Abstract
Tamoxifen, a selective estrogen receptor modulator, has antifibrotic properties; however, whether it can attenuate renal fibrosis is unknown. In this study, we tested the effects of tamoxifen in a model of hypertensive nephrosclerosis (chronic inhibition of nitric oxide synthesis with L-NAME). After 30 days, treated rats had significantly lower levels of albuminuria as well as lower histologic scores for glomerulosclerosis and interstitial fibrosis than untreated controls. Tamoxifen was renoprotective despite having no effect on the sustained, severe hypertension induced by L-NAME. Tamoxifen prevented the accumulation of extracellular matrix by decreasing the expression of collagen I, collagen III, and fibronectin mRNA and protein. These renoprotective effects associated with inhibition of TGF-β1 and plasminogen activator inhibitor-1, and with a significant reduction in α-smooth muscle actin–positive cells in the renal interstitium. Furthermore, tamoxifen abrogated IL-1β– and angiotensin-II–induced proliferation of fibroblasts from both kidney explants and from the NRK-49F cell line. Tamoxifen also inhibited the expression of extracellular matrix components and the production and release of TGF-β1 into the supernatant of these cells. In summary, tamoxifen exhibits antifibrotic effects in the L-NAME model of hypertensive nephrosclerosis, likely through the inhibition of TGF-β1, suggesting that it may have therapeutic use in CKD treatment.
The pathogenesis of most CKD involves a complex mechanism of hemodynamic and inflammatory processes that leads to renal fibrosis and tubulointerstitial scarring with subsequent progression toward ESRD.1 Studies show that therapeutic interventions such as the blockade of the renin–angiotensin–aldosterone system and immunosuppressive drugs slow the progression of renal disease in experimental models2,3 and human CKD clinical trials.4–8 Although these strategies promote renoprotective effects, they do not halt the progression of renal fibrosis and scarring. Considering that interstitial fibrosis represents the final common pathway of CKD, therapeutic intervention with drugs that display antifibrotic properties may represent an attractive choice of therapy for arresting the autonomous fibrogenic process in chronic progressive nephropathies.
In this context, tamoxifen, a selective estrogen receptor modulator (SERM), may represent a novel therapeutic option for promoting the blockade of fibrogenesis. Tamoxifen, a drug clinically used to prevent and treat breast cancer, is effective in treating abnormal healing disorders. Of particular interest are several reports that describe the efficacy of tamoxifen in promoting regression of fibrosis not only in idiopathic retroperitoneal fibrosis,9–11 but also in fibrosclerotic disorders such as desmoid tumors,12,13 encapsulating peritoneal sclerosis,14,15 sclerosing cervicitis, and fibrosing mediastinitis.16 In addition to clinical evidence of fibrosis regression with tamoxifen treatment, in vitro studies also suggest that tamoxifen possesses antifibrotic properties. Tamoxifen suppresses transcription and synthesis of collagen in mesangial cells in culture,17 inhibits proliferation of human dermal fibroblasts,18 decreases fibroblast function,19 and inhibits wound contraction.20
Considering that these fibroproliferative diseases, characterized by increased fibroblast proliferation and excessive deposition of extracellular matrix (ECM) proteins, have common features with the fibrogenic process of progressive renal diseases, we hypothesized that tamoxifen might have a potential benefit in the treatment of abnormal renal scarring. This hypothesis led us to test this drug in an experimental model of chronic progressive renal disease (the NAME model), characterized by severe hypertension, albuminuria, glomerulosclerosis, interstitial fibrosis, and progressive renal injury.21 In addition, considering that TGF-β is an important mediator of renal fibrogenesis, we investigated whether the antifibrotic effect of tamoxifen might be related to TGF-β1 production.
Renal fibroblasts are the major source of ECM production in the kidney. To further investigate whether tamoxifen directly affects the effector cells of renal fibrogenesis, we grew renal fibroblasts in culture, submitted them to specific stimuli, and treated them with tamoxifen. The in vitro stimuli consisted of IL-1β and angiotensin-II (Ang-II) to resemble some of the recognized pathogenic stimuli involved in the process of renal interstitial inflammation and fibrosis.22,23 We analyzed the effect of tamoxifen on cell proliferation, ECM, and TGF-β production in these stimulated cells.
RESULTS
Tamoxifen Treatment Induces Renoprotective Effects
After 30 days of treatment, the body weight of the NAME rats was 18% lower than the control rats (255±27 versus 310±24 g, respectively; P<0.05). Tamoxifen treatment was well tolerated without affecting body weight (255±5 g; P<0.05 versus controls).
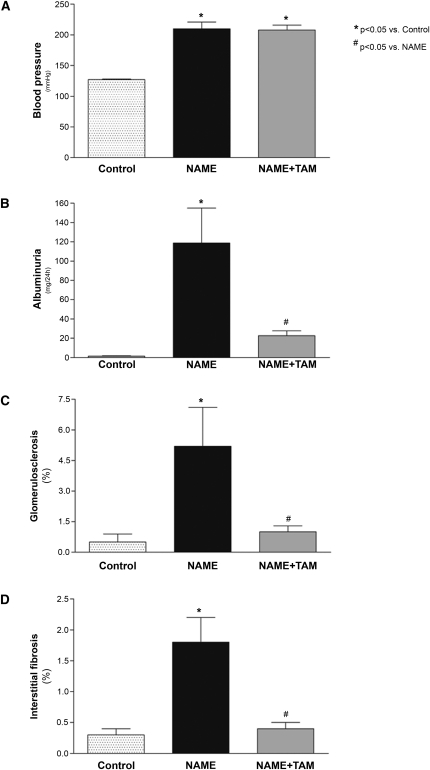
Rats treated with L-NAME developed severe hypertension (Figure 1A and Supplemental Table 1). Tamoxifen treatment had no effect on arterial pressure, and NAME rats receiving tamoxifen displayed sustained hypertension. As expected, the NAME group exhibited a marked increase in albuminuria (Figure 1B and Supplemental Table 1). Although the NAME+TAM group showed severe hypertension, tamoxifen treatment significantly diminished urinary albumin excretion.
Figure 1.
Effect of tamoxifen treatment on NAME rats for 30 days. (A) Tamoxifen had no effect on systolic BP. However, (B) albuminuria, (C) histological scores for glomerulosclerosis, and (D) interstitial fibrosis were significantly lower in tamoxifen-treated rats compared with untreated animals. Control group, animals receiving only high-salt diet; NAME group, animals receiving L-NAME plus high-salt diet; NAME+TAM group, NAME animals treated with tamoxifen.
