Abstract
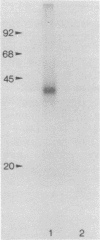
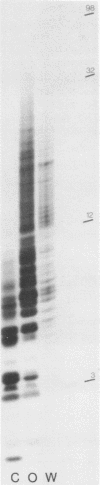
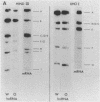
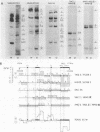
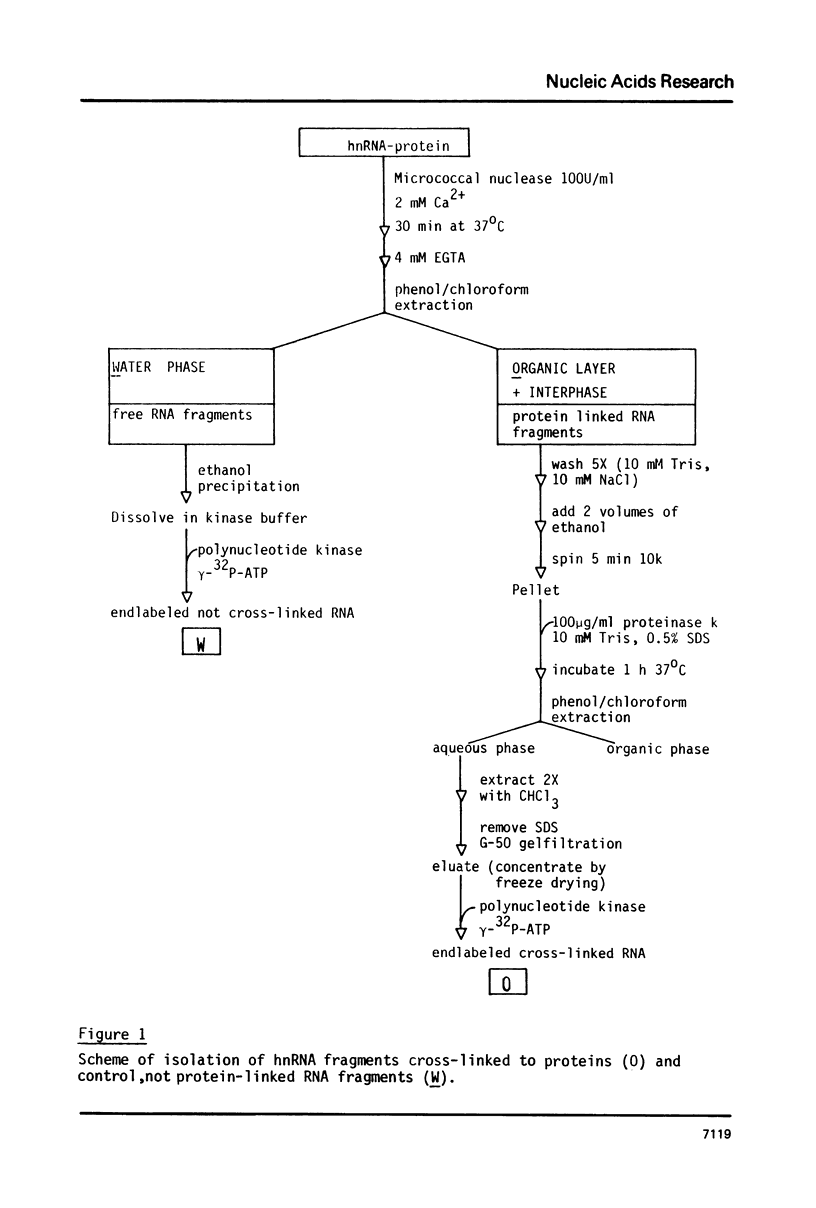
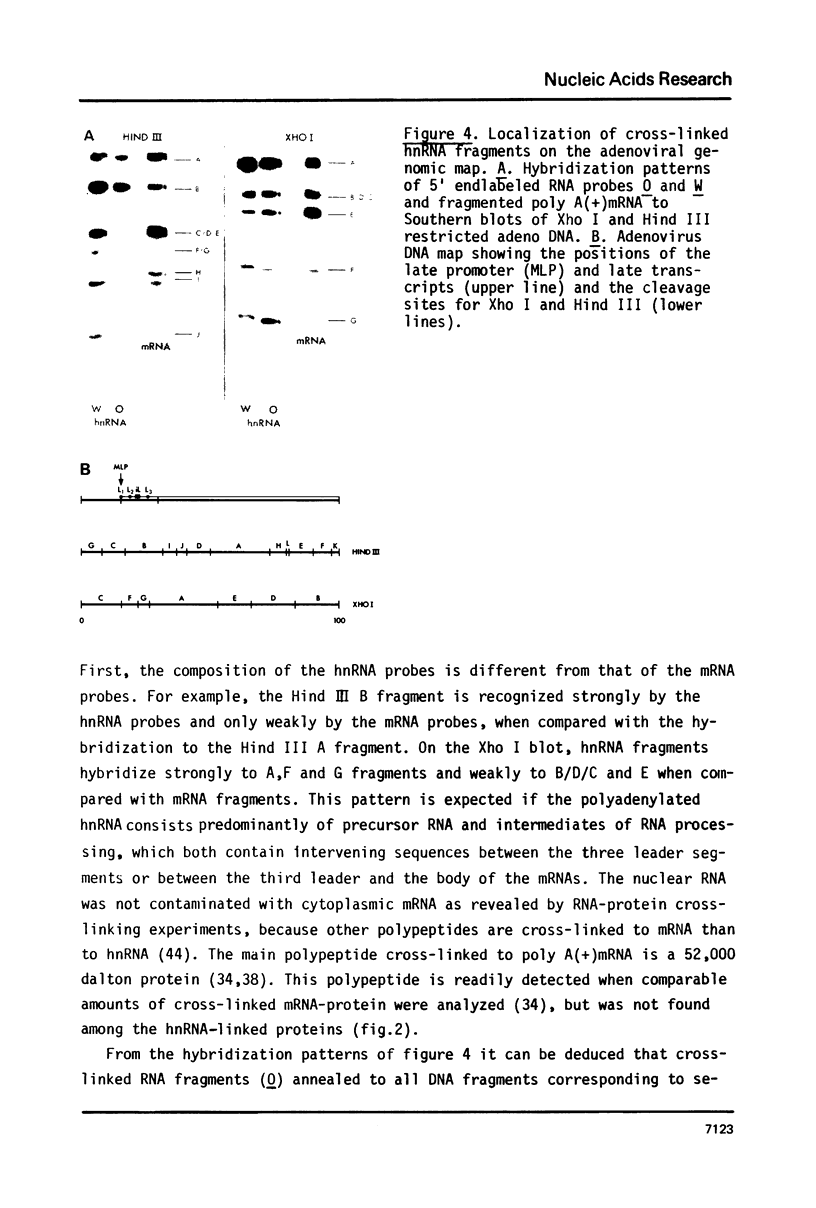
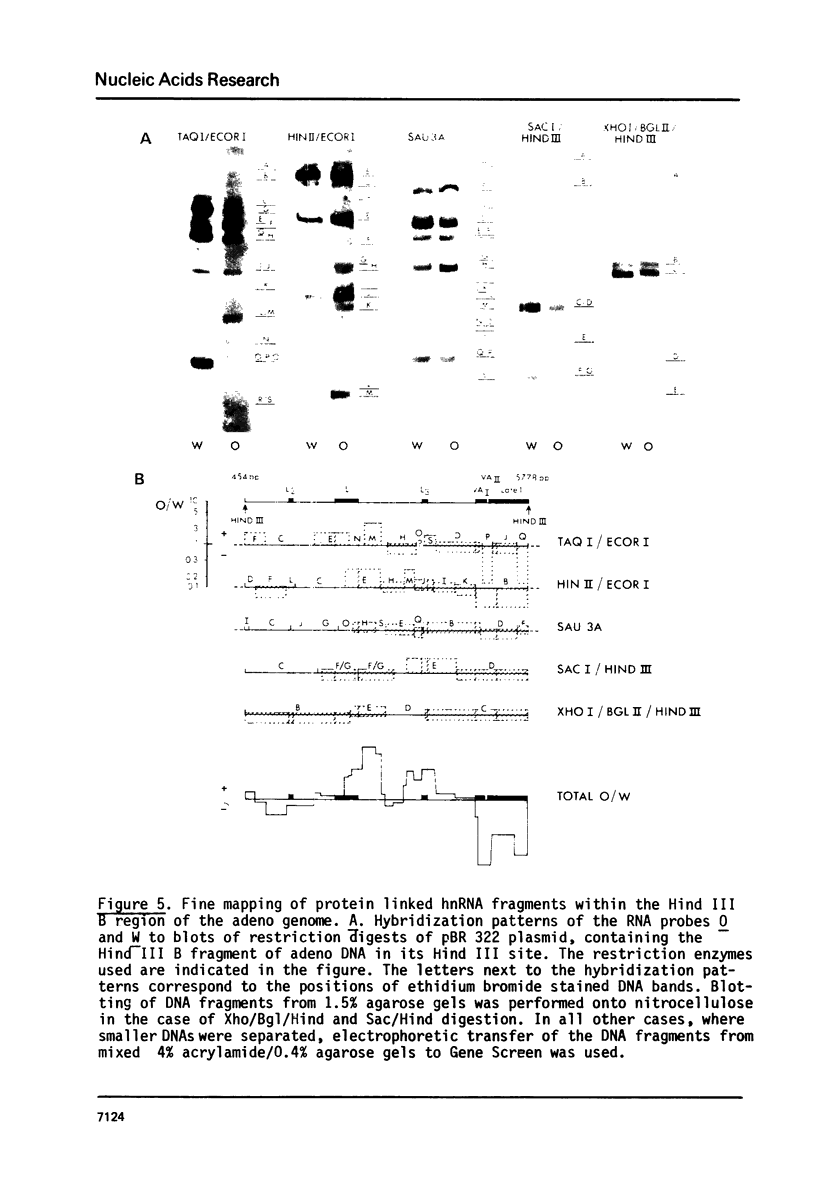
Irradiation with ultraviolet light was used to induce covalent linkage between hnRNA and its associated proteins in intact HeLa cells, late after infection with adenovirus type 2. Covalently linked hnRNA-protein complexes, containing polyadenylated adenoviral RNA, were isolated and their protein moiety characterized. Host 42,000 Mr hnRNP proteins proved to be the major proteins crosslinked to viral hnRNA. To investigate their possible involvement in RNA processing, the localization of these cross-linked polypeptides on adenoviral late transcripts was determined. Sequences of RNA around the attachment sites of the protein were isolated. After in vitro labeling they were hybridized to Southern blots of adeno DNA fragments. The hybridization patterns revealed that the 42,000 Mr polypeptides can be linked to adenoviral transcripts over the entire length of the RNA, corresponding to 16.2-91.5 m.u. of the viral genome. Fine mapping within the Hind III B region (16.8-31.5 m.u.) established, however, that the localization of the cross-linked polypeptides was not random in all parts of the transcript. Sequences around the third leader and the 3' part of the i-leader were overrepresented, whereas the regions encoding VA I and VA II RNA and the late region 1 mRNA bodies were underrepresented in the cross-linked RNA. Using genomic DNA fragments and a cDNA clone containing the tripartite leader it appeared that leader and intervening sequences were represented about equally in cross-linked RNA fragments. Although these results do not support the notion that introns or exons are specifically interacting with one RNP protein, they demonstrate that the 42,000 hnRNP proteins are non randomly positioned on the RNA sequence.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Augenlicht L. H. Protected nucleotide sequences in nuclear ribonucleoprotein. Biochemistry. 