Abstract
ErbB4 receptor tyrosine kinase contributes to the development of the heart, the central nervous system, and the lactating mammary gland, but whether it has a role in the development of the kidney epithelium is unknown. Here, we found that expression of Erbb4 isoforms JM-a CYT-1 and JM-a CYT-2 was first detectable around embryonic day 13 in the mouse, mainly in the collecting ducts and both the proximal and distal tubules. In vitro, overexpression of a relevant ErbB4 isoform promoted proliferation and disturbed polarization of kidney epithelial cells when cultured as three-dimensional structures. We examined ErbB4 function in developing kidney tubules in vivo with Pax8-Cre–mediated conditional overexpression of Rosa26 locus–targeted ERBB4 and with conditional Erbb4 knock-out mice. The Pax8-Cre–driven ERBB4 overexpression enhanced proliferation in the collecting ducts, reduced the size of epithelial duct lumens, and promoted formation of cortical tubular cysts. These defects were associated with changes in the subcellular distribution of markers of epithelial cell polarity. Similarly, the Pax8-Cre–mediated Erbb4 knock-out mice manifested dysfunctional kidneys with larger duct lumens and epithelial cell mispolarization. Taken together, these data suggest that ErbB4 signaling modulates proliferation and polarization, cellular functions critical for the development of epithelial ducts in the kidney.
ErbB4 is a member of the EGF receptor (EGFR) subfamily of receptor tyrosine kinases, which also includes EGFR (ErbB1), ErbB2, and ErbB3.1 Gene targeting studies have revealed critical roles for ErbB4 in embryogenesis. Erbb4-null mice die by embryonic day 11 (E11) as a consequence of defective heart development.2 The embryonic lethality of Erbb4−/− mice can be rescued by ectopic expression of human ERBB4 under an α-myosin heavy-chain promoter revealing additional functions for ErbB4 in the lactating mammary gland and the central nervous system.3 There are also indications for a role in kidney development. ErbB4 is expressed in the developing tubules of the nephrons in vivo,4 and overexpression of a relevant ErbB4 isoform in Madin–Darby canine kidney (MDCK) cells promotes tubulogenesis in vitro.5 ErbB4 expression is also downregulated in human renal cell carcinoma6 and is upregulated in a mouse model of autosomal recessive polycystic kidney disease.7
In this study we addressed the potential role of ErbB4 in mammalian kidney development. We demonstrate that expression of specific Erbb4 isoforms is induced and maintained in the epithelial segments of the kidney, the nephrons, and the collecting ducts. Erbb4 gain- and loss-of-function studies in kidney epithelial cells in vitro and in vivo demonstrated altered ductal epithelial cell proliferation and compromised expression of proteins that are normally expressed in a polarized manner in the ducts. These results are in line with a conclusion that ErbB4 signaling is involved in the control of proliferation and polarization of kidney epithelial cells in the nephrons and collecting ducts during kidney development.
RESULTS
Developing Nephrons and Ureteric Epithelium Selectively Express Cleavable Erbb4 JM-a Isoforms
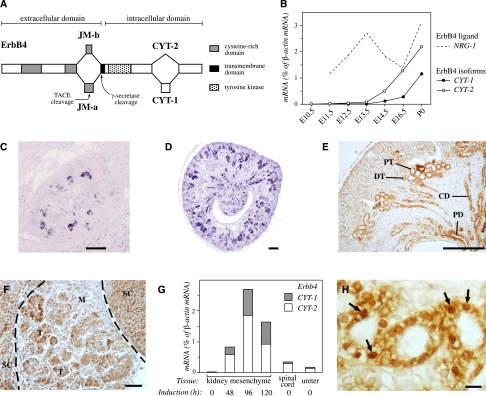
The Erbb4 gene is expressed as four alternatively spliced isoforms with unique signaling characteristics.8,9 The isoforms are composed of alternative extracellular juxtamembrane (JM) and intracellular cytoplasmic (CYT) domains (Figure 1A). The expression of all four Erbb4 isoforms in the kidneys of CD-1 mice was analyzed at developmental stages E10.5 through P0 (newborn) by real-time reverse transcriptase (RT)-PCR (Figure 1B). Erbb4 expression was detected from E12.5 onward, and the expression level increased until birth. Only isoforms JM-a CYT-1 and JM-a CYT-2 were present, and JM-a CYT-2 was more abundant. The gene encoding the ErbB4 ligand Nrg-1 was expressed throughout kidney development (Figure 1B). In addition to Erbb4, Egfr, Erbb2, and Erbb3 were also expressed in varying concentrations (maximally 1.2%, 19.3%, and 1.0% of β-actin mRNA, respectively), indicating that ErbB4 may signal in the developing kidney both as a homodimer and as a heterodimer.
Figure 1.
Erbb4 expression during kidney development. (A) Four ErbB4 isoforms are generated by alternative splicing. The isoforms differ at the extracellular JM or the CYT domains. JM-a isoforms can be cleaved by the tumor necrosis factor-α–converting enzyme (TACE) and γ-secretase to produce a soluble intracellular domain capable of translocating to the nucleus and regulating transcription.10,11,49 CYT-2 isoforms differ from CYT-1 isoforms by lacking 16 amino acids known to include a PI3-K binding site and a proline-rich interaction motif for WW domain–containing proteins.9,43 (B) Expression of transcripts encoding all four Erbb4 isoforms and the ErbB4 ligand Nrg-1 were analyzed at different stages of mouse kidney development by real-time RT-PCR. Only isoforms JM-a CYT-1 and JM-a CYT-2 were detected. β-actin was used as a reference gene to normalize expression levels. (C and D) In situ hybridization analysis of Erbb4 in E14.5 (C) and E17.5 (D) mouse kidneys demonstrated expression in the developing collecting ducts, adjacent epithelializing kidney mesenchymal cells, and relatively well-differentiated nephrons in the medulla. Alkaline phosphatase chromogen 5-bromo-4-chloro-3-indolyl-phosphatase/nitro blue tetrazolium (BCIP/NBT) (Roche Applied Science, Penzberg, Germany) was used to detect the probe. (E) Immunohistochemical analysis of ErbB4 expression in E16.5 mouse kidney demonstrated expression in developing epithelial structures consistent with the in situ hybridization analysis. CD, collecting duct; DT, distal tubule; PD, papillary duct; PT, proximal tubule. (F) Immunohistochemical analysis of ErbB4 expression in kidney mesenchyme induced for 84 hours with spinal cord demonstrated expression in induced epithelial structures. M, mesenchyme; SC, spinal cord; T, tubules developing from renal vesicles. (G) Real-time RT-PCR analysis of Erbb4 JM-a CYT-1 and JM-a CYT-2 isoform expression in uninduced mesenchyme; cultured mesenchyme induced with spinal cord for 48, 96, and 120 hours; spinal cord; and ureter. β-actin was used as a reference gene to normalize expression levels. (H) Immunohistochemical analysis of E16.5 mouse kidney with an antibody (sc-283) recognizing the carboxy-terminus of ErbB4 demonstrated nuclear immunoreactivity in proximal tubule cells (arrows). (E, F, and H) The 3,3′-diaminobenzidine (DAB) peroxidase substrate kit was used to detect the antibodies (Vector Laboratories, Burlingame, CA). Scale bars: (C and D) 250 μm; (E) 200 μm; (F) 50 μm; (H) 10 μm.