Tamoxifen Prevents Glomerulosclerosis and Interstitial Fibrosis
NAME rats developed significant glomerulosclerosis (5.2%±1.9% versus 0.5%±0.4% in controls; P<0.05). Tamoxifen treatment significantly decreased the percentage of glomerulosclerosis (1.0%±0.3%; P<0.05 versus NAME), reaching values similar to controls (Figures 1C and 2, A and B).
Figure 2.
Effect of tamoxifen on glomerulosclerosis, interstitial fibrosis, and α-smooth muscle actin expression on NAME rats. (A) Periodic acid–Schiff staining in kidney sections showing glomerulosclerosis in NAME rats, and (B) amelioration of glomerulosclerosis in NAME rats treated with tamoxifen. (C) Prominent interstitial expansion in NAME rats, analyzed by Masson Trichrome staining. (D) Tamoxifen attenuated the interstitial expansion. (E) Immunohistochemistry for α-SMA. NAME rats presenting an accumulation of myofibroblasts in the interstitium. (F) A striking reduction of myofibroblasts was observed with tamoxifen treatment.
Collapsed glomeruli, characterized by glomerular basement membrane wrinkling and capillary lumen diameter reduction, were increased in the NAME group compared with controls (13.1%±3.4% versus 1.8%±0.6%, respectively; P<0.05), and tamoxifen significantly reduced the percentage of collapsed glomeruli (4.7%±0.7%; P<0.05 versus NAME).
Interstitial fibrosis was markedly increased in the NAME group (1.8%±0.4% versus 0.3%±0.1% in controls; P<0.05). Tamoxifen treatment drastically reduced interstitial fibrosis (0.4%±0.1%; P<0.05 versus NAME) (Figures 1 and 2, C and D).
Tamoxifen Diminishes Renal ECM Protein Expression
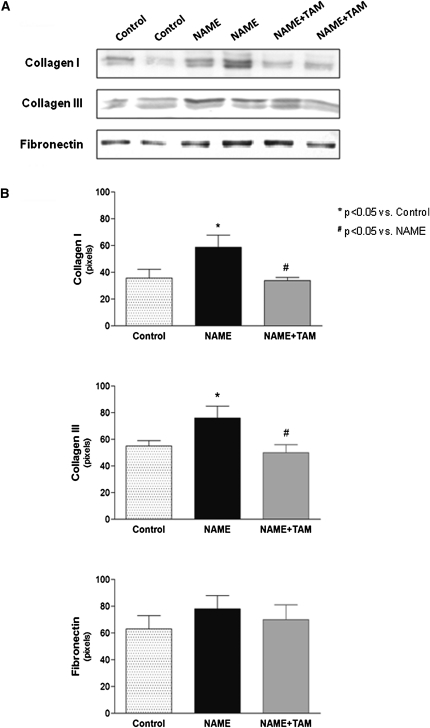
ECM protein expression, as analyzed by real-time PCR, was significantly increased in the NAME group, whereas tamoxifen treatment significantly reduced expression of collagen I and collagen III mRNA (Figure 3 and Supplemental Table 1). Similar results were observed at the protein level (Figure 4 and Supplemental Table 1).
Figure 3.
Expression of ECM components (collagen I, collagen III, and fibronectin) in renal tissue analyzed by real-time PCR. Control group, animals receiving only high-salt diet; NAME group, animals receiving L-NAME plus high-salt diet; and NAME+TAM group, NAME animals treated with tamoxifen.
Figure 4.
Expression of ECM components (collagen I, collagen III, and fibronectin) in renal tissue analyzed by Western blot. (A) Western blot bands; (B) densitometry ratio. Control group, animals receiving only high-salt diet; NAME group, animals receiving L-NAME plus high-salt diet; and NAME+TAM group, NAME animals treated with tamoxifen.
Tamoxifen Inhibits Plasminogen Activator Inhibitor-1 Expression in the Kidney
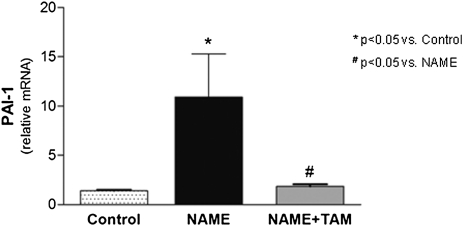
Plasminogen activator inhibitor-1 (PAI-1) expression, analyzed by real-time PCR, was markedly elevated in the NAME rats (Figure 5 and Supplemental Table 1). Tamoxifen treatment significantly decreased PAI-1 expression (by more than 10-fold), maintaining the mRNA levels in the treated group at levels similar to controls.
Figure 5.
PAS-1 expression in renal tissue analyzed by real-time PCR in the different experimental groups. Control group, animals receiving only high-salt diet; NAME group, animals receiving L-NAME plus high-salt diet; and NAME+TAM group, NAME animals treated with tamoxifen.
TGF-β1 Downregulation May Mediate the Antifibrotic Effects of Tamoxifen
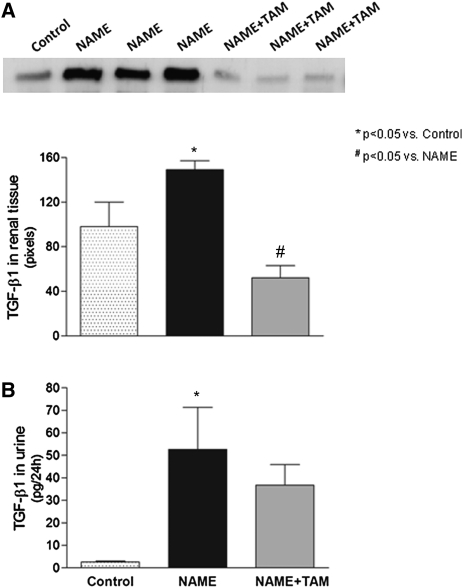
As illustrated in Figure 6A, NAME rats exhibited significantly increased expression of TGF-β1, as assessed by Western blot, in the renal tissue. By contrast, tamoxifen treatment of the diseased rats induced a significant decrease in TGF-β1 expression. Urinary TGF-β1, measured by ELISA, was significantly higher in NAME rats compared with controls (Figure 6B). The effect of tamoxifen in decreasing urinary TGF-β1 excretion was unremarkable.
Figure 6.
Effect of tamoxifen on TGF-β1 in the different experimental groups. (A) Expression of TGF-β1 in renal tissue was analyzed by Western blot. (B) Twenty-four-hour urinary levels of TGF-β1 were analyzed by ELISA. Control group, animals receiving only high-salt diet; NAME group, animals receiving L-NAME plus high-salt diet; NAME+TAM group, NAME animals treated with tamoxifen.