1979 Aug 21;18(17):3780–3786. doi: 10.1021/bi00584a022. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Bouton A. H., Hodge L. D., Miller O. L., Jr Visualization of the major late R strand transcription unit of adenovirus serotype 2. J Mol Biol. 1981 Apr 5;147(2):269–295. doi: 10.1016/0022-2836(81)90441-1. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Bouton A. H., Miller O. L., Jr Correlation of hnRNP structure and nascent transcript cleavage. Cell. 1981 Oct;26(2 Pt 2):155–165. doi: 10.1016/0092-8674(81)90299-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Brunel C., Lelay M. N. Two-dimensional analysis of proteins associated with heterogenous nuclear RNA in various animal cell lines. Eur J Biochem. 1979 Sep;99(2):273–283. doi: 10.1111/j.1432-1033.1979.tb13254.x. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Base-pairing interactions between small nuclear RNAs and nuclear RNA precursors as revealed by psoralen cross-linking in vivo. Cell. 1981 Nov;26(3 Pt 1):363–370. doi: 10.1016/0092-8674(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Nucleoprotein organization of inverted repeat DNA transcripts in heterogeneous nuclear RNA-ribonucleoprotein particles from HeLa cells. J Mol Biol. 1978 Jul 5;122(3):361–378. doi: 10.1016/0022-2836(78)90195-x. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Photochemical cross-linking of secondary structure in HeLa cell heterogeneous nuclear RNA in situ 1. Nucleic Acids Res. 1979;6(5):1993–2001. doi: 10.1093/nar/6.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. The status of small nuclear RNA in the ribonucleoprotein fibrils containing heterogeneous nuclear RNA. Biochim Biophys Acta. 1981 Jan 29;652(1):109–120. doi: 10.1016/0005-2787(81)90214-8. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Gross V., Northemann W., Scheurlen M. Structure and function of nuclear ribonucleoprotein complexes. Rev Physiol Biochem Pharmacol. 1978;81:101–134. doi: 10.1007/BFb0034092. [DOI] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Gattoni R., LeMoullec J. M., Jacob M., Stévenin J. The orderly splicing of the first three leaders of the adenovirus-2 major late transcript. Nucleic Acids Res. 1982 Feb 25;10(4):1215–1229. doi: 10.1093/nar/10.