To define the cells that express Erbb4, in situ hybridization and anti-ErbB4 immunohistochemistry were carried out. In accordance with the real-time RT-PCR data (Figure 1B), Erbb4 expression was detected in epithelial cells at E14.5 (Figure 1C). At later stages of nephrogenesis, the expression was confined to their derivatives, the proximal and distal tubules and the loops of Henle. In addition to the developing nephrons, Erbb4 was expressed in ureteric bud–derived collecting duct epithelium in the medulla and in the papillae (Figure 1, D and E). Tissue recombination experiments with separated E11.5 kidney mesenchymes and a heterologous inducer tissue indicated that Erbb4 was activated in epithelializing pretubular aggregates in the induced mesenchyme (Figure 1F). Erbb4 expression was very low in uninduced kidney mesenchyme but was activated in epithelializing mesenchymal cells after 48 hours of tubule induction (Figure 1G). The expression was further upregulated at 96 and 120 hours in culture, indicating that Erbb4 expression is not activated as an immediate early response to tubule induction but is associated with epithelialization of the mesenchymal cells.
Only the ErbB4 JM-a isoforms can be proteolytically cleaved to produce a soluble intracellular domain that can translocate into the nucleus and regulate transcription.10–12 Indeed, both nuclear and cell membrane immunostaining for ErbB4 was observed in the developing tubules (Figure 1H). Taken together, these findings demonstrate that cleavable Erbb4 isoforms are expressed during kidney ontogeny by the epithelia of the maturing nephrons and the collecting ducts.
ERBB4 Gain-of-Function Disrupts Polarization and Cyst Formation of Tubular Epithelial Cells In Vitro
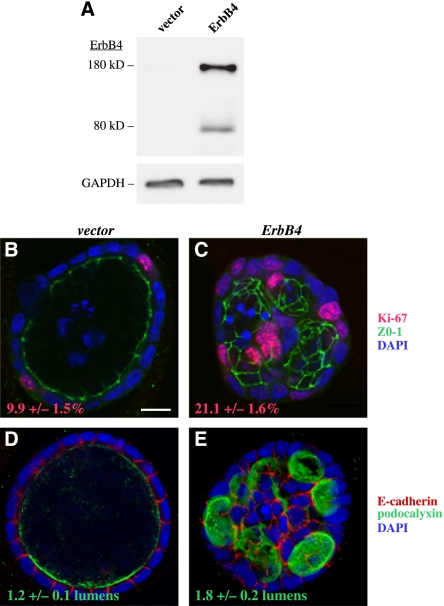
Kidney-derived MDCK epithelial cells were used to address the effects of ErbB4 signaling on kidney epithelium at the cellular level. When cultured in three-dimensional matrices, MDCK cells form cysts, i.e., hollow spheres lined by epithelium, and can be used as a model system to study such events as epithelial morphogenesis, cell polarization, and lumen formation.13,14 The MDCK cells were infected with retroviral constructs encoding ERBB4 JM-a CYT-2 or an empty vector control (Figure 2A), cultured for 5 days in Matrigel (BD Biosciences), and monitored for proliferation and cyst formation (Figure 2, B–E). MDCK cysts overexpressing ERBB4 contained significantly (P<0.001) more Ki-67–positive proliferating cells compared with control cysts (Figure 2, B and C). Unexpectedly, cells overexpressing ERBB4 also exhibited abnormal cyst polarization as they formed significantly (P=0.008) more cysts composed of multiple lumens compared with control cells; a majority of the latter formed cysts with a single lumen (Figure 2, D and E).
Figure 2.
ERBB4 overexpression promotes proliferation and disrupts polarization of epithelial cells in vitro. (A) Western analysis of ErbB4 expression in MDCK cells infected with a retrovirus encoding the ERBB4 JM-a CYT-2 isoform or an empty vector control. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was analyzed as a loading control. (B and C) MDCK cells were cultured in Matrigel for 5 days and stained for Ki-67 (red), ZO-1 (green), and 4′,6-diamidino-2-phenylindole (DAPI) (blue), to visualize proliferating cells, tight junctions, and nuclei, respectively. Cysts formed by cells overexpressing ERBB4 contained significantly more proliferating cells compared with vector control cysts (P<0.001). The percentage of Ki-67–positive cells out of DAPI-stained cells ± SEM is indicated. Scale bar: (B) 10 μm. (D and E) MDCK cysts were stained for E-cadherin (red), podocalyxin (green), and DAPI (blue) to visualize basolateral and apical surfaces and nuclei, respectively. Cysts formed by cells overexpressing ERBB4 formed significantly more lumens compared with the vector control cysts (P=0.008). The number of lumens per cyst ± SEM is indicated.
These findings indicate that ErbB4 controls kidney epithelial cell proliferation, polarization, and cyst formation in vitro.
