Abstract
Notch signaling in podocytes causes proteinuria and glomerulosclerosis in humans and rodents, but the underlying mechanism remains unknown. Here, we analyzed morphologic, molecular, and cellular events before the onset of proteinuria in newborn transgenic mice that express activated Notch in podocytes. Immunohistochemistry revealed a loss of the slit diaphragm protein nephrin exclusively in podocytes expressing activated Notch. Podocyte-specific deletion of Rbpj, which is essential for canonical Notch signaling, prevented this loss of nephrin. Overexpression of activated Notch decreased cell surface nephrin and increased cytoplasmic nephrin in transfected HEK293T cells; pharmacologic inhibition of dynamin, but not depletion of cholesterol, blocked these effects on nephrin, suggesting that Notch promotes dynamin-dependent, raft-independent endocytosis of nephrin. Supporting an association between Notch signaling and nephrin trafficking, electron microscopy revealed shortened podocyte foot processes and fewer slit diaphragms among the transgenic mice compared with controls. These data suggest that Notch signaling induces endocytosis of nephrin, thereby triggering the onset of proteinuria.
Loss of nephrin cell surface expression, either by genetic inactivation or as a consequence of podocyte injury, is associated with slit diaphragm (SD) malformation or destabilization and results in proteinuria in humans and rodents.1–3 A recent study implicated raft-mediated endocytosis of nephrin as a process upregulated after podocyte injury,4 which may result in decreased cell-surface expression of nephrin. A second pool of non-raft, membrane-associated nephrin is reported to be constitutively regulated by dynamin-dependent, clathrin-mediated endocytosis,4,5 although the precise role of the latter process after podocyte injury is poorly understood.
Notch receptors are transmembrane proteins with extracellular domains that bind ligands expressed on the surface of neighboring cells.6 Notch signal transduction is initiated by γ secretase–mediated proteolytic cleavage of ligand-activated Notch,7 which releases a cytoplasmic domain (Notch-IC) that translocates to the nucleus and binds a DNA-binding protein, RBPJκ (encoded by murine Rbpj).8 Transcriptional responses controlled by Notch-IC/RBPJκ complexes are context dependent9 and influence a wide range of cellular processes, including cell proliferation, apoptosis, and cell adhesion.10–12
Evidence in humans and rodents supports a role for Notch activation in proteinuria. For instance, ectopic Notch-IC expression in podocytes was sufficient to cause proteinuria in two independent transgenic mouse models.13,14 In addition, administration of γ secretase inhibitors, which block ligand-activated Notch signaling, prevented proteinuria in rats after single puromycin injection.13 Compelling evidence that Notch plays a role in human proteinuria was provided by the immunohistochemical detection of Notch1-IC and Notch2-IC in podocytes of kidney biopsy samples representative of a wide range of human conditions associated with proteinuria and podocyte injury.13,15 Podocyte apoptosis, cell proliferation, and altered cell differentiation are described as cellular events induced by ectopic Notch signaling.13–15 Research suggests that defects in either cell proliferation or apoptosis, which affect podocyte cell number, are mechanistically coupled to the development of glomerulosclerosis.16 In this context, Notch activation may be considered as a molecular switch that triggers irreversible podocyte injury and promotes disease progression in proteinuric glomerulopathies.17 However, the role played by Notch in initiating glomerular filtration barrier dysfunction and triggering proteinuria remains undefined.
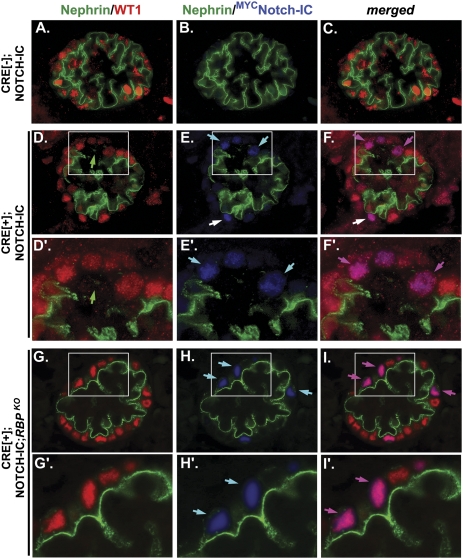
Previously, we used podocyte-specific Cre recombinase (CRE) to induce conditional expression of myc-tagged Notch1-IC (MYCNOTCH-IC) first detected in embryonic mouse podocytes of capillary loop stage glomeruli and persisting in mature glomeruli throughout postnatal life (herein referred to as CRE[+];NOTCH-IC mice).14 Newborn CRE[+];NOTCH-IC mice exhibited normal glomerular architecture on light microscopy and normal mRNA expressions of podocyte-specific markers Nphs1, Nphs2, and Wt1, but developed proteinuria at 2 weeks of life. Our previous analysis of 1-week-old CRE[+];NOTCH-IC mice implicated nephrin protein as a potential target of Notch-IC activity by demonstrating loss of anti-nephrin staining in MYCNOTCH-IC–expressing podocytes.14 Here, we show that nephrin protein is already absent in some but not all MYCNOTCH-IC–expressing podocytes of CRE[+];NOTCH-IC mouse glomeruli at birth (Figure 1, A–F). Within glomeruli containing MYCNOTCH-IC–expressing podocytes, absent or attenuated anti-nephrin staining was spatially restricted to cells that expressed MYCNOTCH-IC (Figure 1, A'–F'). We did not detect a significant decrease in total nephrin protein levels by Western blot analysis of glomerular lysates isolated from newborn CRE[+];NOTCH-IC mice compared with CRE-negative control littermates (Supplemental Figure 1). Because we previously showed that MYCNOTCH-IC was detected in less than one third of podocytes in CRE[+];NOTCH-IC mouse glomeruli within 2 weeks of birth,14 we reasoned that Western blot analysis of whole newborn mouse glomerular lysates may not be sensitive enough to detect changes in nephrin protein levels that occur only in a subset of podocytes.