Analysis of the Renal Inflammatory Infiltrate
We observed a high number of interstitial macrophages and T lymphocytes in the kidneys of NAME animals, predominantly around glomeruli and injured vessels. Tamoxifen treatment had no significant effect on the number of renal macrophages in this model and no influence on the number of T lymphocytes in the renal tissue (Table 1).
Table 1.
Mean number of macrophages (ED-1+), lymphocytes (CD-3+), and myofibroblasts (α-SMA) in the study groups
| Cell Type | Control | NAME | NAME+TAM |
|---|---|---|---|
| ED-1+ (cells/mm2) | 8±2 | 57±11a | 25±7 |
| CD-3+ (cells/mm2) | 32±4 | 65±9a | 84±13a |
| α-SMA (%) | 0.2±0.1 | 11.6±2.9a | 3.6±0.5b |
Control, animals receiving only high-salt diet; NAME group, animals receiving L-NAME plus high-salt diet; NAME+TAM group, NAME animals treated with tamoxifen.
P<0.05 versus control group.
P<0.05 versus NAME group.
Control rats exhibited a constitutive expression of α-smooth muscle actin (α-SMA) in the vessels and an absence of α-SMA in the interstitial compartment. By contrast, NAME rats displayed enhanced α-SMA expression in the interstitium, reflecting an elevated number of myofibroblasts in this compartment (Figure 2E). Tamoxifen treatment significantly reduced the α-SMA expression in the renal interstitium (Figure 2F).
Cell Culture Experiments and Characterization of Renal Fibroblasts
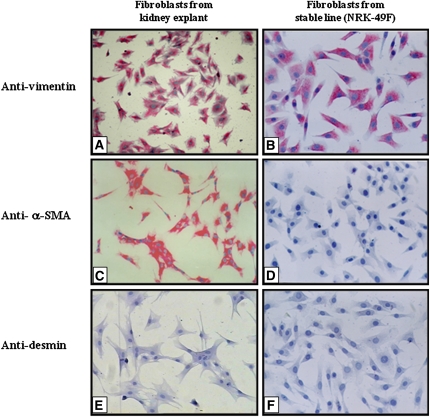
Phenotypic characterization using specific antibodies showed that fibroblasts isolated from rat kidney explants were positive for vimentin (Figure 7A) and α-SMA (Figure 7C) and negative for desmin (Figure 7E), vWf, and pancytokeratin, supporting the hypothesis that these cells were myofibroblasts. However, NRK-49F cells grown in culture were positive for vimentin (Figure 7B) and negative for α-SMA (Figure 7D), desmin (Figure 7F), vWf, and pancytokeratin, which characterized them as fibroblasts from a stable cell line.
Figure 7.
Immunocytochemistry for phenotypic characterization of fibroblasts obtained from primary culture of kidney explants or stable line (NRK-49F). Cells derived from kidney explants were positive for vimentin (A) and α-SMA (C) and negative for desmin (E). NRK-49F cells were positive for vimentin (B) and negative for α-SMA (D) and desmin (F).
Tamoxifen Blocks Renal Fibroblast Proliferation in Culture
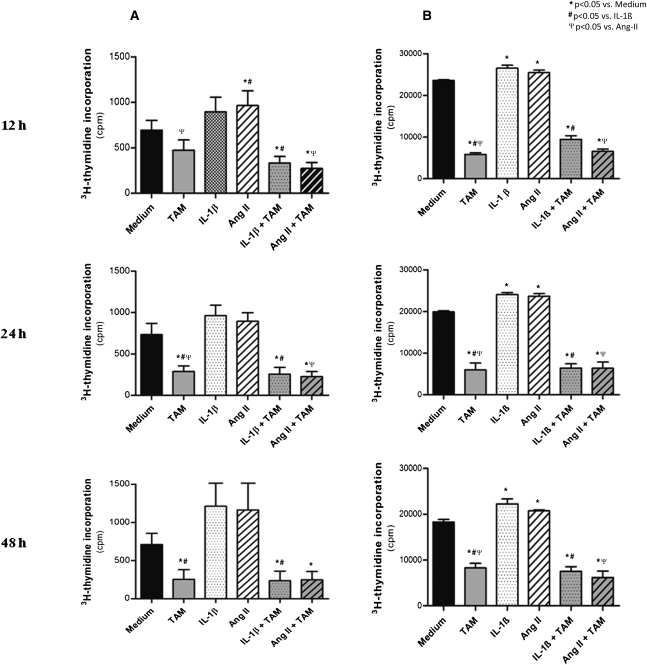
Analysis of the proliferative activity of rat renal fibroblasts, assessed by the 3[H]-thymidine incorporation assay, showed that tamoxifen significantly decreased the proliferation rate of both types of fibroblasts; this effect was already observed at 12 hours of treatment and reached growth inhibition of <60% after 24 hours and 48 hours in culture (Figure 8 and Supplemental Table 2). However, we observed the most striking antiproliferative effect of tamoxifen in fibroblasts previously stimulated with IL-1β and Ang-II. Tamoxifen substantially blocked the mitogenic effect of profibrogenic factors IL-1β and Ang-II on cultured renal fibroblasts at all observation times.
Figure 8.
Tamoxifen blocks renal fibroblast proliferation in culture. [3]H-thymidine incorporation assay of fibroblasts from the primary culture (column A) and from the NRK-49F stable line (column B). Medium, nonstimulated fibroblasts; TAM, fibroblasts incubated with tamoxifen; IL-1β, fibroblasts stimulated with IL-1β; Ang-II, fibroblasts stimulated with angiotensin-II; IL-1β+TAM, fibroblasts stimulated with IL-1β and treated with tamoxifen; Ang-II+TAM, fibroblasts stimulated with angiotensin-II and treated with tamoxifen.
Tamoxifen Decreases the Expression of ECM Components in Cultured Fibroblasts
The effect of tamoxifen on the expression of ECM components (collagen I, collagen III, and fibronectin) was analyzed in NRK-49F fibroblasts (Table 2). Tamoxifen added to the culture at a concentration of 5 µM did not cause any significant change in ECM component expression. However, exposure of IL-1β and Ang-II–stimulated fibroblasts to tamoxifen resulted in a significant decrease in ECM expression at 24 hours.
Table 2.