ERBB4 Gain-of-Function Enhances Proliferation of Collecting-Duct Epithelial Cells, Interferes with the Development of Proximal Tubules, and Promotes Cyst Formation during Kidney Organogenesis In Vivo
To address the effect of ERBB4 gain-of-function on kidney development in vivo, a construct with human ERBB4 JM-a CYT-2 cDNA under a floxed transcriptional stop cassette was inserted into the genomic Rosa26 locus to generate R26ERBB4 mice (Supplemental Figure 1, A and B). The cleavable ERBB4 JM-a CYT-2 isoform was chosen because it was the prevailing isoform expressed in the kidney (Figure 1B). The Pax8-Cre mouse line was used to activate ERBB4 expression in the developing kidney. Cre recombinase under the control of the Pax8 promoter (Pax8-Cre) recombines a floxed reporter gene in the ureteric epithelial cells and the nephron-forming mesenchymal cells after their aggregation from around E11.5 onward (Supplemental Figure 1C).15 Crossing the Pax8-Cre line with the R26ERBB4 line led to the expected gain-of-function expression of human ErbB4 protein in the relevant epithelial structures in the developing kidneys of the R26ERBB4/Pax8-Cre embryos (Supplemental Figure 2, A–D). Analysis of ErbB4-activated signaling demonstrated enhanced ErbB4 and Erk phosphorylation in the mutant mice, while no significant effect on Akt phosphorylation was detected (Supplemental Figure 2, C–E).
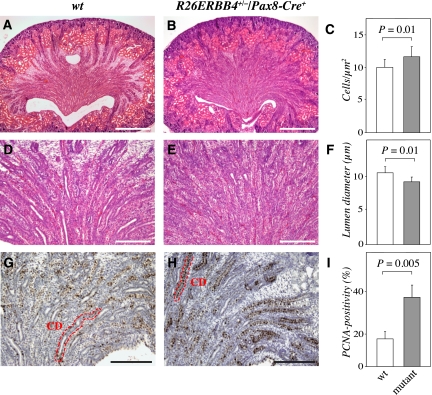
Histologic examination of newborn R26ERBB4+/−/Pax8-Cre+ mouse kidneys revealed characteristic phenotypes in the medulla and the cortex (Figure 3, A and B). Compared with the wild-type kidney, the mutant medulla had an irregular structure with clearly defective collecting duct organization (Figure 3, D and E). Morphometric measurements demonstrated that the cell density in the medullae of the mutant mice was significantly increased compared with wild-type kidneys (Figure 3C). In addition, the average diameter of the collecting duct lumen was significantly reduced (Figure 3F). As ErbB4 activation increased proliferation of kidney epithelial cells in vitro (Figure 2, B and C), newborn R26ERBB4/Pax8-Cre kidneys were analyzed with proliferating cell nuclear antigen (PCNA) immunostaining (Figure 3, G and H). Indeed, mutant papillary epithelial cells included a significantly greater percentage of PCNA-positive cells compared with wild-type samples (Figure 3I).
Figure 3.
ERBB4 gain-of-function leads to defective collecting duct development. Paraffin sections of R26ERBB4/Pax8-Cre kidneys (newborn) were stained with hematoxylin and eosin (A, B, D, and E) or with anti-PCNA and hematoxylin (G and H). R26ERBB4+/−/Pax8-Cre+ mutant mice demonstrated enhanced cellular density in the medulla (A–C), reduced mean diameter of collecting duct lumen (D–F), and increased amount of PCNA-positive tubular epithelial cells (dark brown signals) indicating enhanced proliferation (G–I). CD, collecting duct; wt, R26ERBB4−/−/Pax8-Cre–. Scale bars: (A and B) 500 μm; (D and E) 200 μm; (G and H) 250 μm.
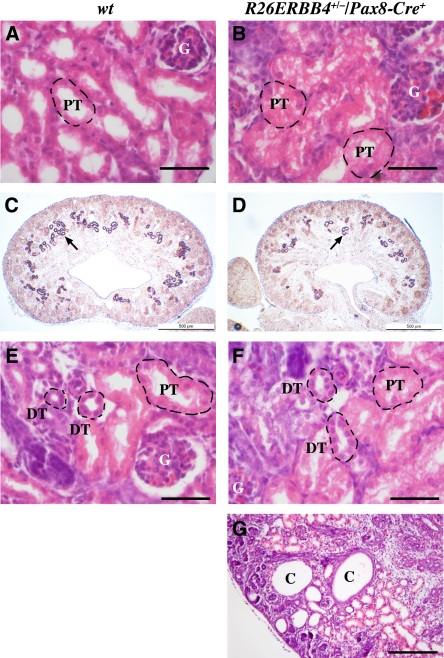
Defects were also observed in the mutant cortices, in particular in the proximal tubules, the epithelial structures most abundantly expressing endogenous ErbB4 (Figure 1, E and H). The proximal tubules of the mutant mice frequently demonstrated abnormal structure with lost or reduced lumens (Figure 4, A and B). In addition, in situ hybridization analysis with the proximal tubule marker Slc3A1 indicated a reduced number of proximal tubules in mutant mice (Figure 4, C and D). The structure of the distal tubules, however, was not affected by ERBB4 gain-of-function (Figure 4, E and F). Of note, abnormal formation of cortical tubular cysts was observed in approximately 50% of the kidneys of newborn mutant mice (Figure 4G). Immunohistochemical analysis demonstrated enhanced ErbB4 expression in the epithelial lining of the cysts but also showed that the expression was gradually lost when the diameter of the cysts increased (data not shown).
Figure 4.
ERBB4 gain-of-function results in defective proximal tubule development and leads to cyst formation. Paraffin sections of R26ERBB4/Pax8-Cre kidneys were stained with hematoxylin and eosin (A, B, E, F, and G) or analyzed by in situ hybridization to detect expression of the proximal tubule marker Slc3A1 (C and D). R26ERBB4+/−/Pax8-Cre+ mutant mice demonstrated aberrant proximal tubule structure with reduced luminal space (P0) (A and B) and a reduced number of proximal tubules, as indicated by Slc3A1 expression (purple indicated by arrow; E16.5) (C and D). The distal tubules, however, appeared normal (P0) (E and F). Tubular cysts were observed in kidneys of mutant mice (P0) (G). C, cyst; DT, distal tubule; G, glomerulus; PT, proximal tubule; wt, R26ERBB4−/−/Pax8-Cre–. Scale bars: (A, B, E, and F) 50 μm; (C and D) 500 μm; (G) 200 μm.