Figure 1.
Nephrin immunostaining patterns in glomeruli of newborn CRE[+];NOTCH-IC mice after podocyte-specific removal of Rbpj. Shown are images of mouse glomeruli after triple indirect immunofluorescence labeling of newborn kidney tissue sections with anti-nephrin (green), anti-WT1 (red), and anti-MYC (blue) antibodies. (A–C) Serial images of a representative glomerulus from a newborn CRE[−];NOTCH-IC (control) mouse. (D–F) Serial images of a representative glomerulus from a newborn CRE[+];NOTCH-IC mouse. (D'–F') Magnified view of region denoted by white boxes in D–F, respectively. (G–I) Serial images of a representative glomerulus from a newborn CRE[+];NOTCH-IC;RBPKO mouse. (G'–I') Magnified views of region denoted by white boxes in G–I, respectively. (A, D, D', G, and G') Green and red channel images depicting immunostaining patterns for anti-nephrin and anti-WT1, respectively. (B, E, E', H, and H') Green and blue channel images depicting immunostaining patterns for anti-nephrin and anti-MYC, respectively. (C, F, F', I, and I') Merged green, red, and blue channel images. (A–C) Glomeruli of CRE[−];NOTCH-IC mice show anti-nephrin staining along the basolateral aspect of WT1-stained podocytes (A) and do not exhibit anti-MYC staining (B). (D–F) White boxes in D–F denote a glomerular segment in which anti-nephrin staining is absent. Light blue arrows in E and E' denote MYC-positive cells, which do not stain positively for nephrin. Of note, some MYCNOTCH-IC–expressing cells retain nephrin expression as indicated by white arrow in E. Pink arrows in F and F' show that anti-MYC–positive cells are also positive for WT1 and are thus MYCNOTCH-IC–expressing podocytes. Merged image shows co-immunodetection of anti-MYC and anti-WT1 in cells (indicated by purple arrows), which also lack anti-nephrin staining. White arrow in F denotes a [MYC,WT1]–double positive cell stained positively for nephrin. (G and H) Nephrin staining is present in WT1-stained podocytes of CRE[+];NOTCH-IC;RBPKO mice (G) with podocyte-specific deletion of Rbpj. Light blue arrows in H and H' demonstrate MYC-positive cells, which also invariably stain positively for nephrin. Pink arrows in I and I' show corresponding [MYC,WT1]–double positive cells stained positively for nephrin.
In contrast to nephrin, the immunostaining patterns for SD-associated proteins Neph1 and ZO1 were not altered by the presence of MYCNOTCH-IC in glomeruli of newborn CRE[+];NOTCH-IC mice (Supplemental Figure 2, A–D). We observed, however, attenuated anti-podocin staining in some MYCNOTCH-IC–expressing podocytes that also lacked nephrin (Supplemental Figure 2, E and F). Conversely, podocin staining was invariably detected in MYCNOTCH-IC–expressing podocytes that retained nephrin immunoreactivity, which suggested that effects on podocin were likely secondary to loss of nephrin in cells with Notch-IC activity.
To more precisely define the spatial relationship between decreased nephrin protein expression and Notch-IC activity, we analyzed anti-nephrin staining in MYCNOTCH-IC–expressing transgenic mouse glomeruli at a single cell resolution. Briefly, the pattern of anti-nephrin staining was analyzed in kidney tissue sections for cells stained positively with both anti-MYC and anti-WT1 antibodies (i.e., [MYC,WT1]–double positive cells). A score was assigned to each [MYC,WT1]–double positive cell based on the pattern of anti-nephrin antibody staining along the cell’s basolateral surface (1=linear, 2=granular, 3=absent; Supplemental Figure 3). The number of [WT1,MYC]–double positive cells having scores of 1, 2, and 3 was subsequently tabulated for each glomerulus. Relative frequencies for scores 1, 2, and 3 were calculated and expressed as a percentage of the total number of [WT1,MYC]–double positive cells per glomerulus. As a point of reference for our analysis of anti-nephrin scoring, 79%±3% of podocytes in 21 glomeruli of three newborn CRE[−];NOTCH-IC (control) mice exhibited an anti-nephrin staining pattern scored as 1, whereas 16%±3% of podocytes from control mice were scored as 2, and 3%±1% were scored as 3.
