Abstract
Upregulation of clusterin occurs in several renal diseases and models of nephrotoxicity, but whether this promotes injury or is a protective reaction to injury is unknown. Here, in the mouse unilateral ureteral obstruction model, obstruction markedly increased the expression of clusterin, plasminogen activator inhibitor-1 (PAI-1), type I collagen, and fibronectin. Compared with wild-type mice, clusterin-deficient mice exhibited higher levels of PAI-1, type I collagen, and fibronectin and accelerated renal fibrosis in response to obstruction. In cultured rat tubular epithelium-like cells, adenovirus-mediated overexpression of clusterin inhibited the expression of TGF-β–stimulated PAI-1, type I collagen, and fibronectin. Clusterin inhibited TGF-β–stimulated Smad3 activity via inhibition of Smad3 phosphorylation and its nuclear translocation. Moreover, intrarenal delivery of adenovirus-expressing clusterin upregulated expression of clusterin in tubular epithelium-like cells and attenuated obstruction-induced renal fibrosis. In conclusion, clusterin attenuates renal fibrosis in obstructive nephropathy. These results suggest that upregulation of clusterin during renal injury is a protective response against the development of renal fibrosis.
Renal fibrosis is the final common pathway of CKD and is characterized by an accumulation of extracellular matrix (ECM) proteins. TGF-β is central to the development of renal fibrosis through its stimulating effect on matrix protein generation and its inhibitory effect on matrix protein removal.1–4 Overexpression of TGF-β isoforms and their receptors has been observed in a variety of renal diseases in both animals and humans, ranging from diabetic nephropathy and GN to tubulointerstitial nephritis.2,5–8 The obstructed kidneys express 20-fold higher levels of TGF-β compared with the unobstructed kidneys and increased levels of TGF-β induce the transcription of genes involved in ECM protein accumulation, including type I collagen and fibronectin.9 Damage of the obstructed kidney is accompanied by progressive interstitial fibrosis, which is caused by the excessive production and deposition of ECM (e.g., fibronectin and collagens) in the interstitium.9,10 In addition, TGF-β stimulates the expression of many ECM proteins in renal cells by stimulating the expression of protease inhibitors, including plasminogen activator inhibitor-1 (PAI-1).11–13 PAI-1 is the main physiologic inhibitor of tissue and urokinase plasminogen activators and is considered to be the most important inhibitor of fibrinolysis.14,15 Recent studies suggest PAI-1 directly promotes tissue fibrosis by promoting the migration of monocytes/macrophages, transdifferentiated tubular epithelia, and myofibroblasts.16–18 PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction.17 PAI-1 inhibits ECM breakdown, which decreases the activation of plasmin, and these effects are linked to the exacerbation of glomerulosclerosis.15,19 PAI-1 is also directly involved in interstitial fibrosis and tubular damage, with early effects on interstitial cell recruitment and late effects associated with decreased urokinase activity.18,19
The clusterin/apolipoprotein J gene is expressed in most human tissues, and the secreted protein is found in all body fluids. The gene encodes two isoforms: a conventional ubiquitous secretory heterodimeric disulfide-linked glycoprotein and a truncated nuclear form.20,21 Clusterin has been functionally implicated in several physiologic processes, including lipid transportation, cell adhesion, morphologic transformation, membrane recycling, cell–cell interactions, and aging, and also in pathologies such as diabetes, atherosclerosis, degenerative disease, and tumorigenesis.22,23 Increased expression of clusterin has been found in several renal disease models such as unilateral ureteral obstruction (UUO) and in nephrotoxicity,24–28 suggesting that clusterin may have an important role in nephropathogenesis.29,30 However, it is not known whether clusterin exerts a protective or a causative effect in the development of renal fibrosis. Several lines of evidence suggest that clusterin exerts a protective role in progressive nephropathy and mesangial cell injury.31,32 In this report, loss of clusterin is shown to accelerate renal fibrosis induced by UUO, whereas its overexpression is shown to prevent UUO-induced renal fibrosis.