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Heterogeneous nuclear RNA secondary structure: oligo (U) sequences base-paired with poly (A) and their possible role as binding sites for heterogeneous nuclear RNA-specific proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1426–1430. doi: 10.1073/pnas.74.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Eekelen C. A., Reinders R. J., Berns A. J., van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with the host nuclear matrix during splicing. J Mol Biol. 1982 Jan 5;154(1):103–119. doi: 10.1016/0022-2836(82)90420-x. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Scherrer K. Characterization of pre-messenger-RNA-containing nuclear ribonucleoprotein particles from avian erythroblasts. Eur J Biochem. 1979 Sep;99(2):225–238. doi: 10.1111/j.1432-1033.1979.tb13249.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
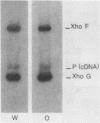
- Ohlsson R. I., van Eekelen C., Philipson L. Non-random localization of ribonucleoprotein (RNP) structures within an adenovirus mRNA precursor. Nucleic Acids Res. 1982 May 25;10(10):3053–3068. doi: 10.1093/nar/10.10.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Davis N. G. Messenger RNA processing and nuclear structure: isolation of nuclear ribonucleoprotein particles containing beta-globin messenger RNA precursors. J Cell Biol. 1980 Oct;87(1):47–54. doi: 10.1083/jcb.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P. RNA processing comes of age. J Cell Biol. 1981 Dec;91(3 Pt 2):28s–38s. doi: 10.1083/jcb.91.3.28s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Seifert H., Scheurlen M., Northemann W., Heinrich P. C. Low molecular weight RNAs as components of nuclear ribonucleoprotein particles containing heterogeneous nuclear RNA. Biochim Biophys Acta. 1979 Aug 29;564(1):55–66. doi: 10.1016/0005-2787(79)90188-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Kamen R. Arrangement of 30S heterogeneous nuclear ribonucleoprotein on polyoma virus late nuclear transcripts. Mol Cell Biol. 1981 Jan;1(1):21–34. doi: 10.1128/mcb.1.