Expression of ECM components (collagen I, collagen III, and fibronectin) and TGF-β1 from rat renal fibroblast cell line NRK-49F after 24 hours in culture
| ECM Components | Control | TAM | IL-1β | Ang-II | IL-1β+TAM | Ang-II+TAM |
|---|---|---|---|---|---|---|
| Collagen I (ratio/GAPDH) | 1.00±0.45 | 0.89±0.40 | 2.62±0.26a | 2.83±0.22b | 1.12±0.26c | 0.94±0.53d |
| Collagen III (ratio/GAPDH) | 1.00±0.80 | 0.60±0.50 | 1.75±0.17 | 2.72±0.17b | 0.79±0.20 | 0.74±0.34d |
| Fibronectin (ratio/GAPDH) | 1.00±0.69 | 1.02±0.33 | 3.08±0.20a | 2.06±0.55 | 1.06±0.24c | 0.98±0.49 |
| TGF-β1 (ratio/GAPDH) | 1.00±0.33 | 0.91±0.13 | 2.37±0.21a | 2.16±0.21b | 1.02±0.35c | 0.51±0.21d |
Control, nonstimulated renal fibroblasts; TAM, renal fibroblasts treated with tamoxifen; IL-1β, renal fibroblasts stimulated with IL-1β; Ang-II, renal fibroblasts stimulated with angiotensin-II; IL-1β+TAM, IL-1β–stimulated renal fibroblasts treated with tamoxifen; Ang-II+TAM, angiotensin-II–stimulated renal fibroblasts treated with tamoxifen; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
P<0.05 versus control and TAM groups.
P<0.05 versus control and TAM groups.
P<0.05 versus IL-1β (one-way ANOVA between control, TAM, IL-1β, and IL-1β+TAM groups).
P<0.05 versus Ang-II (one-way ANOVA between control, TAM, Ang-II, and Ang-II+TAM groups).
Tamoxifen Decreases TGF-β Synthesis and Secretion in Cultured Fibroblasts
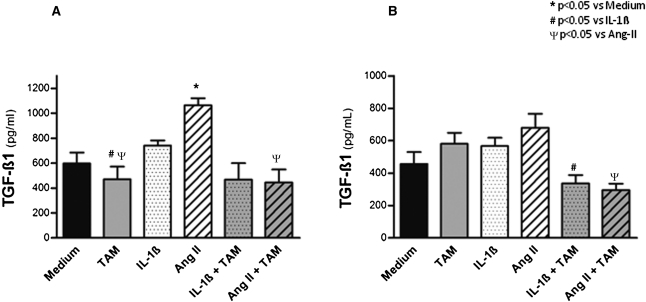
Exposure of IL-1β or Ang-II–stimulated rat renal fibroblasts to tamoxifen in culture had a significant effect in reducing TGF-β1 mRNA expression at 24 hours (Table 2). These findings parallel the measurements of TGF-β1 protein in the supernatants of both the primary culture of renal fibroblasts and fibroblasts of the NRK-49F cell line that were stimulated with IL-1β and Ang-II (Figure 9 and Supplemental Table 2). Hence, these findings suggest that the downregulation of TGF-β1 may mediate the antifibrotic effects of tamoxifen.
Figure 9.
Effect of tamoxifen on TGF-β1 production in cultured fibroblasts. Concentration of TGF-β1 protein in the supernatant was measured after 48 hours of primary culture of renal fibroblasts (A) and culture of fibroblasts of the NRK-49F cell line (B), both stimulated with IL-1β and Ang-II. Medium, nonstimulated fibroblasts; TAM, fibroblasts incubated with tamoxifen; IL-1β, fibroblasts stimulated with IL-1β; Ang-II, fibroblasts stimulated with angiotensin-II; IL-1β+TAM, fibroblasts stimulated with IL-1β and treated with tamoxifen; Ang-II+TAM, fibroblasts stimulated with angiotensin-II and treated with tamoxifen.
DISCUSSION
In this study, we demonstrated that tamoxifen administration induces renoprotective and potent antifibrogenic effects in an experimental model of chronic progressive renal disease. In the NAME model, tamoxifen promoted a reduction in urinary albumin levels of >80% and caused a marked decrease in the histologic parameters of glomerular and tubulointerstitial damage, reducing the degree of glomerulosclerosis and collapsed glomeruli as well as the severity of interstitial fibrosis. It is noteworthy that tamoxifen displayed no effects on arterial pressure levels. These findings are remarkable because the renoprotective effects were achieved even in a setting of sustained, severe hypertension. The negative effect of sustained hypertension was likely overcome by the protective effects of tamoxifen on renal architecture, resulting in a final balance of renoprotection.
A significant finding of this study was the marked reduction in the relative area of renal interstitial fibrosis achieved with tamoxifen treatment, reaching values similar to the control group. Considering that the magnitude of interstitial fibrosis strongly predicts the degree and progression to renal failure,24,25 the antifibrotic effect of tamoxifen in this compartment may possibly be crucially relevant in attenuating the progression of renal disease. The effect of tamoxifen in ameliorating tubulointerstitial fibrosis seems directly related to a reduction in the synthesis of major ECM components, as demonstrated by the diminished production of collagen I, collagen III, and fibronectin in kidney tissue of tamoxifen-treated NAME rats. The effect of tamoxifen in decreasing renal fibrosis was also demonstrated in rats with unilateral urethral obstruction, a model characterized by severe renal fibrosis.26
The decreased production of collagen I, collagen III, and fibronectin associated with tamoxifen treatment may be a consequence of its effect on myofibroblasts. In fact, immunohistochemical analysis demonstrated a significant decrease in α-SMA expression. Although studies show that tamoxifen decreases inflammation and disease severity in NZB/W F1 mice,27 we observed no substantial effect on the number of macrophages or T cells in this study.
The antifibrotic effects observed in kidneys of tamoxifen-treated NAME rats were further analyzed in vitro by investigating the direct effect of tamoxifen on the effector cells of renal fibrogenesis. Renal myofibroblasts isolated from rat kidney and renal fibroblasts from a rat cell line (NRK-49F) were stimulated with IL-1β or Ang-II to mimic the inflammatory and profibrogenic environment involved in the development of renal fibrogenesis. In fact, both IL-1β and Ang-II induced in vitro fibroblast proliferation. Exposure to tamoxifen in the conditioning media promoted pronounced inhibitory effects on fibroblast proliferation, which were particularly remarkable in the activated fibroblasts stimulated with IL-1β and Ang-II.