RESULTS
Loss of Clusterin Increases PAI-1, Type I Collagen, and Fibronectin Expression and Accelerates Renal Fibrosis after UUO
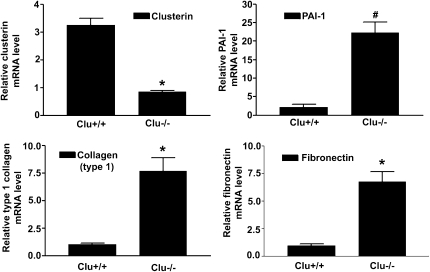
The effect of clusterin loss on the renal expression of PAI-1, type I collagen, and fibronectin and on renal fibrosis was examined. In the kidneys of clusterin knockout (Clu−/−) mice, PAI-1, type I collagen, and fibronectin mRNA levels were increased compared with those in the kidneys of wild-type (Clu+/+) mice (Figure 1).
Figure 1.
Loss of clusterin increased PAI-1, type 1 collagen, and fibronectin expression. Representative real-time RT-PCR analysis of PAI-1, type I collagen, and fibronectin expression in kidneys of wild-type (Clu+/+) and Clu KO (Clu−/−) mice. Real-time RT-PCR was performed using RNA from three different Clu+/+ or Clu−/− mouse kidneys. GAPDH mRNA was used as an internal control. Data are the mean ± SEM of three independent measurements (three separate experiments). *P<0.01 and #P<0.001 compared with wild-type mice.
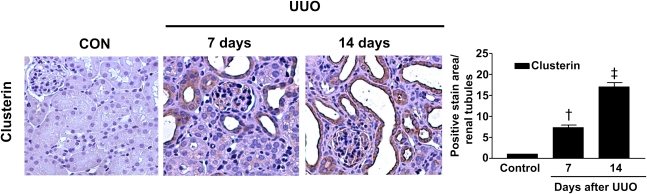
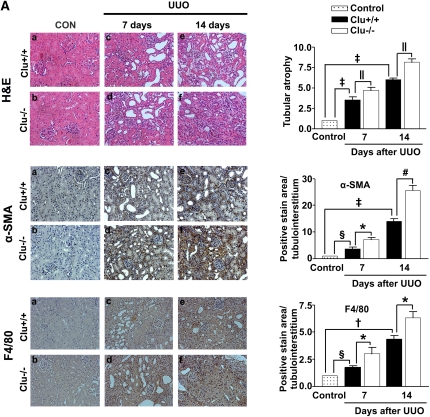
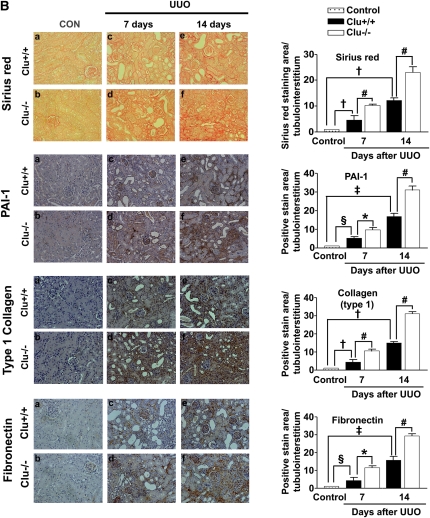
These data prompted us to examine whether loss of clusterin accelerates renal fibrosis in the UUO model. In wild-type mice, clusterin expression was markedly increased in the renal tubule area after UUO (Figure 2). As expected, the architecture of tubules was dramatically altered in UUO kidneys, resulting in tubular atrophy and the development of interstitial fibrosis. Hematoxylin and eosin (H&E) staining showed that, in comparison with the wild-type mice, the Clu−/− mice exhibited markedly increased tubulointerstitial damage after UUO (Figure 3A). Macrophage infiltration, demonstrated by anti-F4/80 antibody, was used as an indicator of inflammation of the kidney. As shown in Figure 3A, F4/80-positive macrophage infiltration in the kidneys of Clu−/− mice 7 days after UUO was higher than in wild-type mice. Moreover, in the kidneys of Clu−/− mice 7 days after UUO, the frequency of α-smooth muscle actin (SMA)-positive myofibroblasts was increased compared with wild-type mice (Figure 3A). Sirius red staining showed, in comparison with wild-type mice, the Clu−/− mice had a marked increase in renal fibrosis 7 days after UUO (Figure 3B). Immunohistochemical staining for PAI-1, type I collagen, and fibronectin revealed that renal fibrosis in the UUO kidneys of Clu−/− mice was more extensive than in wild-type mice (Figure 3B). The significant differences in tubulointerstitial damage, inflammation, renal fibrosis, and the expression of profibrotic genes between wild-type mice and Clu−/− mice persisted at 14 days after UUO (Figure 3, A and B). These data suggest that clusterin plays an important role in ECM accumulation and renal fibrosis in obstructive nephropathy.