ERBB4 Gain-of-Function Disrupts Polarization and Lumen Formation in Collecting Ducts and Proximal Tubules In Vivo
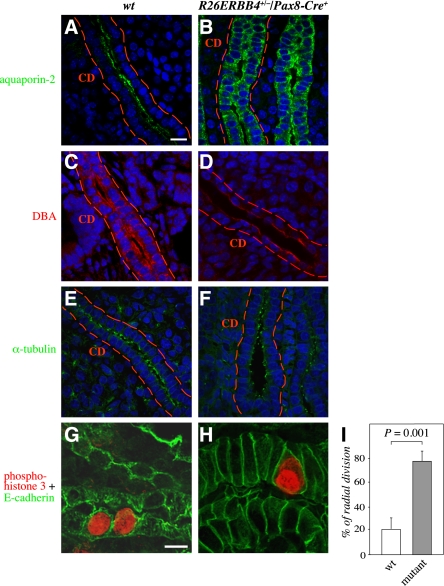
To address whether the observed phenotypes associated with defects in epithelial cell polarization, sections representing R26ERBB4/Pax8-Cre newborn kidneys were immunostained for aquaporin-1, aquaporin-2,16 Dolichos bifloris agglutinin (DBA),17 acetylated α-tubulin,18 and thiazide-sensitive NaCl cotransporter.19
Normally the collecting duct epithelium is composed of a single layer of cuboidal cells that are well lined next to each other (Figure 5, A and C). The ERBB4 gain-of-function changed this organization, and many of the ductal cells were morphologically abnormal and irregularly organized within the epithelial cell layer (Figure 5, B and D). Aquaporin-2 expression in the wild-type collecting ducts was polarized to the apical membranes of the epithelial cells (Figure 5A). In contrast, ERBB4 gain-of-function changed this pattern, and aquaporin-2 became localized into intracellular vesicles dispersed throughout the cytosol of epithelial cells (Figure 5B). Polarization of another collecting duct marker, DBA, was also altered, and its localization became restricted to apical surfaces in the mutant mice, whereas both apical and basolateral expression was seen in wild-type mice (Figure 5, C and D). ERBB4 gain-of-function also reduced the expression levels of DBA. Moreover, polarization of the tight-junction marker ZO-1 to the lateral membranes of the collecting-duct epithelial cells was partially disrupted in response to ERBB4 gain-of-function (data not shown). Immmunostaining for acetylated α-tubulin, in turn, indicated that the primary cilia of the mutant collecting ducts were not dramatically affected (Figure 5, E and F).
Figure 5.
ERBB4 gain-of-function disrupts polarization and enhances radial cell division of collecting-duct epithelial cells. (A–H) Immunohistochemical analyses of R26ERBB4/Pax8-Cre kidneys (P0) visualized by confocal microscopy. Staining for aquaporin-2 (green) and DBA (red) demonstrated altered polarization and disorganization of R26ERBB4+/−/Pax8-Cre+ mutant collecting duct epithelium (A–D). Expression of α-tubulin (green), in epithelial cell cilia of mutant collecting ducts was not significantly altered (E and F). The sections were stained for phospho-histone H3 (red) to define the axis of mitoses and with E-cadherin (green) to visualize cell borders (G and H). When the orientation of mitosis in collecting ducts was scored as longitudinal or radial (non-axis parallel) divisions, the mutant mice demonstrated significantly more radial cell divisions compared with the wild-type controls (I). Nuclei were stained with DAPI (blue). CD, collecting duct; wt, R26ERBB4−/−/Pax8-Cre–. Scale bars: (A) 20 μm; (G) 10 μm.
One characteristic feature of a developing duct is the regulation of the orientation of cell division. Division along the longitudinal axis of the duct increases the length of the duct, and radial division dilates the duct.20,21 To address whether the observed changes in the lumen size (Figures 3F and 4, A and B), proliferation (Figure 3I), and polarity (Figure 5, A–D) of the collecting duct epithelia were associated with changes in the axis of epithelial cell division, sections of R26ERBB4/Pax8-Cre newborn kidneys were immunostained with antibodies recognizing phosphorylated histone H3, and the axis of mitosis in relation to the axis of the lumen of the duct was determined under the microscope.21 Indeed, ERBB4 overexpression significantly increased the percentage of dividing collecting duct epithelial cells demonstrating radial axis of mitosis, compared with the control mice, which mostly demonstrated longitudinal cell division (Figure 5, G–I).
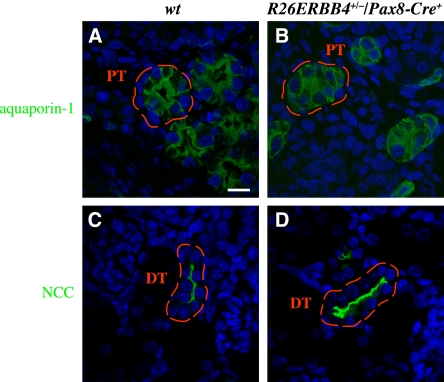
Similar to aquaporin-2 localization in the collecting ducts, aquaporin-1 in the mutant proximal tubule epithelia was localized throughout the cytosol as opposed to the apical cell surface polarization observed in the wild-type controls (Figure 6, A and B). The polarization of NaCl cotransporter in the mutant distal tubules, in contrast, appeared undisturbed and similar to that of wild-type tissues (Figure 6, C and D). These findings about the tubular cell polarization are consistent with the defects in the structure of the proximal (Figure 4, A and B) but not distal (Figure 4, E and F) tubule cells.
Figure 6.
ERBB4 gain-of-function disrupts polarization of proximal tubule epithelial cells. (A–D) Immunohistochemical analyses of R26ERBB4/Pax8-Cre kidneys (P0) visualized by confocal microscopy. Aquaporin-1 staining (green) demonstrated altered polarization and reduced lumen size of mutant proximal tubule epithelium (A and B). NaCl cotransporter (NCC) staining (green) indicated normal polarization and structure of mutant distal tubule epithelial cells (C and D). Nuclei were stained with DAPI (blue). DT, distal tubule; PT, proximal tubule; wt, R26ERBB4−/−/Pax8-Cre–. Scale bar: (A) 20 μm.
Taken together, these data suggest that ERBB4 gain-of-function in the developing kidney epithelium leads to defective polarization in collecting duct and proximal tubule epithelium, as well as to changes in the axis of cell division in the developing collecting duct.
