevention of Cardiovascular Death in Uraemia and End Stage Renal Disease (GENECURE) project, a Specific Targeted Research or Innovation Project (STREP), funded by the European Commission under the Sixth Framework Programme as FP6-037696.
Portions of these results were presented at the American Society of Nephrology Renal Week 2010, November 16–21, 2010, in Denver, Colorado.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sodium Intake, ACE Inhibition, and Progression to ESRD,” on pages 10–12.
References
- 1.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 2.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G: Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 352: 1252–1256, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Perna A, Remuzzi G. GISEN Group Investigators: Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK, Perna A, Remuzzi G: Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 19: 1213–1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Bettinaglio P, Pinares F, Remuzzi G: Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin J Am Soc Nephrol 3: 1511–1525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navis G, de Jong PE, Donker AJ, van der Hem GK, de Zeeuw D: Moderate sodium restriction in hypertensive subjects: Renal effects of ACE-inhibition. Kidney Int 31: 815–819, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Buter H, Hemmelder MH, Navis G, de Jong PE, de Zeeuw D: The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant 13: 1682–1685, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekinci EI, Thomas G, Thomas D, Johnson C, Macisaac RJ, Houlihan CA, Finch S, Panagiotopoulos S, O’Callaghan C, Jerums G: Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care 32: 1398–1403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD. HOlland NEphrology STudy Group: Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 343: d4366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabris B, Jackson B, Johnston CI: Salt blocks the renal benefits of ramipril in diabetic hypertensive rats. Hypertension 17: 497–503, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Allen TJ, Waldron MJ, Casley D, Jerums G, Cooper ME: Salt restriction reduces hyperfiltration, renal enlargement, and albuminuria in experimental diabetes. Diabetes 46: 19–24, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Wapstra FH, Van Goor H, Navis G, De Jong PE, De Zeeuw D: Antiproteinuric effect predicts renal protection by angiotensin-converting enzyme inhibition in rats with established adriamycin nephrosis. Clin Sci (Lond) 90: 393–401, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Kreutz R, Kovacevic L, Schulz A, Rothermund L, Ketteler M, Paul M: Effect of high NaCl diet on spontaneous hypertension in a genetic rat model with reduced nephron number. J Hypertens 18: 777–782, 2000 [DOI] [PubMed] [Google Scholar]
- 17.De’Oliveira JM, Price DA, Fisher ND, Allan DR, McKnight JA, Williams GH, Hollenberg NK: Autonomy of the renin system in type II diabetes mellitus: dietary sodium and renal hemodynamic responses to ACE inhibition. Kidney Int 52: 771–777, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Cianciaruso B, Bellizzi V, Minutolo R, Tavera A, Capuano A, Conte G, De Nicola L: Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab 24: 296–301, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, Garini G, Sessa A, Basile C, Alpa M, Scanziani R, Sorba G, Zoccali C, Remuzzi G. REIN-2 Study Group: Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): Multicentre, randomised controlled trial. Lancet 365: 939–946, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Epstein M: Aldosterone blockade: An emerging strategy for abrogating progressive renal disease. Am J Med 119: 912–919, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Lakkis J, Lu WX, Weir MR: RAAS escape: A real clinical entity that may be important in the progression of cardiovascular and renal disease. Curr Hypertens Rep 5: 408–417, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH: Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 47: 1936–1939, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Shiigai T, Shichiri M: Late escape from the antiproteinuric effect of ace inhibitors in nondiabetic renal disease. Am J Kidney Dis 37: 477–483, 2001 [DOI] [PubMed] [Google Scholar]
- 24.du Cailar G, Ribstein J, Mimran A: Dietary sodium and target organ damage in essential hypertension. Am J Hypertens 15: 222–229, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Houlihan CA, Allen TJ, Baxter AL, Panangiotopoulos S, Casley DJ, Cooper ME, Jerums G: A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care 25: 663–671, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Krikken JA, Lely AT, Bakker SJ, Navis G: The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int 71: 260–265, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA: Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: A randomized control trial. Hypertension 46: 308–312, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Verhave JC, Hillege HL, Burgerhof JG, Janssen WM, Gansevoort RT, Navis GJ, de Zeeuw D, de Jong PE. PREVEND Study Group: Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med 256: 324–330, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Krikken JA, Laverman GD, Navis G: Benefits of dietary sodium restriction in the management of chronic kidney disease. Curr Opin Nephrol Hypertens 18: 531–538, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Largo R, Gómez-Garre D, Soto K, Marrón B, Blanco J, Gazapo RM, Plaza JJ, Egido J: Angiotensin-converting enzyme is upregulated in the proximal tubules of rats with intense proteinuria. Hypertension 33: 732–739, 1999 [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation: Guideline 1: Goals of antihypertensive therapy in CKD. Am J Kidney Dis 43: S65–S73, 2004 [Google Scholar]
- 32.Vennegoor MA: Salt restriction and practical aspects to improve compliance. J Ren Nutr 19: 63–68, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Gerdts E, Lund-Johansen P, Omvik P: Reproducibility of salt sensitivity testing using a dietary approach in essential hypertension. J Hum Hypertens 13: 375–384, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Mazouz H, Kacso I, Ghazali A, El Esper N, Moriniere P, Makdassi R, Hardy P, Westeel PF, Achard JM, Pruna A, Fournier A: Risk factors of renal failure progression two years prior to dialysis. Clin Nephrol 51: 355–366, 1999 [PubMed] [Google Scholar]
- 35.Wolfe RA, Strawderman RL: Logical and statistical fallacies in the use of Cox regression models. Am J Kidney Dis 27: 124–129, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Frost CD, Law MR, Wald NJ: By how much does dietary salt reduction lower blood pressure? II—Analysis of observational data within populations. BMJ 302: 815–818, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Nicola L, Minutolo R, Chiodini P, Zoccali C, Castellino P, Donadio C, Strippoli M, Casino F, Giannattasio M, Petrarulo F, Virgilio M, Laraia E, Di Iorio BR, Savica V, Conte G. TArget Blood Pressure LEvels in Chronic Kidney Disease (TABLE in CKD) Study Group: Global approach to cardiovascular risk in chronic kidney disease: Reality and opportunities for intervention. Kidney Int 69: 538–545, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Intersalt Cooperative Research Group: Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flack JM, Grimm RH, Jr, Staffileno BA, Dnsc, Elmer P, Yunis C, Hedquist L, Dudley A: New salt-sensitivity metrics: Variability-adjusted blood pressure change and the urinary sodium-to-creatinine ratio. Ethn Dis 12: 10–19, 2002 [PubMed] [Google Scholar]
- 40.Rizopoulos D: JM: An R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 35: 1–33, 2010. 21603108 [Google Scholar]
- 41.Fox J, Laughton CD: Cox Proportional-Hazards Regression for Survival Data. An R and S-PLUS Companion to Applied Regression, Thousand Oaks, CA, Sage Publications Inc, 2002 [Google Scholar]
- 42.Duenhas MR, Draibe SA, Avesani CM, Sesso R, Cuppari L: Influence of renal function on spontaneous dietary intake and on nutritional status of chronic renal insufficiency patients. Eur J Clin Nutr 57: 1473–1478, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Venables WN, Ripley BD: Modern Applied Statistics with S, New York, Springer, 2002 [Google Scholar]