Figure 2.
Clusterin expression was increased in the renal tubular area after UUO. Representative images of immunohistochemical staining for clusterin expression in kidneys from obstructed Clu+/+ and Clu−/− mice at 7 and 14 days after UUO. Kidney sections were analyzed using computer-based morphometry. Data from the UUO kidneys were normalized to the control (=1) and in the bar graph were expressed as fold increases in clusterin expression relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). †P<0.01 and ‡P<0.001 compared with control. Original magnification, ×400.
Figure 3.
Loss of clusterin accelerated renal fibrosis after UUO. (A) Representative images of H&E and immunohistochemical staining for α-SMA and F4/80 of the renal cortex in kidney tissue sections from unobstructed Clu+/+ and Clu−/−, Clu+/+ and Clu−/− at 7 days after UUO, and Clu+/+ and Clu−/− at 14 days after UUO. The number of atrophic tubules was determined by measurement of the abnormal irregular and dilated tubular basement membranes in H&E-stained sections of kidneys in five random fields under high-power magnification. Areas of positive immunostaining with α-SMA and F4/80 were quantified by computer-based morphometric analysis. All data were normalized to the control (=1) and in all bar graphs were expressed as fold increase relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). Original magnification, ×200. (B) Representative images of renal cortex sections stained with Sirius red and immunohistochemical staining for PAI-1, fibronectin, and type I collagen from unobstructed Clu+/+ and Clu−/−, Clu+/+ and Clu−/− at 7 days after UUO, and Clu+/+ and Clu−/− at 14 days after UUO. All data obtained by computer-based morphometric analysis of positive areas in the UUO kidneys were normalized to the control (=1) and in all bar graphs were expressed as the fold increase relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). †P<0.01, §P<0.05, and ‡P<0.001 compared with control and *P<0.01, ‖P<0.05, and #P<0.001 compared with wild-type mice. Original magnification, ×200.
Clusterin Inhibits TGF-β–Stimulated PAI-1, Type I Collagen, and Fibronectin Expression
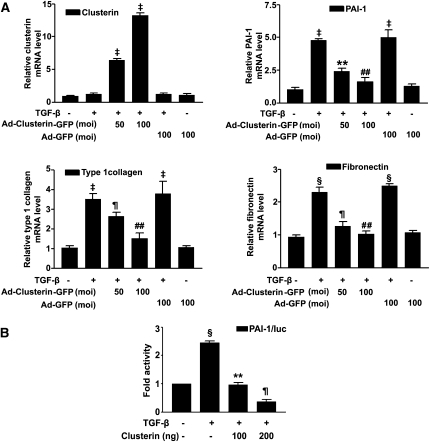
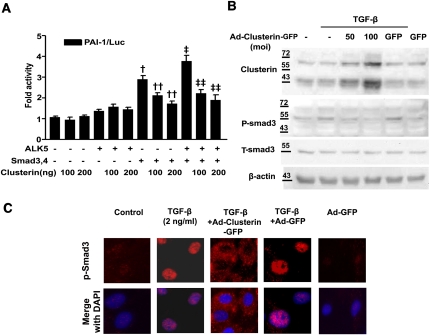
Next, the ability of clusterin to inhibit TGF-β–stimulated profibrotic target gene expression in cultured renal tubular epithelium-like cells, which increase clusterin expression after UUO (Figure 2), was examined. Infection efficiency and cell viability after infection of adenovirus (Ad) were measured (Supplemental Figure 1, A and B). Real-time RT-PCR analysis showed that Ad-clusterin–mediated overexpression of clusterin in NRK-52E cells inhibited the expression of TGF-β–stimulated PAI-1, type I collagen, and fibronectin mRNA in a dose-dependent manner (Figure 4A). However, Ad-clusterin did not influence the basal mRNA expression of PAI-1, type I collagen, or fibronectin in TGF-β–untreated NRK-52E cells (Supplemental Figure 2). Transient cotransfection of NRK-52E cells with clusterin and PAI-1 promoter luciferase showed that clusterin inhibited the TGF-β–stimulated PAI-1 promoter activity (Figure 4B), which suggested that clusterin inhibits profibrotic gene expression through the downregulation of TGF-β–stimulated transcription factor activity. To determine whether clusterin directly inhibits TGF-β–stimulated Smad3 activation, clusterin inhibition of ALK5 and Smad3/4-stimulated PAI-1 promoter activity was examined. Consistent with the real-time RT-PCR results, clusterin did not influence basal PAI-1 promoter activity. Transfection with ALK5 alone did not significantly increase PAI-1 promoter activity, and clusterin did not decrease the PAI-1 promoter activity that was not stimulated by ALK5. Transfection with Smad3/4 alone, however, increased PAI-1 promoter activity, and clusterin inhibited PAI-1 promoter activity stimulated by Smad3/4. Cotransfection with ALK5 and Smad3/4 resulted in a greater increase in PAI-1 promoter activity than with Smad3/4 transfection alone, and this increase in activity was also decreased by clusterin (Figure 5A).