Erbb4 Loss-of-Function Disrupts Polarization and Lumen Formation in Collecting Ducts and Proximal Tubules In Vivo
To further address the role of ErbB4 in regulating kidney tubulogenesis, ErbB4 signaling was conditionally inactivated using Cre recombinase under the Pax8 promoter to generate Erbb4Flox/Flox/Pax8-Cre+ mice. The Erbb4Flox/Flox/Pax8-Cre+ genotype was over-represented in prematurely terminated resorbed embryos at E11.5–E16.5 (8 mutants out of a total of 12 dead embryos; P<0.001). Moreover, at birth, significantly fewer viable pups had the Erbb4Flox/Flox/Pax8-Cre+ genotype (12.9%) than expected (25%) from the normal Mendelian distribution (P=0.02) (Table 1). Because Pax8 expression is also detectable in the developing heart22 and the Erbb4 null mice die by E11 with defective heart development,2 it is possible that heart-specific effects contributed to the compromised viability of the Erbb4Flox/Flox/Pax8-Cre+ mice. To minimize the contribution of possible developmental heart defects and subsequent changes in tissue perfusion on kidney development and function, the born mutant mice were not examined until adulthood (4 months of age). Four-month-old mutant mice did not exhibit signs of heart failure, such as edema at autopsy, and the lack of hypoxia in the mutant kidneys was documented by immunohistochemical analysis of hypoxia-inducible factor-1α expression (data not shown).
Table 1.
Genotype distribution of Erbb4Flox/Pax8-Cre mice in different developmental stages
| Stage | Mice Alive/Dead (n/n) | ||||
|---|---|---|---|---|---|
| FF Cre+ | F+ Cre+ | FF Cre− | F+ Cre− | Total | |
| E11.5 | 12/5 | 16/0 | 21/1 | 15/0 | 64/6 |
| E12.5 | 3/1 | 6/0 | 2/1 | 4/0 | 15/2 |
| E16.5 | 5/2 | 6/0 | 7/0 | 2/1 | 20/3 |
| P0 | 9/0 | 23/0 | 25/0 | 13/1 | 70/1 |
| All | 29/8 | 51/0 | 55/2 | 34/2 | 169/12 |
Erbb4Flox/Flox mice were bred with Erbb4Flox/+/Pax8-Cre+ mice. Heterozygous Pax-Cre–mediated Erbb4 knock-out resulted in fewer live pups at birth (P0) compared with expected (25%) Mendelian distribution (P=0.02). Significantly more dead Erbb4Flox/Flox/Pax8-Cre+ embryos were also observed (E11.5–P0) compared with other genotypes (P<0.001). FF Cre+, Erbb4Flox/Flox/Pax8-Cre+; F+ Cre+, Erbb4Flox/+/Pax8-Cre+; FF Cre-, Erbb4Flox/Flox/Pax8-Cre–; F+ Cre-, Erbb4Flox/+/Pax8-Cre–.
The kidneys of the surviving Erbb4Flox/Flox/Pax8-Cre+ mice demonstrated significantly reduced or lost ErbB4 expression (Supplemental Figure 3, A–E), and no signal for phosphorylated ErbB4 was detected (Supplemental Figure 3E). Analysis of Erk and Akt downstream signaling pathways indicated modestly reduced Akt phosphorylation in response to Erbb4 loss-of-function (Supplemental Figure 3E).
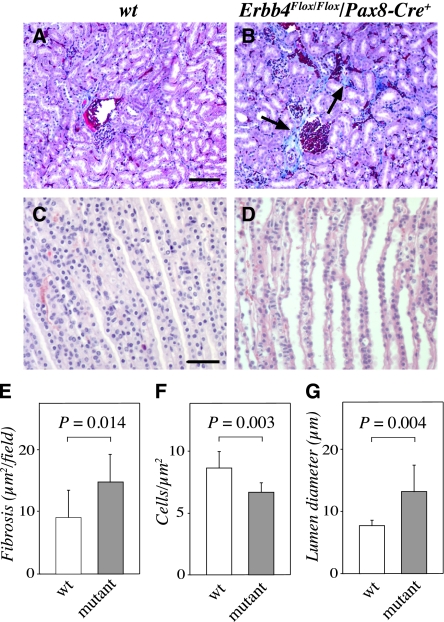
The mutant mice demonstrated abnormal kidney histologic features, with significantly more fibrotic tissue surrounding the Bowman capsules compared with wild-type kidneys (Figure 7, A, B, and E). Consistent with the gain-of-function data that indicated increased cell density as a result of ERBB4 overexpression, the density of cells in the medullae of the Erbb4 loss-of-function mice was significantly reduced (Figure 7F). Furthermore, the surviving mice manifested significantly dilated collecting ducts (Figure 7, C, D, and G).
Figure 7.
Erbb4 loss-of-function reduces medullar cell density and increases collecting duct lumen size. Paraffin sections of Erbb4Flox/Flox/Pax8-Cre (adult) kidneys were stained with Masson trichrome staining to visualize fibrosis (A and B) or with hematoxylin and eosin to visualize structure (C and D). Mutant kidneys demonstrated significantly increased fibrosis around the glomeruli (blue color indicated with arrows in B) (A, B, and E), and irregular collecting-duct structure compared with the wild-type controls (C and D). (F and G) Morphometric analyses demonstrated that the cell density in the medulla was reduced (F), whereas the mean diameter of the collecting duct lumens was increased (G) in the kidneys with Erbb4 loss-of-function. wt, Erbb4Flox/Flox/Pax8-Cre–. Scale bars: (A) 100 μm; (C) 50 μm.
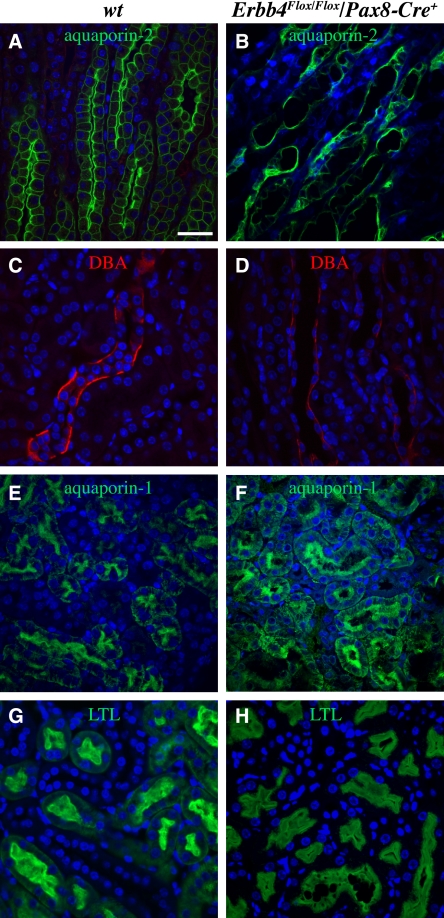
Immunohistochemical analyses of the Erbb4 loss-of-function mice with antibodies recognizing aquaporin-2 and DBA indicated mispolarization of the collecting duct epithelial cells (Figure 8, A–D). Similarly, analyses with antibodies recognizing aquaporin-1 and another proximal tubule marker, Lotus tetragonolobus lectin, indicated mispolarization of epithelial cells in the proximal tubules (Figure 8, E–H). These data indicate that, consistent with the observations based on the Pax8-Cre–mediated gain-of-function, the Pax8-Cre–mediated loss-of-function of Erbb4 leads to defective control of tubular epithelial cell growth and polarization.