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenin J., Gattoni R., Gallinaro-Matringe H., Jacob M. Nuclear ribonucleoprotein particles contain specific proteins and unspecific non-histone nuclear proteins. Eur J Biochem. 1978 Mar 15;84(2):541–549. doi: 10.1111/j.1432-1033.1978.tb12197.x. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Keohavong P., Jacob M. Mild nuclease treatment as a probe for a non-random distribution of adenovirus-specific RNA sequences and of cellular RNA in nuclear ribonucleoprotein fibrils. J Mol Biol. 1982 Mar 5;155(3):185–205. doi: 10.1016/0022-2836(82)90001-8. [DOI] [PubMed] [Google Scholar]
- Van Eekelen C. A., Mariman E. C., Reinders R. J., Van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with host cell proteins. Eur J Biochem. 1981 Oct;119(3):461–467. doi: 10.1111/j.1432-1033.1981.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Wahrman M. Z., Augenlicht L. H. Limited nuclease digestion of heterogeneous nuclear ribonucleoprotein in nuclei. Biochem Biophys Res Commun. 1979 Mar 30;87(2):395–402. doi: 10.1016/0006-291x(79)91809-6. [DOI] [PubMed] [Google Scholar]
- Walker B. W., Lothstein L., Baker C. L., LeStourgeon W. M. The release of 40S hnRNP particles by brief digestion of HeLa nuclei with micrococcal nuclease. Nucleic Acids Res. 1980 Aug 25;8(16):3639–3657. doi: 10.1093/nar/8.16.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., Riemen T., van Venrooij W. J. Specificity in the interaction of hnRNA and mRNA with proteins as revealed by in vivo cross linking. FEBS Lett. 1981 Aug 3;130(2):223–226. doi: 10.1016/0014-5793(81)81125-8. [DOI] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C., Buijtels H., Linné T., Ohlsson R., Philipson L., van Venrooij W. Detection of a cellular polypeptide associated with adenovirus-coded VA RNA using in vitro labeling of proteins cross-linked to RNA. Nucleic Acids Res. 1982 May 25;10(10):3039–3052. doi: 10.1093/nar/10.10.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Janssen D. B. HnRNP particles. Mol Biol Rep. 1978 Feb 28;4(1):3–8. doi: 10.1007/BF00775172. [DOI] [PubMed] [Google Scholar]