To determine whether loss of nephrin expression required formation of Notch-IC/RBPJκ complexes, we examined the effect of deleting Rbpj in MYCNOTCH-IC–expressing podocytes on nephrin protein expression in Podocin-CRE;IC-Notch1;RbpjloxP/del mice (also known as CRE[+];NOTCH-IC;RBPKO mice).14 Previously, we showed that podocyte-specific Rbpj inactivation alone did not impair podocyte function in Podocin-CRE;RbpjloxP/del transgenic mice (which neither express MYCNOTCH-IC nor RBPJκ), and prevented proteinuria and glomerulosclerosis in CRE[+];NOTCH-IC;RBPKO mice (which express MYCNOTCH-IC yet lack RBPJκ in podocytes). Blocking canonical Notch-IC activity through conditional Rbpj inactivation resulted in a 23%±9% increase in the number of MYCNOTCH-IC–expressing podocytes exhibiting a linear pattern of anti-nephrin staining (i.e., score 1) compared with CRE[+];NOTCH-IC mouse glomeruli (Table 1; see also Figure 1, D–I). Conversely, whereas 12%±2% of [WT1,MYC]–double positive podocytes in 56 glomeruli of CRE[+];NOTCH-IC mice exhibited absent anti-nephrin staining (i.e., score 3), no [WT1,MYC]–double positive podocytes showed absent anti-nephrin staining in 23 glomeruli of CRE[+];NOTCH-IC;RBPKO mice (Table 1). Indeed, results of anti-nephrin scores in podocytes of CRE[+];NOTCH-IC;RBPKO mouse glomeruli were comparable with results obtained after scoring CRE[−];NOTCH-IC glomeruli (CRE[+];NOTCH-IC;RBPKO versus CRE[−];NOTCH-IC – score 1: 77%±7% versus 79%±3%, P=0.73; score 2: 22%±7% versus 16%±3%, P=0.44; score 3: 0%±0% versus 3%±1%, P=0.002). Moreover, quantitative reverse transcription PCR analysis of isolated glomeruli revealed that mRNA levels of Nphs1 and other SD-associated genes (e.g., Nphs2, Kirrel1 [encoding Neph1], Tjp1 [encoding ZO-1], Fat1, Cd2ap) were not significantly altered in CRE[+];NOTCH-IC mice compared with age-matched CRE-negative controls (Supplemental Figure 4). These results strongly implicated an RBPJκ-dependent Notch signaling mechanism in control over nephrin at the protein level in podocytes.
Table 1.
Scoring of anti-nephrin antibody staining in [WT1,MYC]–double positive cells of newborn CRE(+);NOTCH-IC and CRE(+);NOTCH-IC;RBPKO mice
| Genotype | Nephrin Score = 1 | Nephrin Score = 2 | Nephrin Score = 3 |
|---|---|---|---|
| CRE(+);NOTCH-IC, % | 54±5 | 32±4 | 12±2 |
| CRE(+);NOTCH-IC;RBPKO, % | 77±7a | 22±7 | 0b |
n=3 mice per genotype.
Versus CRE(+);NOTCH-IC; P=0.004.
Versus CRE(+);NOTCH-IC; P=0.0005.
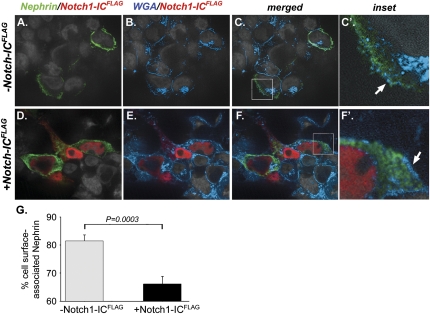
To examine mechanisms that might explain how Notch signaling decreases nephrin protein in podocytes, we determined effects of Notch-IC on nephrin protein expression in vitro using transfected HEK293T cells. In cells transfected with a myc-tagged nephrin-expressing plasmid alone (pNephrinMYC), nephrin protein was predominantly detected along the cell perimeter (Figure 2, A–C). In contrast, there was strong cytoplasmic staining for nephrin in cells transfected with pNephrinMYC and a FLAG-tagged Notch1-IC–expressing plasmid (pNOTCH1-ICFLAG; Figure 2, D–F). Analysis of anti-nephrin cell surface immunofluorescence intensity revealed a 15% decrease in nephrin surface localization in cells expressing both NephrinMYC and NOTCH1-ICFLAG compared with cells expressing NephrinMYC alone (% cell-surface nephrin, nephrin/NOTCH1-ICFLAG versus nephrin alone: 66%±3% versus 81%±2%; P=0.0003; Figure 2G). These data suggested that Notch inhibited expression of nephrin at the cell surface.
Figure 2.
Notch1-IC activity decreases cell surface nephrin localization in transfected HEK293T cells. Shown are representative, merged images of HEK293T cells stained with anti-nephrin (green), anti-FLAG (red), and WGA (blue) to detect NephrinMYC, NOTCH1-ICFLAG, and cell membrane, respectively; original magnification, ×600. (A and D) Representative dual channel images of cells stained for nephrin and NOTCH1-ICFLAG. (B and E) Dual channel images of the same cells stained for WGA and NOTCH1-ICFLAG. (C and F) Merged three-channel images of the same cells. (C' and F') Magnified views of corresponding areas in C and F, respectively, denoted by white boxes. (A, B, C, and C') HEK293T cells expressing NephrinMYC alone. (D, E, F, and F') HEK293T cells expressing both pNephrinMYC and pNOTCH1-ICFLAG. (A–C′) In cells expressing NephrinMYC alone, anti-nephrin staining is predominantly detected along the peripheral margins of cells and co-localizes with WGA (C', white arrow). (D–F) Conversely, in cells expressing both NephrinMYC and NOTCH1-ICFLAG, anti-nephrin staining is primarily detected within the cytoplasmic compartment and does not predominantly co-localize with WGA (F', white arrow). (G) Histogram depicting nephrin cell surface localization in HEK293T cells expressing either NephrinMYC alone (gray bar) or expressing both NephrinMYC and NOTCH1-ICFLAG (black bar) as determined by measuring anti-nephrin immunofluorescence intensity. Shown are mean values of cell surface–associated nephrin calculated as the difference between total and cytoplasmic anti-nephrin immunofluorescence intensities in single cells and expressed as a percentage of total cell anti-nephrin immunofluorescence intensity. Error bars denote SEM. Experiments were performed in duplicate. Gray bar, n=20 cells; black bar, n=27 cells.