These results parallel our in vivo findings on the effect of tamoxifen in decreasing myofibroblast proliferation in NAME rats. Similarly, previous reports documented that tamoxifen negatively affects the proliferation of vascular smooth muscle cells28 and dermal fibroblasts,18 with the antiproliferative effect occurring in a dose-dependent manner. In this study, both in vivo and in vitro antiproliferative effects of tamoxifen and the downregulation of ECM component synthesis were demonstrated using a relatively low concentration of tamoxifen.29
The exact antifibrotic mechanisms of tamoxifen are not clear. However, both in vivo and in vitro findings of this study provide cellular and molecular evidence that these mechanisms may rely on the blockade of TGF-β1, a key profibrotic growth factor involved in renal fibrogenesis. In the renal tissue of diseased NAME rats, tamoxifen significantly reduced the upregulated expression of TGF-β1, suggesting a direct effect of tamoxifen on the TGF-β–mediated mechanisms. In addition, the urinary levels of TGF-β1 were substantially reduced in tamoxifen-treated NAME rats. In parallel, tamoxifen exposure also markedly reduced the synthesis of TGF-β1 by IL-1β or Ang-II–stimulated renal fibroblasts in culture.
Studies focused on elucidating the pathophysiologic mechanisms of keloid, a fibroproliferative skin disorder characterized by overproduction of collagen proteins, provide evidence that tamoxifen downregulates TGF-β production. Researchers showed that tamoxifen downregulates TGF-β1 in keloid fibroblasts in a dose-dependent manner.30,31 These studies proposed that the antifibrotic effect afforded by tamoxifen in activated fibroblasts could be mediated by TGF-β1 downregulation, corroborating our results. In contrast, some tumor cell lines and breast cancer cells have a different response to tamoxifen, consisting of an increase in TGF-β production.32,33 Thus, the exact mechanisms involved in these discordant effects have not been completely clarified.
The action of PAI-1, a major inhibitor of ECM degradation, could also mediate the antifibrotic effect afforded by tamoxifen.33 Although diseased NAME rats overexpressed PAI-1 mRNA, tamoxifen treatment induced a marked downregulation of PAI-1 in renal tissue. PAI-1 inhibition associated with tamoxifen treatment may contribute to amelioration of ECM turnover.
Clinical use of tamoxifen to treat breast cancer seems to rely on its anti-estrogenic effect in this tissue. Paradoxically, tamoxifen has estrogenic effects in other tissues, and thus has been more correctly classified as a SERM.34,35 Considering that tamoxifen is an estrogen receptor (ER) modulator, its renoprotective effects may be related to its influence on the estrogen signaling pathway. A large body of evidence points to the protective effect of estrogens on renal disease progression. Women with CKD show a slower decline in renal function over time compared with men with CKD.17,36–39 In addition, premenopausal women have a lower prevalence of CKD compared with age-matched men, but the incidence of the disease increases after menopause.40 Experimental models of renal disease are widely established in male animals, which develop a more severe disease at a more rapid rate than female animals.41 It is not clear whether these effects are caused by the low levels of estrogens or the presence of testosterone. However, modifications of sex hormone status, such as estrogen deficiency induced by ovariectomy, are shown to worsen renal damage and accelerate the progression of renal disease in several models of kidney disease.42–46
Studies using 17β-estradiol, 2-hydroxyestradiol, or estriol replacement provide evidence that estrogen or estrogen metabolites have a beneficial effect ameliorating renal damage.43–48 For example, the data showed that estrogen or estrogen metabolites reduced albuminuria, attenuated renal lesions, and prevented disease progression in a wide variety of experimental models of renal disease. Renoprotective effects were observed even when estrogen supplementation was initiated after disease onset.49 The favorable effects of estrogens in the kidney are possibly related to their known effects on mesangial cells, as demonstrated both in vitro and in vivo, by reducing proliferation50 and synthesis of collagen and other ECM components,43,47,51,52 which contributes to preventing the development of glomerulosclerosis42–47 and tubulointerstitial fibrosis.43–45,48 In addition, estrogens induce a reduction in PAI-1 levels.50 The estrogen-like antifibrotic effects are possibly related to the reduction in TGF-β expression.43–45,49,50,53,54 It is interesting to note that these reported renoprotective effects of estrogens parallel the findings observed for tamoxifen in this study.47
All effects of estrogens are mediated via ER, intracellular transcription factors that regulate the transcription of target genes. There are two ER subtypes, ER-α and ER-β, with quite different tissue distributions.55 The biologic relevance of the two subtypes is still unclear, but they may determine the distinct cellular effects of estrogens in different tissues.55 In the kidney, ER-α is the predominant receptor subtype, particularly expressed in mesangial cells of the glomerulus,55,56 suggesting that this ER subtype mediates the renoprotective effects of estrogens.57 Accordingly, ER-α−/− knockout animals, lacking ER-α but not ER-β, develop GN, proteinuria, and tubular cell destruction.58
Similarly to estrogen, tamoxifen binds to ER in a specific region called the C-terminal ligand-binding domain.59 The complex ER/ligand (estrogen, tamoxifen, or other SERMs) binds to the respective estrogen-response element displayed in the promoter region of the estrogen-responsive genes, recruiting coactivators or corepressors that activate or inhibit, respectively, the transcription machinery.60 Thus, the combination of ER with transcription coactivators and corepressors has been suggested as the determinant mechanism that explains the different effects triggered by these receptors.60
Efficacy and safety make tamoxifen a useful drug in breast cancer treatment. The most important side effects reported in patients undergoing long-term tamoxifen treatment consist of visual impairment, cataracts,61 and risk of thromboembolic events.62,63 Tamoxifen has weak estrogenic properties that can induce endometrial cell proliferation and, consequently, endometrial cancer.64 In addition, tamoxifen seems to increase the risk of radiation-induced lung and skin fibrosis.65–67 This paradoxical effect aggravating pulmonary fibrosis is likely dependent on the concurrent exposition to radiotherapy. Thus, it seems reasonable to assume that tamoxifen may not be appropriate for all fibrotic diseases.
In summary, our study demonstrated that tamoxifen ameliorates renal injury in a model of chronic nephropathy (the NAME model), preventing albuminuria, glomerulosclerosis, and interstitial fibrosis. These effects, demonstrated both in vivo and in vitro, were related to the direct action of tamoxifen in the myofibroblasts and consequent action on ECM synthesis. This study strongly supports the hypothesis that tamoxifen inhibits renal fibrogenesis by TGF-β1 blockade, providing evidence that tamoxifen is a potentially useful antifibrotic therapy in the kidney.