Figure 8.
Erbb4 loss-of-function disrupts polarization of tubular epithelial cells. (A–H) Immunohistochemical analyses of Erbb4Flox/Pax8-Cre kidneys (adult) visualized by confocal microscopy. Aquaporin-2 (A and B; green) and DBA (B and C; red) staining demonstrated aberrant structure and polarization of collecting ducts of Erbb4 knock-out mice. Aquaporin-1 (E and F; green) and Lotus tetragonolobus lectin (LTL) (G and H; green) staining demonstrated enlarged lumens and disrupted polarization of proximal tubules of Erbb4 knock-out mice. Nuclei were stained with DAPI (blue). wt, Erbb4Flox/Flox/Pax8-Cre–. Scale bar (A): 50 μm.
DISCUSSION
The ERBB4 gene is actively expressed in the mature functional kidney.4,23 Previous in vitro work has demonstrated that the cleavable ErbB4 JM-a CYT-2 isoform promotes tubule formation in kidney epithelial cells.5 According to our real-time RT-PCR, in situ hybridization, and immunohistochemical analyses, Erbb4 was also expressed during kidney development in vivo. Expression of cleavable Erbb4 isoforms JM-a CYT-1 and JM-a CYT-2 was activated starting at E12.5 in association with epithelialization of the embryonic kidney mesenchymal cells during nephrogenesis. In addition, ErbB4 was present in the developing ureteric bud that generates the collecting-duct cells and the ureter. The observed ErbB4 expression pattern suggests that this signaling protein has a role in the development of kidney epithelium.
Functional gain-of-function and loss-of-function studies indicated a role for ErbB4 in orchestrating nephrogenic epithelial cell proliferation. Increased epithelial cell proliferation in vitro (by 2.1-fold) was observed in kidney-derived MDCK epithelial cells overexpressing the relevant ERBB4 isoform, JM-a CYT-2. Moreover, transgenic mice with Pax8 promoter-driven overexpression of ERBB4 JM-a CYT-2 in the developing kidney resulted in significantly increased cell density in the medulla (by 1.14-fold) and in enhanced proliferation (by 2.6-fold) of the epithelial cells of the collecting ducts. Finally, data from mice with conditional Erbb4 loss-of-function under the Pax8 promoter demonstrated a significant decrease in cell density in the medulla (by 1.3-fold). These findings are in line with previous data suggesting that the cleavable ErbB4 JM-a CYT-2 isoform selectively promotes proliferation in vitro11,24 and in the mammary epithelium in vivo.25
In addition to enhanced proliferation, disturbed epithelial cell polarization was observed in the MDCK cells overexpressing ERBB4, as well as in both gain- and loss-of-function mouse models. Because polarization of one of the used markers, aquaporin-2, has been shown to be regulated by the hydration status of the body,26 the analyses were also carried out with aquaporin-2–independent markers: DBA, LT-1, aquaporin-1, and ZO-1. The observation that plasma creatinine and electrolyte concentrations did not significantly differ between the knock-out and wild-type mice (data not shown) also indicated that the hydration status of the mice was similar. Furthermore, ERBB4 overexpression induced changes in kidney epithelial cell polarization in vitro, demonstrating that ErbB4 activity is sufficient to affect polarization under conditions with controlled water balance.
An intriguing finding was that the orientation of epithelial cell proliferation was more radial in relation to the axis of the collecting-duct lumen in mice with ERBB4 gain-of-function compared with wild-type controls. This finding is consistent with the observed changes in the lumen diameter of the collecting ducts in both the gain- and loss-of-function mouse models because radial orientation of cell division increases the width of the kidney collecting duct epithelium, whereas an increase of cell division along the longitudinal axis increases the length of the duct.20,21 Of note, misorientation of cell division has also been implicated as having a role in cyst formation in the kidney,20,27,28 and epithelial cortical cysts were occasionally observed in our model of ERBB4 gain-of-function. Taken together, these findings indicate that ErbB4 regulates normal kidney development by controlling polarization and orientation of cell division, whereas deregulated ErbB4 signaling may lead to epithelial cystogenesis.
ErbB signaling has also been associated with formation of renal cysts in previous studies. Indeed, an ERBB4 allele has been reported to be lost in a patient with a polycystic kidney and early myoclonic encephalopathy.29 In addition, increased ErbB4 expression has been shown in a mouse model of autosomal recessive polycystic kidney disease.7 Of the other ErbBs, deregulated EGFR and ErbB2 signaling has been associated with human autosomal recessive polycystic kidney disease (PKD),30,31 autosomal dominant PKD,32,33 and with rodent models of both forms of PKD.34,35 Furthermore, ErbB2 inhibition reduces cyst formation in a mouse model of PKD and rescues the impaired migration of epithelial cell lines isolated from patients with autosomal dominant PKD.34 Thus, several lines of evidence imply a role for ErbB signaling in the generation of renal cysts both in mouse models and in human disease.
In summary, we demonstrate that specific isoforms of the Erbb4 receptor tyrosine kinase are induced in both mesenchyme- and ureteric bud–derived epithelia of the embryonic kidney. In addition, these findings indicate a functional role for ErbB4 signaling pathway in establishing and maintaining the kidney epithelium by controlling proliferation, polarization, and orientation of cell division.
CONCISE METHODS
Animals
Erbb4Flox/Flox,36 Pax8-Cre,15,37 and Rosa26LacZ37 mice were used to generate Erbb4Flox/Pax8-Cre mice. CD-1 mice were used to examine Erbb4 expression in wild-type embryos and newborn mice (Figure 1), whereas all the mutant mice were maintained and bred in C57BL/6 background.