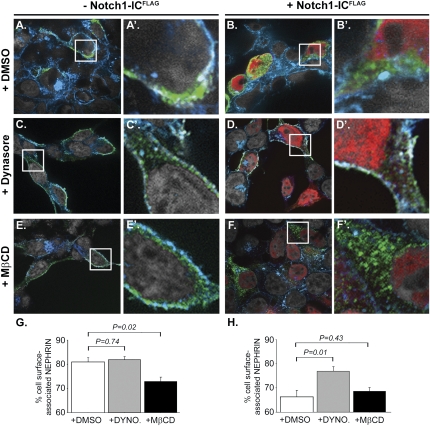
To determine if Notch-IC internalized nephrin by a dynamin-dependent mechanism, we analyzed nephrin expression in NOTCH1-ICFLAG–expressing HEK293T cells pretreated with Dynasore, which is a pharmacological inhibitor of dynamin GTPase.18 Confirmation that Dynasore effectively blocked dynamin-dependent trafficking was provided in untransfected cells cultured in the presence of AlexaFluor488-labeled transferrin (Supplemental Figure 5), which is known to be internalized with its cognate cell surface receptor by dynamin-dependent endocytosis.19 In the absence of NOTCH1-ICFLAG, Dynasore pretreatment did not substantially increase nephrin cell surface expression (Dynasore versus DMSO: 82%±1% versus 81%±1%; P=0.74; Figure 3, A versus C, and G, white versus gray bars). In contrast, Dynasore blocked nephrin internalization and increased nephrin surface expression in NOTCH1-ICFLAG–expressing cells by 11% compared with NOTCH1-ICFLAG–expressing cells pre-incubated with vehicle alone (Dynasore versus DMSO: 77%±2% versus 66%±3%; P=0.01; Figure 3, B versus D, and H, white versus gray bars).
Figure 3.
Notch-IC activity promotes nephrin internalization through a dynamin-dependent, raft-independent process. Shown are representative, merged images of HEK293T cells stained with anti-nephrin (green), anti-FLAG (red), and WGA (blue) to detect NephrinMYC, NOTCH1-ICFLAG, and cell membrane, respectively; original magnification, ×600. Cells were preincubated with either DMSO vehicle (A, A', D, and D'), Dynasore 160 μM (C, C', D, and D'), or MβCD 10 mM (E, E', F, and F'). (A, C, and E) Multichannel merged images depicting nephrin staining in NephrinMYC-expressing HEK293T cells. (B, D, and F) Multichannel merged images of nephrin and Notch-ICFLAG staining in HEK293T cells expressing both NephrinMYC and NOTCH1-ICFLAG. (A'–F') Enlarged view of areas represented by white boxes in corresponding panels A–F. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (recolorized as gray). (A, C, and E) Nephrin staining co-localizes with WGA along the peripheral margins of cells expressing NephrinMYC alone (A and A') and is unaffected by preincubation with Dynasore (C and C'). MβCD pretreatment resulted in a small increase in cytoplasmic staining for nephrin in cells expressing NephrinMYC alone (E and E') compared with cells preincubated with vehicle (A and A'). (B, D, and F) In the presence of vehicle alone (B), nephrin staining is strongly detected within the cytoplasm of cells, which also show nuclear staining for NOTCH1-ICFLAG (B', white arrow). Pretreatment with Dynasore (D) decreased the amount of cytoplasmic nephrin staining in NOTCH1-ICFLAG–expressing cells (D'), whereas MβCD did not block nephrin internalization (F) as revealed by comparable patterns of anti-nephrin staining within the cytoplasm of NOTCH1-ICFLAG–expressing cells (red nuclei) in both the absence (B') and presence (F') of MβCD. (G and H) Histograms showing the effect of Dynasore and MβCD on nephrin cell-surface localization as quantified by anti-nephrin immunofluorescence intensity in NephrinMYC-expressing cells (G) and in cells expressing both NephrinMYC and NOTCH1-ICFLAG (H). Shown are mean values of cell surface–associated nephrin calculated as the difference between total and cytoplasmic anti-nephrin immunofluorescence intensities in single cells and expressed as a percentage of total cell anti-nephrin immunofluorescence intensity. Error bars denote SEM. Experiments were performed in duplicate. White bars, DMSO treated; (G), n=28 cells; (H), n=20 cells. Gray bars, Dynasore treated; (G), n=25 cells; (H), n=22 cells. Black bars, MβCD treated; (G), n=18 cells; (H), n=15 cells.