CONCISE METHODS
Animal Model and Experimental Groups
In this study, we used 35 adult male Wistar rats, weighing 240–270 g, obtained from an established colony at the University of São Paulo, São Paulo, Brazil. All experimental procedures were conducted in accordance with institutional guidelines. The animals received a 3.2% high-salt diet (Nuvital, São Paulo, Brazil) for 2 weeks and were then divided into three groups: the control group, receiving only the high-salt diet; the NAME group, receiving L-NAME (Sigma Chemical Company, St. Louis, MO), 200 mg/L dissolved in drinking water, and the high-salt diet; and the NAME+TAM group, receiving L-NAME, the high-salt diet, and tamoxifen citrate treatment 10 mg/kg per day (Nolvadex; AstraZeneca, London, UK) by gavage. The groups were followed for 30 days and then sacrificed. One day before sacrifice, rats were maintained in metabolic cages for 24-hour urine collection to determine urinary albumin excretion (by radial immunodiffusion) and urinary TGF-β1 excretion (ELISA technique; Promega, San Luis Obispo, CA). Systolic BP was determined by tail cuff manometry with tail plethysmography (Harvard Apparatus, Eden Bridge, UK). Euthanasia was performed by anesthesia with sodium pentobarbital 25–50 mg/kg intraperitoneally, and renal tissue was collected.
Renal Histology
One midcoronal section of the left kidney was fixed in Dubosq-Brazil solution for 45 minutes and then postfixed in buffered 10% formaldehyde solution. We stained 2- to 3-μm-thick sections with periodic acid–Schiff reagent and with the Masson Trichrome technique. The extent of glomerulosclerosis was evaluated by attributing a score to each glomerulus according to the extent of sclerotic lesions.3 Collapsed glomeruli were defined as glomeruli reduced in size and exhibiting basement membrane wrinkling with collapsed segmental capillary loops. The frequency of each category of glomerular injury was expressed as the percentage of the total number of glomeruli examined. The extent of interstitial expansion was quantitatively evaluated in Masson-stained sections by a point-counting technique.68 All morphometric evaluations were performed in a blinded manner by a single observer.
Immunohistochemistry
Paraffin sections of renal tissue were cut at 4-µm thickness and subjected to microwave irradiation in citrate buffer to enhance antigen retrieval. The following monoclonal antibodies were used as primary antibodies: anti-rat ED-1 (Serotec, Oxford, UK), anti-rat CD-3 (Seralab, Oxford, UK), and anti-rat α-SMA (Sigma Chemical Company) to identify macrophages, lymphocytes, and myofibroblasts, respectively. After incubation with the primary antibodies, the slides were submitted to a second reaction either with rat-adsorbed biotinylated anti-mouse IgG (Vector Labs, Burlingame, CA) or with biotinylated anti-rabbit IgG (Vector Labs). To complete the sandwich, sections were incubated with streptavidin-biotin-alkaline phosphatase complex (Dako, Glostrup, Denmark) for ED-1, CD-3, and α-SMA. Finally, sections were incubated with a freshly prepared substrate, consisting of naphtol-AS-MX-phosphate (Sigma Chemical Company) and fast red dye (Sigma Chemical Company). Proliferating cell nuclear antigen staining was performed as previously described.3
We conducted quantitative analysis of ED-1 and CD-3–positive cells in a blinded fashion under ×200 microscopic magnification, expressed as cells per millimeter squared. The fraction of the cortical interstitium positive for α-SMA was quantified by a point-counting technique.68
Real-Time PCR and Western Blot
We used real-time PCR to analyze expression of collagen type I, collagen type III, fibronectin, and PAI-1 in kidney samples. Total RNA was extracted by the guanidinium thiocyanate-phenol-chloroform method,69 and cDNA was synthesized by Moloney murine leukemia virus reverse transcriptase enzyme (Promega). The SsoFast EvaGreen Supermix (Bio-Rad Laboratories, Hercules, CA) and the StepOne real-time PCR system (Applied Biosystems, Foster City, CA) were used to analyze collagen type I, collagen type III, and fibronectin. Briefly, quantitative PCR experiments were conducted in 20-μl reactions containing 3 µl of cDNA, 1 µl of each primer (10 µM) (Table 3), 10 µl of SsoFast EvaGreen Supermix 2×, and water. We used the following PCR cycle profile: 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C for denaturation, 20 seconds at 60°C for combined annealing, and 10 seconds at 72°C for extension. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping control (Table 3).
Table 3.
Primers used for real-time PCR
| Gene Target | Sense and Antisense (5′-3′) | Product (bp) |
|---|---|---|
| Collagen type I | CACCTCCGGACGGAGCAGGA | 80 |
| CTCTTTGCGGCTGGGGTGGG | ||
| Collagen type III | ATCTGAGGGCTCGCCCGGT | 92 |
| CAATGGCAGCACCGCCACCA | ||
| Fibronectin | TGACCCAGACTTACGGTGGCA | 80 |
| GGAGTAGAAGGTCCTACCGTTGTAGTG | ||
| TGF-β1 | CAACCCGGGTGCTTCCGCAT | 96 |
| TGCTCCACCTTGGGCTTGCG | ||
| PAI-1 | GACTGACATCTTCAGCTCAACCC | 101 |
| TCACCTCGATCTTGACCTTTTGT | ||
| β-actin | AGGAGTACGATGAGTCCGGCCC | 70 |
| GCAGCTCAGTAACAGTCCGCCT |
To analyze PAI-1 expression, PCR reactions were performed in the ABI Prism 7700 Sequence Detection System using Syber Green PCR Master Mix (Applied Biosystems), as previously described.70
Western blot was used to analyze the expression of collagen type I, collagen type III, fibronectin, and TGF-β1, performed as previously described.21 Briefly, 30 µg of total protein extracted from renal tissue was denatured and separated on 8% (for collagen type I and III) or 14% (for TGF-β1) SDS-PAGE gels, and transferred to a nitrocellulose membrane by electroblotting. Mouse anti-rat collagen type I (Sigma Chemical Company), mouse anti-rat collagen type III (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-rat fibronectin (Calbiochem), and mouse anti-rat TGF-β1 primary antibodies (Genzyme Corp, Cambridge, MA) were used in 1:200, 1:250, 1:250, and 1:500 dilutions, respectively. After washing, the blots were incubated in a 1:5000 dilution of goat anti-mouse IgG horseradish peroxidase–conjugated antibody (Santa Cruz Biotechnology). Blots were detected by enhanced chemiluminescence. The band density was semi-quantified using ImageMaster software (version 2.0; Pharmacia Biotech, Buckinghamshire, UK).
Cell Culture Experiments
We performed cell culture experiments by using renal fibroblasts obtained from kidney explants as primary culture, as well as by using a commercially available renal fibroblast cell line (NRK-49F; American Type Culture Collection, Manassas, VA).