Real-Time RT-PCR
Real-time RT-PCR was carried out using primers and probes listed in Supplemental Table 1 or described earlier.38
In Situ Hybridization
In situ hybridization analysis of Erbb4 expression was carried out at Max Planck Institute of Biophysical Chemistry (Gene Paint database; http://www.genepaint.org). Slc3A1 expression was analyzed as described elsewhere.39
Immunostaining
Proliferation was assessed from paraffin-embedded sections using a PCNA staining kit (Invitrogen, Carlsbad, CA). ErbB4 immunohistochemistry was carried out, as described elsewhere,38 except that sc-283 (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the primary antibody. HIF-1α immunohistochemistry was done with H1alpha67 (Abcam, Cambridge, UK) primary antibody using HistoMouse-MAX kit (Invitrogen). To identify fibrotic tissue, paraffin sections were stained with Masson trichrome.40
Immunofluorescence staining of paraffin sections was carried out using primary antibodies against aquaporin-1 (Millipore, Billerica, MA), aquaporin-2 (Sigma-Aldrich, St. Louis, MO), thiazide-sensitive NaCl cotransporter (Millipore), phospho-histone H3 (Millipore), acetylated α-tubulin (Sigma-Aldrich), DBA (rhodamine labeled) (Vector Laboratories, Burlingame, CA), Lotus tetragonolobus lectin (fluorescein labeled) (Vector Laboratories), anti–ZO-1 (Invitrogen), or E-cadherin (BD Biosciences, Franklin Lakes, NJ), and Alexa Fluor 488- or 546-conjugated secondary antibodies (Invitrogen). Nuclei were stained with 4',6-diamidino-2-phenylindole (Sigma Pharmaceuticals).
Immunofuorescence staining of MDCK cells was carried out, as described elsewhere,14 using the following primary antibodies: anti–E-cadherin (rrl),41 anti-podocalyxin (gp135),42 anti–ZO-1 (Invitrogen), and anti–Ki-67 (Invitrogen). Anti-podocalyxin and anti–E-cadherin were gifts from Dr. Kai Simons (MPI-CBG, Dresden, Germany). The primary antibodies were detected using Alexa Fluor 488- or 546-conjugated secondary antibodies and the nuclei were stained with 4',6-diamidino-2-phenylindole.
To evaluate the orientation of cell division, sections of newborn R26ERBB4/Pax8-Cre mouse kidneys were immunostained with anti–phospho-histone H3 and anti–E-cadherin antibodies.
Western Blotting
Proteins were extracted from MDCK cells or embryonic and newborn kidneys and analyzed by Western blotting using anti-ErbB4 (E200) (Abcam), anti–phospho-ErbB4 (#4757; Cell Signaling, Danvers, MA), anti-Akt (sc-1618; Santa Cruz Biotechnology), anti–phospho-Akt (#9271; Cell Signaling), anti-Erk (#9102; Cell Signaling), anti–phospho-Erk (#9101; Cell Signaling), anti–β-actin (sc-1616; Santa Cruz Biotechnology), and anti–glyceraldehyde 3-phosphate dehydrogenase (Millipore) antibodies, as described elsewhere.43
Three-Dimensional Culture of MDCK Cells
MDCK cells were transduced with retroviral constructs encoding ERBB4 JM-a CYT-2 (pBABE-puroErbB4JM-aCYT-2) or an empty vector.44 The transduced cells were cultured for 5 days in Matrigel and fixed with 4% paraformaldehyde.45
Tissue Culture
For kidney mesenchyme tubule induction assays, E11.5 mesenchymes were separated from the ureteric buds and cultured for 0, 48, 96, or 120 hours in combination with a heterologous tubule inducer tissue, a dorsal piece of E11.5 embryonic spinal cord.46
Targeting of Human ERBB4 cDNA into the Rosa26 Locus to Generate a Mouse Model for Conditional Expression
Human ERBB4 JM-a CYT-2 cDNA was amplified by PCR (Supplemental Table 2, PCR I) from a vector pcDNA3.1ErbB4JM-aCYT-2.11 The amplified PCR fragment was cloned into a pRosa26-DEST vector.47 This generated a targeting vector, pRosa26-DEST-ERBB4, that contained the ERBB4 insert under a floxed transcriptional stop cassette. The targeting vector was electroporated into a SV-129 mouse embryonic stem cells. Targeting to the Rosa26 locus (Supplemental Figure 1A) was identified by Southern blotting48 and by PCR (Supplemental Table 2, PCR II; Supplemental Figure 1B) and was confirmed by sequencing.
Selected embryonic stem cells were injected into blastocyst stage C57BL/6 mouse embryos to obtain chimeras. The chimeric mice were screened for germline transmission to obtain F1 progeny of the R26ERBB4+/− mice that were heterozygous for the ERBB4 JM-a CYT-2 cDNA under a floxed transcriptional stop cassette inserted to the Rosa26 locus. The F1 mice were bred with C57BL/6 mice to produce F2 progeny. The heterozygous R26ERBB4+/− mice were further crossed with the Pax8-Cre recombinase-positive mice15 to obtain R26ERBB4/Pax8-Cre mice (Supplemental Figure 2).
Statistical Analyses
A t test was used to statistically analyze morphometric and immunohistochemical data. A chi-squared test was used to determine whether the number of ErbB4Flox/Flox/Pax8-Cre+ pups and embryos deviated from the expected Mendelian ratios. The statistical analyses were done using the SPSS Statistics program (Version 19.0.0; IBM, Armonk, NY).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Hannele Härkman, Johanna Kekolahti-Liias, Jaana Kujala, Merja Lakkisto, and Tuula Oivanen for excellent technical assistance. We also thank Ilkka Paatero for assistance with HIF immunohistochemistry and Ana Martinez-Hernandez at the Max Planck Institute of Biophysical Chemistry for help with the Erbb4 in situ hybridization.
This work was supported by the Academy of Finland, European Union, Finnish Cancer Organizations, Foundation for the Finnish Cancer Institute, Sigrid Jusélius Foundation, and Turku University Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Polarity and Renal Cystogenesis,” on pages 4–5.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011020160/-/DCSupplemental.