Figure 4.
Clusterin inhibited TGF-β–stimulated PAI-1, type 1 collagen, and fibronectin expression. (A) Representative real-time RT-PCR analysis of the expression of PAI-1, type I collagen, and fibronectin in TGF-β–stimulated NRK-52E cells. Cells were incubated with TGF-β (2 ng/ml) after 24-hour serum starvation and then infected with the indicated doses of Ad-clusterin. GAPDH mRNA was used as an internal control. Data are the mean ± SEM of three independent measurements (three separate experiments). §P<0.05 and ‡P<0.001 compared with control and ¶P<0.01, **P<0.05, and ##P<0.001 compared with TGF-β alone. (B) Effects of clusterin on TGF-β-stimulated PAI-1 promoter activity in NRK-52E cells. Cells were treated with TGF-β (2 ng/ml) for 3 hours with or without 24-hour pretreatment with the indicated amounts of clusterin expression vector. Data are the mean ± SEM of three independent measurements (three separate experiments). §P<0.05 compared with control and ¶P<0.01 and **P<0.05 compared with TGF-β alone.
Figure 5.
Clusterin inhibited TGF-β–stimulated Smad3 activation. (A) Effect of clusterin on ALK5/Smad3-stimulated PAI-1 promoter activity in NRK-52E cells. Cells were cotransfected with the PAI-1 promoter (p800neo-Luc) and expression vectors for Smad3/4 (pRK5) or ALK5 (pcDNA). Data are the mean ± SEM of three independent measurements (three separate experiments). †P<0.01 and ‡P<0.001 compared with control, ††P<0.001 compared with Smad3/4, and ‡‡P<0.001 compared with Smad3/4 and ALK5. (B) Representative Western blot analysis of the expression of p-Smad3 in TGF-β–stimulated NRK-52E cells. Cells were incubated with TGF-β (2 ng/ml) after 24-hour serum starvation and then infected with the indicated doses of Ad-clusterin or 100 moi of Ad-GFP. (C) Effect of clusterin on p-Smad3 nuclear translocalization in NRK-52E cells. Cells were incubated with TGF-β (2 ng/ml) after 24-hour serum starvation and then infected with Ad-clusterin or Ad-GFP. Control cells were untreated. The cellular localization of p-Smad3 was examined by fluorescence microscopy of p-Smad3 (red) and 4′-6-diamidino-2-phenylindole nuclear staining (blue). Original magnification, ×400.
Phosphorylation of Smad3 and its subsequent nuclear translocation are critical steps in TGF-β signaling33; therefore, the effect of clusterin on the phosphorylation of Smad3 and on the nuclear translocation of phosphorylated Smad3 (p-Smad3) was examined. As shown in Figure 5B, phosphorylation of Smad3 in NRK-52E cells that had been treated with TGF-β was inhibited by Ad-mediated overexpression of clusterin. Upon TGF-β stimulation, p-Smad3 accumulated in the nucleus, but overexpression of clusterin blocked p-Smad3 translocation into the nucleus, and p-Smad3 was distributed in the cytoplasm (Figure 5C). Clusterin also inhibited TGF-β–stimulated p-Smad3 translocation into the nucleus when a higher dose of TGF-β (5 ng/ml) was used (Supplemental Figure 3). Taken together, these data suggest that clusterin negatively affects TGF-β–mediated transcription via the inhibition of Smad3 phosphorylation and nuclear translocation.