We hypothesized that if Notch signaling additionally induced raft-mediated endocytosis, then pretreating HEK293T cells with methyl-β-cyclodextrin (MβCD), a chemical compound that depletes plasma membrane cholesterol,20 would block nephrin internalization in cells with Notch-IC activity. We confirmed spectrophotometrically that MβCD pretreatment reduced total cell cholesterol in HEK293T cells in a dose-dependent manner (Supplemental Figure 6). In cells expressing NephrinMYC alone, MβCD had a modest inhibitory effect (7% decrease) on nephrin cell surface expression (MβCD versus DMSO: 74%±2% versus 81%±2%; P=0.02; Figure 3, A versus E, and G, white versus black bars). We interpreted this 7% decrease in nephrin cell surface expression in cells without Notch-IC activity as representative of the baseline effect of cholesterol depletion in our experimental system. With that in mind, we subsequently determined the effect of MβCD in cells with Notch-IC activity.
In contrast to Dynasore, MβCD was ineffective at blocking nephrin internalization in NOTCH1-ICFLAG–expressing cells (MβCD versus DMSO: 66%±2% versus 66%±3%; P=0.43; Figure 3, B versus F, and H, white versus black bars). Moreover, when comparing results in MβCD-treated cells in the absence and presence of NOTCH1-ICFLAG (Figures 3, E versus F, respectively), the presence of NOTCH1-ICFLAG resulted in an additional 8%±3% decrease in nephrin cell-surface expression (Figure 3, G and H, black bars: 74%±2% versus 66%±2%; P=0.01). This result suggested that raft-mediated endocytosis could not fully account for effects of Notch signaling on nephrin internalization. Thus, a dynamin-dependent, raft-independent mechanism for nephrin endocytosis seemed to mediate the effects of Notch-IC on nephrin trafficking.
To explore the pathophysiological relevance of Notch signaling on decreased nephrin expression in vivo, we used scanning and transmission electron microscopy (SEM and TEM, respectively) to examine effects of Notch-IC activity on the morphologic appearance of podocyte SDs and foot processes (FPs) in glomeruli of newborn CRE[+];NOTCH-IC mice and littermate CRE[−];NOTCH-IC controls. By SEM, CRE[+];NOTCH-IC mouse glomeruli appeared less compacted compared with controls (Supplemental Figure 7, A and B). FPs appeared shorter and wider than corresponding structures in CRE[−];NOTCH-IC mice, which rendered a more simplified pattern of FP interdigitation than that observed in control glomeruli (Supplemental Figure 7, C and D). Analysis of newborn CRE[+];NOTCH-IC mouse glomeruli by TEM revealed focal areas that showed irregular-shaped or broad FPs (Supplemental Figure 7, E and F), narrowing or obliteration of the space between neighboring FPs, and disappearance of electron-dense SDs (Supplemental Figure 7, G and H). Semi-quantitative analysis of the number of SDs and FPs per unit length (in micrometers) of glomerular basement membrane (GBM) in 209 TEM imaged fields of seven glomeruli from two newborn CRE[+];NOTCH-IC and 204 TEM imaged fields of six glomeruli from two littermate control mice revealed fewer SDs per micrometer GBM in CRE[+];NOTCH-IC mouse glomeruli (median 1.1; interquartile range, 0.7–1.9) compared with control mice (median 1.5; interquartile range, 0.9–2.4; P=0.0004). Likewise, the number of FPs per micrometer of GBM was significantly lower in newborn CRE[+];NOTCH-IC mice (median 1.9; interquartile range, 0.7–2.6) compared with controls (median 2.4; interquartile range, 1.5–3.1; P=0.0001). From these data, together with our analyses of nephrin cell surface localization in HEK293T cells, we conclude that ectopic Notch activation may be a molecular trigger for SD destabilization and/or disassembly, and consequently, proteinuria.
Altered patterns of nephrin distribution have been demonstrated in experimental models of proteinuria (e.g., after puromycin injection),21 as well as in human biopsy samples from cases of heritable and acquired, proteinuric glomerulopathy.2,22,23 In acquired disease, the cause of secondary loss of nephrin expression is often unclear and may relate to the cause of podocyte injury. For heritable conditions, functional analyses of NPHS1 missense mutations in humans with congenital nephrotic syndrome revealed retained nephrin protein in the ER or Golgi.22,23 These genetic studies suggest that defective nephrin transport to the cell surface may be sufficient to induce loss of glomerular filtration barrier perm-selectivity. Results from our study suggest that ectopic Notch signaling activates a dynamin-dependent mechanism that promotes nephrin internalization. We recognize that nephrin endocytosis may not be a specific effect of Notch activation because other nonspecific cellular stress stimuli have been shown to cause nephrin internalization. For example, ER stress and altered glycosylation have been proposed as mechanisms that result in decreased nephrin trafficking to the cell surface, causing retention within intracellular compartments.24,25 In mouse kidney tissue sections from CRE[+];NOTCH-IC mice, we did not observe increased staining for anti-Grp78 (Supplemental Figure 8), a marker of ER stress,26 suggesting that ER stress is unlikely to be a mitigating factor in trafficking nephrin to the surface in Notch-activated cells. Further studies are required to determine if Notch activation regulates nephrin glycosylation in addition to endocytosis in podocytes.