REFERENCES
- 1.Yarden Y, Sliwkowski MX: Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G: Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378: 390–394, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP: Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA 100: 8281–8286, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ: Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol 185: 236–245, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Zeng F, Zhang MZ, Singh AB, Zent R, Harris RC: ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol Cell 18: 4446–4456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomasson M, Hedman H, Junttila TT, Elenius K, Ljungberg B, Henriksson R: ErbB4 is downregulated in renal cell carcinoma—a quantitative RT-PCR and immunohistochemical analysis of the epidermal growth factor receptor family. Acta Oncol 43: 453–459, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Nemo R, Murcia N, Dell KM: Transforming growth factor alpha (TGF-alpha) and other targets of tumor necrosis factor-alpha converting enzyme (TACE) in murine polycystic kidney disease. Pediatr Res 57: 732–737, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junttila TT, Sundvall M, Määttä JA, Elenius K: Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc Med 10: 304–310, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Sundvall M, Korhonen A, Paatero I, Gaudio E, Melino G, Croce CM, Aqeilan RI, Elenius K: Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc Natl Acad Sci USA 105: 4162–4167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni CY, Murphy MP, Golde TE, Carpenter G: gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294: 2179–2181, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Määttä JA, Sundvall M, Junttila TT, Peri L, Laine VJ, Isola J, Egeblad M, Elenius K: Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol Biol Cell 17: 67–79, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G: Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell 127: 185–197, 2006 [DOI] [PubMed] [Google Scholar]
- 13.O’Brien LE, Zegers MM, Mostov KE: Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Torkko JM, Manninen A, Schuck S, Simons K: Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci 121: 1193–1203, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bouchard M, Souabni A, Busslinger M: Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 38: 105–109, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Devuyst O, Burrow CR, Smith BL, Agre P, Knepper MA, Wilson PD: Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol 271: F169–F183, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ: Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current Biology. 12::R378. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt R, Ellison DH, Farman N, Rossier BC, Reilly RF, Reeves WB, Oberbäumer I, Tapp R, Bachmann S: Developmental expression of sodium entry pathways in rat nephron. Am J Physiol 276: F367–F381, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon APA: A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136: 161–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Lai D, Huang X, Shi X, Gao Z, Huang F, Zhou X, Geng YJ, et al. The defects in development and apoptosis of cardiomyocytes in mice lacking the transcriptional factor Pax-8. [published online ahead of print September 17, 2010] Int J Cardiol. . 10.1016/j.ijcard.2010.08.057 [DOI] [PubMed] [Google Scholar]
- 23.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M: A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem 272: 26761–26768, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Sundvall M, Veikkolainen V, Kurppa K, Salah Z, Tvorogov D, van Zoelen EJ, Ageilan R, Elenius K, et al. Cell death or survival promoted by alternative isoforms of ErbB4. Mol Biol Cell 21:4275–4286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muraoka-Cook RS, Sandahl MA, Strunk KE, Miraglia LC, Husted C, Hunter DM, Elenius K, Chodosh LA, Earp HS, 3rd: ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Mol Cell Biol 29: 4935–4948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T: Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol 130: 197–209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST: Primary cilia and signaling pathways in mammalian development, health and disease. Nephron, Physiol 111: 39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backx L, Ceulemans B, Vermeesch JR, Devriendt K, Van Esch H: Early myoclonic encephalopathy caused by a disruption of the neuregulin-1 receptor ErbB4. Eur J Hum Genet 17: 378–382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweeney WE, Jr, Avner ED: Functional activity of epidermal growth factor receptors in autosomal recessive polycystic kidney disease. Am J Physiol 275: F387–F394, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi K, Sweeney W, Jr, Avner ED: Segment-specific c-ErbB2 expression in human autosomal recessive polycystic kidney disease. J Am Soc Nephrol 12: 379–384, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Wilson PD, Du J, Norman JT: Autocrine, endocrine and paracrine regulation of growth abnormalities in autosomal dominant polycystic kidney disease. Eur J Cell Biol 61: 131–138, 1993 [PubMed] [Google Scholar]
- 33.Du J, Wilson PD: Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol 269: C487–C495, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Wilson SJ, Amsler K, Hyink DP, Li X, Lu W, Zhou J, Burrow CR, Wilson PD: Inhibition of HER-2(neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim Biophys Acta 1762:647–655, 2006 [DOI] [PubMed]
- 35.Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED: Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol 19: 1331–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hennighausen L, Jones FE: Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development 130: 5257–5268, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Jokela T, Vainio S: Conditional tamoxifen Cre induced mutagenesis in the embryonic kidney in organ culture. Genesis 45: 757–761, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Junttila TT, Laato M, Vahlberg T, Söderström KO, Visakorpi T, Isola J, Elenius K: Identification of patients with transitional cell carcinoma of the bladder overexpressing ErbB2, ErbB3, or specific ErbB4 isoforms: Real-time reverse transcription-PCR analysis in estimation of ErbB receptor status from cancer patients. Clin Cancer Res 9: 5346–5357, 2003 [PubMed] [Google Scholar]
- 39.Zhang S, Lin Y, Itäranta P, Yagi A, Vainio S: Expression of Sprouty genes 1, 2 and 4 during mouse organogenesis. Mech Dev 109: 367–370, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sheehan DC, Hrapchak BB: Theory and Practice of Histotechnology, St. Louis, London, Mosby, 1980 [Google Scholar]
- 41.Gumbiner B, Simons K: A functional assay for proteins involved in establishing an epithelial occluding barrier: Identification of a uvomorulin-like polypeptide. J Cell Biol 102: 457–468, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojakian GK, Schwimmer R: The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol 107: 2377–2387, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kainulainen V, Sundvall M, Määttä JA, Santiestevan E, Klagsbrun M, Elenius K: A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxis. J Biol Chem 275: 8641–8649, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Tvorogov D, Sundvall M, Kurppa K, Hollmén M, Repo S, Johnson MS, Elenius K: Somatic mutations of ErbB4: selective loss-of-function phenotype affecting signal transduction pathways in cancer. J Biol Chem 284: 5582–5591, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Friedrichs J, Torkko JM, Helenius J, Teräväinen TP, Füllekrug J, Muller DJ, Simons K, Manninen A: Contributions of galectin-3 and -9 to epithelial cell adhesion analyzed by single cell force spectroscopy. J Biol Chem 282: 29375–29383, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP: Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 122: 3627–3637, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Hohenstein P, Slight J, Ozdemir DD, Burn SF, Berry R, Hastie ND: High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Pathogenetics 1: 3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Rio C, Buxbaum JD, Peschon JJ, Corfas G: Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem 275: 10379–10387, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.