Ad-Mediated Overexpression of Clusterin Ameliorates Renal Fibrosis after UUO
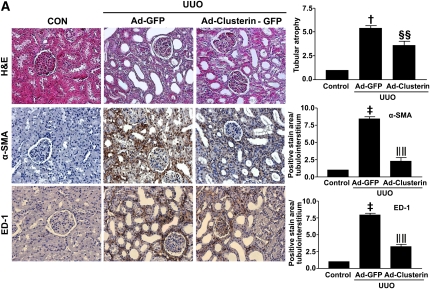
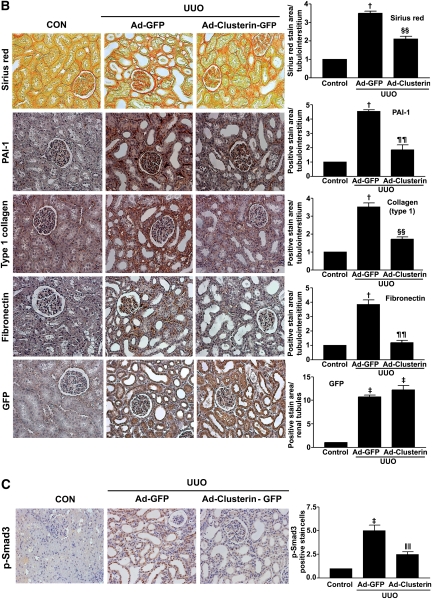
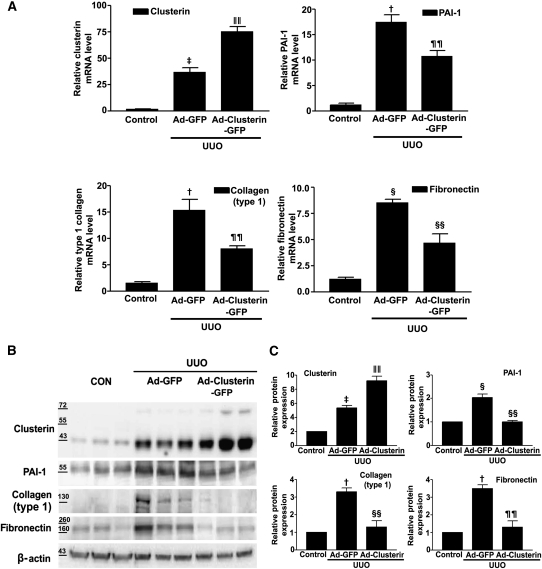
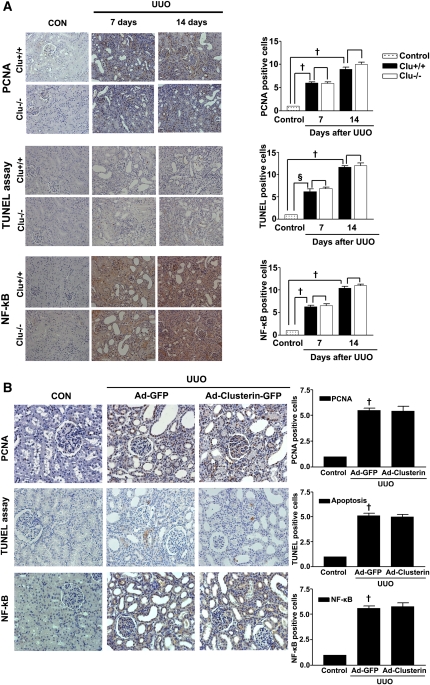
To examine further the beneficial effect of clusterin on UUO-induced renal fibrosis, Ad-clusterin or Ad encoding green fluorescent protein (Ad-GFP) was infused intrarenally into the left kidneys of rats after ligation of the left ureter. Clusterin expression in the renal tubular epithelium-like cells was further increased after Ad-clusterin delivery (Supplemental Figure 4A). Ad-mediated overexpression of clusterin in the kidney resulted mainly in the expression of the secreted form (Supplemental Figure 4B). H&E staining showed that Ad-clusterin reduced UUO-induced renal damage (Figure 6A). Ad-clusterin reduced UUO-induced ED-1–positive macrophage infiltration (Figure 6A). Moreover, Ad-clusterin reduced the number of UUO-induced α-SMA–positive myofibroblasts (Figure 6A). Sirius red staining showed that Ad-clusterin markedly reduced UUO-induced renal fibrosis (Figure 6B). Immunohistochemical staining showed that UUO kidneys had increased expression of PAI-1, type I collagen, and fibronectin. In contrast, Ad-clusterin–infected UUO kidneys showed decreased expression of all these proteins (Figure 6B). Successful adenoviral-mediated gene expression (GFP and clusterin-GFP) was confirmed by immunohistochemical staining for anti-GFP antibody (Figure 6B). Moreover, Ad-clusterin decreased UUO-induced p-Smad3 nuclear localization in vivo (Figure 6C). The changes in mRNA and protein expression were further examined by real-time RT-PCR and Western blot analysis (Figure 7). In UUO kidneys, clusterin, PAI-1, type I collagen, and fibronectin mRNA and protein expression were increased. However, a further increase of clusterin expression caused by intrarenal infusion of Ad-clusterin led to a decrease in UUO-induced renal profibrotic gene and protein expression (Figure 7, A–C). To confirm that the adenoviral infection had no effect on the outcome and that the overexpression of clusterin did not result in any changes under nonpathologic conditions, the effect of Ad-GFP or Ad-clusterin on sham-operated kidneys was examined (Supplementary Figure 5). Furthermore, to