In HEK293T cells, we used the cholesterol-depleting agent, MβCD, to disrupt lipid rafts and block raft-mediated endocytosis. We reasoned that if Notch-mediated nephrin internalization were dependent on raft-mediated endocytosis, then pretreatment with MβCD would result in increased nephrin localization at the surface in NOTCH1-ICFLAG–expressing cells. Rather, we observed no increase in nephrin cell-surface expression in NOTCH1-ICFLAG–expressing cells treated with MβCD compared with cells treated with vehicle alone. This led to the conclusion that a dynamin-dependent, endocytic mechanism, such as clathrin-mediated endocytosis, was more likely to be responsible for decreased cell surface nephrin expression in cells with Notch-IC activity. This possibility may be more precisely ascertained in vitro in knockdown experiments in which clathrin function can be inhibited in Notch-activated cells by using specific small interfering RNA.27 Moreover, we recognize that a Notch-IC–independent effect on nephrin internalization may be present, which caused a 7% decrease in surface expression in MβCD-treated cells that did not express Notch-ICFLAG (Figure 3, E and G, white bar versus black bar) and was not observed in MβCD-treated, NOTCH1-ICFLAG–expressing cells (Figure 3H, white bar versus black bar). One possibility is that Notch activity may mitigate effects of MβCD on nephrin internalization, possibly by directly interacting with and stabilizing formation of lipid microdomain protein complexes as observed for Notch-IC in other contexts (e.g., with active γ secretase).28,29 However, because NOTCH1-ICFLAG did not localize to the cytoplasm or cell surface in our in vitro experiments (Figure 2, D–F), we favor a mechanism for Notch that does not involve direct protein–protein interaction between Notch-IC and cell surface proteins.
Recently, Notch signaling has also been found to regulate endocytosis in HeLa cells after small interfering RNA knockdown of several human pathway genes including NOTCH4, PSEN2, MAML1, and RBPJ, which blocked internalization of early and late endosomal markers, transferrin and EGFR, respectively.30 Because RBPJ and MAML encode proteins that form part of active human Notch-IC/RBPJκ complexes,8,31 this report supports the notion that Notch signaling may be involved in regulating transcription of genes encoding endocytic proteins. Additional evidence in support of this concept is provided by a recent demonstration in Drosophila that orthologous Notch-IC/Su(H)–responsive cis-regulatory elements were recently identified within an intronic region of the numb gene,32 which encodes an evolutionary conserved, clathrin-associated adaptor protein implicated in endocytic trafficking of several proteins, including Notch.33,34 Our in vivo demonstration that nephrin staining is not attenuated in RBPJκ-deficient, MYCNOTCH-IC–expressing podocytes of CRE[+];NOTCH-IC;RBPKO further supports a Notch-dependent transcriptional mechanism for controlling nephrin trafficking in this context.
Until now, Notch signaling in podocytes was arguably viewed as a mechanism for glomerular disease progression through its deleterious effects on podocyte cell number.13,14 Our previous analysis of CRE[+];NOTCH-IC mice revealed a quantitative and progressive increase in podocyte cell proliferation exclusively in MYCNOTCH-IC–expressing podocytes that was temporally associated with development of glomerulosclerosis.14 Conversely, Niranjan et al. found in cultured human podocytes transduced with retroviral constructs expressing Notch1-IC that Notch activation induced apoptosis,13 which was not observed in our analysis of this cellular event in podocytes of newborn CRE[+];NOTCH-IC mice, as well as in mice at advanced stages of glomerulosclerosis.14 These opposing effects of Notch, which result in cell proliferation in one instance and cell death in another, is in character with the diverse growth regulatory roles for Notch signaling in other organ systems (e.g., skin), in which Notch functions either as a tumor suppressor or as an oncogene in a context-dependent manner.11 Mechanisms that account for these contextual responses may rely on molecular cross-talk between Notch and different downstream pathways as revealed recently for Notch and vascular endothelial growth factor signaling in glucose-stressed, cultured human podocytes, which was associated with decreased nephrin and increased apoptosis.35 Alternatively, contextual differences may involve induction of unique RBPJκ-dependent transcriptional responses (e.g., Hes1/Hey113; Hes1/HeyL [Wu MYJ, Onay T, Piscione TD, 2010, unpublished raw data]). Our future studies to define molecular responses downstream of Notch-IC/RBPJκ complexes will render insight into mechanisms responsible for downregulating nephrin cell surface expression before onset of proteinuria and disrupting cell-cycle regulation later in injured podocytes once proteinuria ensues. Defining these pathways will have significant implications for development of therapeutic approaches targeting Notch that may selectively promote glomerular filtration barrier repair (i.e., stop proteinuria) or prevent terminal podocyte injury (i.e., attenuate disease progression).
Concise Methods
Breeding, Genotyping, and Nephrectomy of NOTCH-IC Transgenic and Rbpj Mouse Strains
All transgenic mice were previously generated,36–39 maintained on mixed backgrounds, and genotyped as previously described.14 Bilateral nephrectomies were performed under sterile conditions on newborn mice. Experiments complied with ethical standards of The Hospital for Sick Children Research Institute Animal Care Committee.