For the primary culture, kidneys from rats were surgically and aseptically removed. Kidney pieces 1 mm3 in size were seeded in 25-cm2 bottles and cultured in DMEM (Gibco Corp, Carlsbad, CA), supplemented with 20% FCS (Cultilab, Campinas, Brazil) and antibiotics (amphotericin, 2.5 μg/ml; ampicillin, 100 μg/ml; and streptomycin, 100 μg/ml; all from Gibco Corp), at 37°C in a humidified atmosphere of 5% CO2. When cell outgrowth from the explants began, the remaining tissue was removed. After the cells reached confluence, they were harvested and split at a 1:3 ratio. The NRK-49F rat renal fibroblasts were grown in high glucose DMEM with 5% FCS.
After four to six passages, the cells displayed typical fibroblast morphology. Cells were phenotypically characterized on the basis of immunocytochemistry. We used the following antibodies: mouse anti-α-SMA, mouse anti-vimentin, mouse anti-desmin, mouse anti-pancytokeratin (Sigma Aldrich), and rabbit anti-vWf (Dako).
Cells were stimulated with IL-1β (200 pg/ml; Biosource, Camarillo, CA) or Ang-II (10−7 mM; Sigma Chemical Company), and were incubated with tamoxifen citrate (5 µM) for 12, 24, and 48 hours.
We conducted cellular proliferation assays with 3[H]-thymidine; used RT-PCR for collagen type I, collagen type III, and fibronectin; and measured TGF-β1 in the supernatant using the ELISA technique.
Statistical Analyses
Data are presented as mean±SEM, and statistical analyses were performed with the Prism statistical program (GraphPad, San Diego, CA). We used one-way ANOVA with pairwise comparisons according to the Newman-Keuls formulation. P<0.05 was considered significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Wagner Vasquez Domingues and Rosana Domingues for excellent technical assistance. We thank Dr. Niels Olsen Saraiva Camara and Dr. Alvaro Pacheco-Silva Filho for the PAI-1 analysis by real-time PCR.
This work was supported by the São Paulo Foundation for Research Support (FAPESP) Grants 01/01452-5 and 01/05837-9 and by the National Council for Scientific and Technological Development (CNPq) Grant 476963/2003-6.
Footnotes
Deceased.
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011010046/-/DCSupplemental.
REFERENCES
- 1.Noronha IL, Fujihara CK, Zatz R: The inflammatory component in progressive renal disease—Are interventions possible? Nephrol Dial Transplant 17: 363–368, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Anderson S, Rennke HG, Brenner BM: Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 77: 1993–2000, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujihara CK, Malheiros DM, Zatz R, Noronha IL: Mycophenolate mofetil attenuates renal injury in the rat remnant kidney. Kidney Int 54: 1510–1519, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The Collaborative Study Group: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G: Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet 352: 1252–1256, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW: Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Clark CP, Vanderpool D, Preskitt JT: The response of retroperitoneal fibrosis to tamoxifen. Surgery 109: 502–506, 1991 [PubMed] [Google Scholar]
- 10.Loffeld RJ, van Weel TF: Tamoxifen for retroperitoneal fibrosis. Lancet 341: 382, 1993 [DOI] [PubMed] [Google Scholar]
- 11.van Bommel EF, Hendriksz TR, Huiskes AW, Zeegers AG: Brief communication: Tamoxifen therapy for nonmalignant retroperitoneal fibrosis. Ann Intern Med 144: 101–106, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kinzbrunner B, Ritter S, Domingo J, Rosenthal CJ: Remission of rapidly growing desmoid tumors after tamoxifen therapy. Cancer 52: 2201–2204, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Sportiello DJ, Hoogerland DL: A recurrent pelvic desmoid tumor successfully treated with tamoxifen. Cancer 67: 1443–1446, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Allaria PM, Giangrande A, Gandini E, Pisoni IB: Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: Tamoxifen as a new therapeutic agent? J Nephrol 12: 395–397, 1999 [PubMed] [Google Scholar]
- 15.Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, Betjes MGH. investigators of the Dutch Multicentre EPS Study: Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: Results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant 26: 691–697, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Savelli BA, Parshley M, Morganroth ML: Successful treatment of sclerosing cervicitis and fibrosing mediastinitis with tamoxifen. Chest 111: 1137–1140, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Neugarten J, Acharya A, Lei J, Silbiger S: Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am J Physiol Renal Physiol 279: F309–F318, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ruffy MB, Kunnavatana SS, Koch RJ: Effects of tamoxifen on normal human dermal fibroblasts. Arch Facial Plast Surg 8: 329–332, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kuhn MA, Wang X, Payne WG, Ko F, Robson MC: Tamoxifen decreases fibroblast function and downregulates TGF(beta2) in dupuytren’s affected palmar fascia. J Surg Res 103: 146–152, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Hu D, Hughes MA, Cherry GW: Topical tamoxifen—a potential therapeutic regime in treating excessive dermal scarring? Br J Plast Surg 51: 462–469, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Graciano ML, Cavaglieri RC, Dellê H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL: Intrarenal renin-angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol 15: 1805–1815, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fernández L, Mosquera JA: Interleukin-1 increases fibronectin production by cultured rat cardiac fibroblasts. Pathobiology 70: 191–196, 2002-2003-2003 [DOI] [PubMed] [Google Scholar]
- 23.Lijnen PJ, Petrov VV, Fagard RH: Angiotensin II-induced stimulation of collagen secretion and production in cardiac fibroblasts is mediated via angiotensin II subtype 1 receptors. J Renin Angiotensin Aldosterone Syst 2: 117–122, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Bohle A, Müller GA, Wehrmann M, Mackensen-Haen S, Xiao JC: Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int Suppl 54[Suppl]: S2–S9, 1996 [PubMed] [Google Scholar]
- 25.el Nahas AM, Muchaneta-Kubara EC, Essawy M, Soylemezoglu O: Renal fibrosis: Insights into pathogenesis and treatment. Int J Biochem Cell Biol 29: 55–62, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Shirazi M, Noorafshan A, Kroup M, Tanideh N: Comparison of the effects of captopril, tamoxifen and L-carnitine on renal structure and fibrosis after total unilateral ureteral obstruction in the rat. Scand J Urol Nephrol 41: 91–97, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wu WM, Lin BF, Su YC, Suen JL, Chiang BL: Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol 52: 393–400, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Grainger DJ, Weissberg PL, Metcalfe JC: Tamoxifen decreases the rate of proliferation of rat vascular smooth-muscle cells in culture by inducing production of transforming growth factor beta. Biochem J 294: 109–112, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karimian E, Chagin AS, Gjerde J, Heino T, Lien EA, Ohlsson C, Sävendahl L: Tamoxifen impairs both longitudinal and cortical bone growth in young male rats. J Bone Miner Res 23: 1267–1277, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Chau D, Mancoll JS, Lee S, Zhao J, Phillips LG, Gittes GK, Longaker MT: Tamoxifen downregulates TGF-beta production in keloid fibroblasts. Ann Plast Surg 40: 490–493, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Mikulec AA, Hanasono MM, Lum J, Kadleck JM, Kita M, Koch RJ: Effect of tamoxifen on transforming growth factor beta1 production by keloid and fetal fibroblasts. Arch Facial Plast Surg 3: 111–114, 2001 [DOI] [PubMed] [Google Scholar]