examine whether the antifibrotic effect of clusterin is a primary consequence of the suppression of TGF-β signaling or a secondary consequence of apoptosis or renal tubular epithelium-like cell proliferation, immunohistochemical staining for proliferating cell nuclear antigen (PCNA), terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL), and NF-kB activity was performed. UUO markedly increased the number of PCNA, TUNEL, and NF-kB–positive cells, but these increases were not affected by clusterin (Figure 8).
Figure 6.
Adenovirus-mediated clusterin overexpression ameliorates renal fibrosis after UUO. (A) Representative images of H&E and immunohistochemical staining for α-SMA and ED-1 of kidneys from rats killed 7 days after UUO surgery and infected with Ad-GFP or Ad-clusterin-GFP. The number of atrophic tubules was determined by the measurement of abnormal irregular and dilated tubular basement membranes in H&E-stained sections of kidneys in five random fields of each kidney (n=5 in each group) under high-power magnification. Areas of positive immunostaining with α-SMA and ED-1 were quantified by computer-based morphometric analysis. All data were normalized to the control (=1) and in all bar graphs were expressed as fold increases relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). †P<0.01 and ‡P<0.001 compared with control and §§P<0.05 and ‖‖P<0.001compared with Ad-GFP. Original magnification, ×200. (B) Representative images of Sirius red and immunohistochemical staining for PAI-1, type I collagen, and fibronectin expression in UUO kidneys infected with Ad-GFP or Ad-clusterin-GFP. All data were normalized to the control (=1) and in all bar graphs were expressed as fold increases relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). †P<0.01 and ‡P<0.001 compared with control and ¶¶P<0.01 and §§P<0.05 compared with Ad-GFP. Original magnification, ×200. (C) Representative images of immunohistochemical staining for p-Smad3 expression in UUO kidneys infected with Ad-GFP or Ad-clusterin-GFP. All data were normalized to the control (=1) and in the bar graph were expressed as fold increases relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). ‡P<0.001 compared with the control and ‖‖P<0.001 compared with Ad-GFP. Original magnification, ×200.
Figure 7.