SEM and TEM, Imaging, and Morphometric Analysis of Mouse Glomeruli
The Imaging Centre, Toronto Centre for Comparative Models of Human Disease, Mount Sinai Hospital, Toronto, Ontario, Canada, performed SEM and TEM. For SEM, kidney cortex fragments were immersed in 2% glutaraldehyde/0.1 M sodium cacodylate buffer, pH 7.3, followed by 1-hour postfixation in 1% osmium tetroxide/0.1 M sodium cacodylate buffer, pH 7.3. Gold-impregnated samples (Denton Desk II) were examined at 3 μm thickness under a scanning electron microscope (XL30, FEI) at an acceleration potential of 20 kV. For TEM, samples were fixed and dehydrated as above, treated with propylene oxide, and embedded in Spurr-Quetol resin. Uranyl acetate/lead citrate was used to stain 100-nm sections, which were observed under a transmission electron microscope (CM100, FEI) at 75 kV. Glomeruli (postcapillary loop stage) were imaged at two levels 100 μm apart. Fifty photomicrographs were obtained at random locations along glomerular capillary loops at ×46,000 magnification. SDs contiguous with GBM were counted for each imaged field. Corresponding GBM length was measured using image analysis software (Soft Imaging Systems).
Antibodies and Lectins
Primary antibodies used for multilabeling immunofluorescence staining of mouse kidney tissue sections were mouse monoclonal anti-MYC (1:1000; Invitrogen), rabbit polyclonal anti-WT1 (1:50; Santa Cruz), guinea pig polyclonal anti-nephrin (1:100; Fitzgerald, Concord, MA), rabbit polyclonal anti-podocin (1:100; Sigma), rabbit polyclonal anti-Neph1 (1:150; Santa Cruz), and rabbit polyclonal anti-ZO1 (1:100; Zymed). For multilabeling immunofluorescence staining of HEK293T cells, primary antibodies were guinea pig anti-nephrin (1:1000; Fitzgerald) and mouse monoclonal anti-FLAG (1:1000; Sigma). AlexaFluor 594- or 647-conjugated wheat germ agglutinin (WGA) (1:1000; Invitrogen) was used as a membrane marker.
Multilabeling Immunofluorescence Staining of Mouse Kidney Tissue Sections
Dual and triple immunofluorescence antibody staining was performed on formalin-fixed, paraffin-embedded 5-μm tissue sections as previously described.14 Primary antibody incubations were carried out simultaneously. AlexaFluor 488–, AlexaFluor 594–, or Cy5-conjugated secondary antibodies (1:1000; Invitrogen) were used for multilabeling immunodetection. Sections were counterstained with 4'-6-diamidino-2-phenylindole and imaged and photographed by fluorescence microscopy as described.14
Cell Culture, Plasmids, and Cell Transfections
HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI media 1640 (Invitrogen) supplemented with 5% FBS (Hyclone). The following plasmids were used for cell transfection: pcDNA3.1-hNephrin-MYC (referred to as pNephrinMYC)37 and pcDNA3.1-hNotch1-IC-FLAG (referred to as pNOTCH1-ICFLAG). pNOTCH1-ICFLAG was designed and generated using reverse transcription PCR to produce human Notch1-IC (amino acids 1749–2531), which was fused at its C terminus to FLAG polypeptide (Phe-Val-Ile-Ile-Ile-Leu-Val-Ile) and subcloned into pcDNA3.1. Cells were transiently transfected using LipoD293 (SinnaGen Laboratories) according to the manufacturer’s instructions, washed, and plated onto glass coverslips at a density of 500,000 cells per coverslip.
Nephrin Cell Surface Expression Assays in HEK293T cells
Analysis of cell surface protein expression was conducted on transfected cells as previously described, with minor modifications.27 Briefly, HEK293T cells were plated onto glass coverslips at 50% confluence and either doubly transfected with pNephrinMYC and pNOTCH1-ICFLAG plasmids or singly transfected with pNephrinMYC plasmid alone. Transfected cells were cultured for 24 hours at 37°C. In some experiments, transfectants were incubated with Dynasore (160 μm; Sigma) or MβCD (10 mM; Sigma) for 30 minutes at 37°C. Cells were washed in PBS, pH 7.4 (PBS), fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton. Fixed, permeabilized cells were co-incubated with anti-nephrin and anti-FLAG primary antibodies for 1 hour at room temperature, washed three times with PBS, and co-incubated for 1 hour at room temperature with AlexaFluor 488– and 594–conjugated secondary antibodies for anti-nephrin and anti-FLAG immunodetection, respectively, and AlexaFluor 647–conjugated WGA for membrane labeling. After washes, coverslips were mounted on glass slides with DAKO fluorescent mounting medium and examined using a spinning disk Leica DMIRE2 confocal microscope with Hamamatsu back-thinned EM-CCD camera. Single-channel images were acquired using appropriate excitation and emission filters and a ×60 oil immersion objective. Optical sections at 0.25 μm were obtained and analyzed using Volocity software (Improvision).
Cell surface nephrin expression was quantified by measuring immunofluorescence intensity of anti-nephrin staining using image analysis software as previously described with modifications.27 In brief, cells were inspected for anti-nephrin staining and immunofluorescence intensity values were obtained for total and cytoplasmic cell compartments in a single optical section through each cell. Total cell area corresponded to the region circumscribed by cell perimeter WGA staining within a single optical section. The cytoplasmic compartment was defined as the interior region of the cell that excluded the WGA-stained cell surface as well as the nucleus and nuclear membrane. Total and cytoplasmic anti-nephrin immunofluorescence intensities were subsequently measured within these regions as described.27 Cell surface NephrinMYC expression was consequently determined for each imaged cell by calculating the difference between total and cytoplasmic anti-nephrin immunofluorescence intensities. Mean values were expressed as a percentage of total cell immunofluorescence intensity.