- 32.MacCallum J, Keen JC, Bartlett JM, Thompson AM, Dixon JM, Miller WR: Changes in expression of transforming growth factor beta mRNA isoforms in patients undergoing tamoxifen therapy. Br J Cancer 74: 474–478, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benson JR, Wakefield LM, Baum M, Colletta AA: Synthesis and secretion of transforming growth factor beta isoforms by primary cultures of human breast tumour fibroblasts in vitro and their modulation by tamoxifen. Br J Cancer 74: 352–358, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomooka S, Border WA, Marshall BC, Noble NA: Glomerular matrix accumulation is linked to inhibition of the plasmin protease system. Kidney Int 42: 1462–1469, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Lonard DM, Smith CL: Molecular perspectives on selective estrogen receptor modulators (SERMs): Progress in understanding their tissue-specific agonist and antagonist actions. Steroids 67: 15–24, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Donadio JV, Jr, Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M, Ilstrup DM, Chu CP: Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int 33: 708–715, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Gretz N, Zeier M, Geberth S, Strauch M, Ritz E: Is gender a determinant for evolution of renal failure? A study in autosomal dominant polycystic kidney disease. Am J Kidney Dis 14: 178–183, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Rekola S, Bergstrand A, Bucht H: Deterioration of GFR in IgA nephropathy as measured by 51Cr-EDTA clearance. Kidney Int 40: 1050–1054, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Antus B, Liu S, Yao Y, Zou H, Song E, Lutz J, Heemann U: Effects of progesterone and selective oestrogen receptor modulators on chronic allograft nephropathy in rats. Nephrol Dial Transplant 20: 329–335, 2005 [DOI] [PubMed] [Google Scholar]
- 40.U.S. Renal Data System: USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 41.Lombet JR, Adler SG, Anderson PS, Nast CC, Olsen DR, Glassock RJ: Sex vulnerability in the subtotal nephrectomy model of glomerulosclerosis in the rat. J Lab Clin Med 114: 66–74, 1989 [PubMed] [Google Scholar]
- 42.Elliot SJ, Karl M, Berho M, Potier M, Zheng F, Leclercq B, Striker GE, Striker LJ: Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am J Pathol 162: 1441–1448, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, Hamar P, Amann K, Ritz E: Beneficial Effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats. J Am Soc Nephrol 15: 348–358, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Maric C, Sandberg K, Hinojosa-Laborde C: Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Mankhey RW, Bhatti F, Maric C: 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol 288: F399–F405, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, Sato H, Haneda M, Kashiwagi A, Koya D: Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol 166: 1629–1636, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karl M, Berho M, Pignac-Kobinger J, Striker GE, Elliot SJ: Differential effects of continuous and intermittent 17beta-estradiol replacement and tamoxifen therapy on the prevention of glomerulosclerosis: Modulation of the mesangial cell phenotype in vivo. Am J Pathol 169: 351–361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller V, Szabó A, Viklicky O, Gaul I, Pörtl S, Philipp T, Heemann UW: Sex hormones and gender-related differences: their influence on chronic renal allograft rejection. Kidney Int 55: 2011–2020, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Dixon A, Maric C: 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am J Physiol Renal Physiol 293: F1678–F1690, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silbiger S, Lei J, Neugarten J: Estradiol suppresses type I collagen synthesis in mesangial cells via activation of activator protein-1. Kidney Int 55: 1268–1276, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, Silbiger S: Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int 50: 1173–1179, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Guccione M, Silbiger S, Lei J, Neugarten J: Estradiol upregulates mesangial cell MMP-2 activity via transcription factor AP-2. Am J Physiol Renal Physiol 282: F164–F169, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Lei J, Silbiger S, Ziyadeh FN, Neugarten J: Serum-stimulated alpha 1 type IV collagen gene transcription is mediated by TGF-β and inhibited by estradiol. Am J Physiol 274: F252–F258, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu F: Cross-talk between transforming growth factor-β and estrogen receptor signaling through Smad3. J Biol Chem 276: 42908–42914, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA: Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863–870, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Potier M, Elliot SJ, Tack I, Lenz O, Striker GE, Striker LJ, Karl M: Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol 12: 241–251, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C: Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med 2: 227–237, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Shim GJ, Kis LL, Warner M, Gustafsson JA: Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci USA 101: 1720–1724, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mader S, Chambon P, White JH: Defining a minimal estrogen receptor DNA binding domain. Nucleic Acids Res 21: 1125–1132, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones KA, Kadonaga JT: Exploring the transcription-chromatin interface. Genes Dev 14: 1992–1996, 2000 [PubMed] [Google Scholar]
- 61.Gerner EW: Ocular toxicity of tamoxifen. Ann Ophthalmol 21: 420–423, 1989 [PubMed] [Google Scholar]
- 62.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N. National Surgical Adjuvant Breast and Bowel Project (NSABP): Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295: 2727–2741, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Cushman M, Costantino JP, Bovill EG, Wickerham DL, Buckley L, Roberts JD, Krag DN: Effect of tamoxifen on venous thrombosis risk factors in women without cancer: The Breast Cancer Prevention Trial. Br J Haematol 120: 109–116, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L: Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst 91: 1654–1662, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J: Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 88: 918–922, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Koc M, Polat P, Suma S: Effects of tamoxifen on pulmonary fibrosis after cobalt-60 radiotherapy in breast cancer patients. Radiother Oncol 64: 171–175, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Bese NS, Umay C, Yildirim S, Ilvan S, Dirican A, Salar S, Altug T, Ober A: The effects of tamoxifen on radiation-induced pulmonary fibrosis in Wistar albino rats: results of an experimental study. Breast 15: 456–460, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Jepsen FL, Mortensen PB: Interstitial fibrosis of the renal cortex in minimal change lesion and its correlation with renal function. A quantitative study. Virchows Arch A Pathol Anat Histol 383: 265–270, 1979 [DOI] [PubMed] [Google Scholar]
- 69.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 70.Pereira MG, Câmara NO, Campaholle G, Cenedeze MA, de Paula Antunes Teixeira V, dos Reis MA, Pacheco-Silva A: Pioglitazone limits cyclosporine nephrotoxicity in rats. Int Immunopharmacol 6: 1943–1951, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.