Statistical Analyses
Unless otherwise specified, statistical analyses were performed by the Mann–Whitney U test using Statview 5.0 software (Stata Corp, College Station, TX). For comparisons of cell surface protein expression in transfected cells, a one-tailed test was used to analyze reductions in immunofluorescence intensity.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank S. Quaggin (Samuel Lunenfeld RI, Mount Sinai Hospital, Toronto, Canada) for providing Nephrin-CRE and Podocin-CRE mice and C. Lobe and J. Liu (formerly of Sunnybrook and Women’s College Health Science Centre, Toronto, Canada) for providing IC-Notch1 transgenic mice. We gratefully acknowledge B. Cohen of the Egan laboratory and S.-J. Kim and H. Su of the Piscione laboratory for technical assistance in the preparation of this manuscript. We also thank R. Temkin, Advanced Bioimaging Centre, and L. Morikawa, CMHD, Mount Sinai Hospital, Toronto, Ontario, for technical assistance in electron microscopy and histopathology, respectively.
This work was supported by a RESTRACOMP award from The Hospital for Sick Children Research Institute (A.M.W.) and by an American Society of Nephrology Norman Siegel Research Scholar Award and a Kidney Foundation of Canada Biomedical Research Grant (T.D.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011010027/-/DCSupplemental.
REFERENCES
- 1.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G: Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158: 1723–1731, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furness PN, Hall LL, Shaw JA, Pringle JH: Glomerular expression of nephrin is decreased in acquired human nephrotic syndrome. Nephrol Dial Transplant 14: 1234–1237, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T: Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron M: An overview of the Notch signalling pathway. Semin Cell Dev Biol 14: 113–119, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Schroeter EH, Kisslinger JA, Kopan R: Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A: Signalling downstream of activated mammalian Notch. Nature 377: 355–358, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Meier-Stiegen F, Schwanbeck R, Bernoth K, Martini S, Hieronymus T, Ruau D, Zenke M, Just U: Activated Notch1 target genes during embryonic cell differentiation depend on the cellular context and include lineage determinants and inhibitors. PLoS One 5: e11481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson A, Radtke F: Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett 580: 2860–2868, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Dotto GP: Notch tumor suppressor function. Oncogene 27: 5115–5123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grammont M: Adherens junction remodeling by the Notch pathway in Drosophila melanogaster oogenesis. J Cell Biol 177: 139–150, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K: The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 14: 290–298, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD: Ectopic Notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol 19: 1139–1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma M, Callen S, Zhang D, Singhal PC, Vanden Heuvel GB, Buch S: Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS 24: 2161–2170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Niranjan T, Murea M, Susztak K: The pathogenic role of Notch activation in podocytes. Nephron, Exp Nephrol 111: e73–e79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T: Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Jing SQ, Spencer T, Miller K, Hopkins C, Trowbridge IS: Role of the human transferrin receptor cytoplasmic domain in endocytosis: Localization of a specific signal sequence for internalization. J Cell Biol 110: 283–294, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilangumaran S, Hoessli DC: Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 335: 433–440, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F: Cloning of rat nephrin: Expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Doné SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, Tryggvason K: Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: Insight into the mechanisms of congenital nephrotic syndrome. Hum Mol Genet 10: 2637–2644, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Liu XL, Doné SC, Yan K, Kilpeläinen P, Pikkarainen T, Tryggvason K: Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J Am Soc Nephrol 15: 1731–1738, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Yan K, Khoshnoodi J, Ruotsalainen V, Tryggvason K: N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol 13: 1385–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Nakajo A, Khoshnoodi J, Takenaka H, Hagiwara E, Watanabe T, Kawakami H, Kurayama R, Sekine Y, Bessho F, Takahashi S, Swiatecka-Urban A, Tryggvason K, Yan K: Mizoribine corrects defective nephrin biogenesis by restoring intracellular energy balance. J Am Soc Nephrol 18: 2554–2564, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE: Coupling endoplasmic reticulum stress to the cell death program: Role of the ER chaperone GRP78. FEBS Lett 514: 122–128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YW, Su P, Liu GY, Crow MR, Chaukos D, Yan H, Robinson LA: Constitutive endocytosis of the chemokine CX3CL1 prevents its degradation by cell surface metalloproteases. J Biol Chem 284: 29644–29653, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ: Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem 283: 22529–22540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urano Y, Hayashi I, Isoo N, Reid PC, Shibasaki Y, Noguchi N, Tomita T, Iwatsubo T, Hamakubo T, Kodama T: Association of active gamma-secretase complex with lipid rafts. J Lipid Res 46: 904–912, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Collinet C, Stöter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, Habermann B, Buchholz F, Henschel R, Mueller MS, Nagel WE, Fava E, Kalaidzidis Y, Zerial M: Systems survey of endocytosis by multiparametric image analysis. Nature 464: 243–249, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD: MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 26: 484–489, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Rebeiz M, Miller SW, Posakony JW: Notch regulates numb: Integration of conditional and autonomous cell fate specification. Development 138: 215–225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP: Numb is an endocytic protein. J Cell Biol 151: 1345–1352, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGill MA, McGlade CJ: Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem 278: 23196–23203, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Lin CL, Wang FS, Hsu YC, Chen CN, Tseng MJ, Saleem MA, Chang PJ, Wang JY: Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes 59: 1915–1925, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788–793, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Lobe CG: Cre-conditional expression of constitutively active Notch1 in transgenic mice. Genesis 45: 259–265, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T: Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol 3: 443–450, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.