Adenovirus-mediated clusterin overexpression inhibited PAI-1, type 1 collagen, and fibronectin expression after UUO. (A) Representative real-time RT-PCR analysis of PAI-1, type I collagen, and fibronectin mRNA expression in kidneys from rats infected with Ad-GFP or Ad-clusterin-GFP and killed 7 days after UUO surgery. GAPDH mRNA was used as an internal control. Data are the mean ± SEM of three independent measurements (n=5 in each group). †P<0.01, §P<0.05, and ‡P<0.001 compared with control, and ¶¶P<0.01, §§P<0.05, and ‖‖P<0.001 compared with Ad-GFP. (B) Representative Western blot analysis of PAI-1, type I collagen, and fibronectin protein expression in UUO kidneys with Ad-GFP or Ad-clusterin-GFP. (C) Quantification of Western blot analysis. Data are expressed as the mean ± SEM of three independent experiments (n=5 in each group). β-Actin levels were analyzed as an internal control. †P<0.01, §P<0.05, and ‡P<0.001 compared with control, and ¶¶P<0.01, §§P<0.05, and ‖‖P<0.001 compared with Ad-GFP.
Figure 8.
Clusterin had no effect on tubular epithelial cell proliferation and apoptosis after UUO. (A) Representative images of immunohistochemical staining for PCNA, NF-kB, and TUNEL assay in the kidneys of wild-type (Clu+/+) and Clu KO (Clu−/−) mice at 7 and 14 days after UUO. The numbers of PCNA-positive, NF-κB–positive and apoptotic cells were determined by counting positively stained tubular epithelial cells of renal tubules in five random fields of each kidney (n=5 in each group) under high-power magnification. All data were normalized to the control (=1) and, in the bar graph, were expressed as fold increases in the numbers of these cells relative to the control. †P<0.01 and §P<0.05 compared with control; no effect compared with Clu+/+ mice. Original magnification, ×200. (B) Representative images of immunohistochemical staining for PCNA, NF-κB, and TUNEL in UUO kidneys after Ad-GFP or Ad-clusterin delivery. The numbers of PCNA-positive, NF-κB–positive, and apoptotic cells were determined by counting positively stained tubular epithelial cells of renal tubules kidneys in five random fields of each kidney (n=5 in each group) under high-power magnification. All data were normalized to the control (=1) and in the bar graph were expressed as fold increases in the numbers of these cells relative to the control. *P<0.01 compared with control; no effect compared with Ad-GFP. Original magnification, ×200.
Clusterin Prevented the UUO-Induced Decrease of E-Cadherin Expression
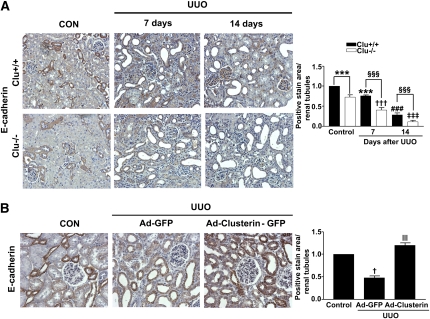
To investigate whether clusterin improves tubular integration, the E-cadherin level in the kidneys after UUO was examined. On day 7 after UUO, the kidneys were characterized by the loss of E-cadherin. The loss was more evident at 14 days after UUO (Figure 9A). E-cadherin expression showed a greater decrease in the kidneys of Clu−/− than in the kidneys of wild-type mice. Moreover, Ad-clusterin prevented UUO-induced loss of E-cadherin (Figure 9B).
Figure 9.
Clusterin prevented the UUO-induced decrease of E-cadherin expression. (A) Representative images of immunohistochemical staining for E-cadherin in kidney from wild-type (Clu+/+) and Clu KO (Clu−/−) mice at 7 days and 14 days after UUO. Data obtained by computer-based morphometric analysis on days 7 and 14 after UUO were normalized to the data obtained from Clu+/+ or to Clu−/− control mice (=1) and in the bar graph were expressed as fold increases relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). ***P<0.05 and ###P<0.001 compared with Clu+/+ control, †††P<0.01 and ‡‡‡P<0.001 compared with Clu−/− control, and §§§P<0.001 compared with Clu+/+ UUO. Original magnification, ×200. (B) Representative images of immunohistochemical staining for E-cadherin expression in kidneys of rats infected with Ad-GFP or Ad-clusterin-GFP and killed 7 days after UUO surgery. All data were normalized to the control (=1) and in all bar graphs were expressed as fold increases relative to the control. Data are the mean ± SEM of five random fields of each kidney (n=5 in each group). †P<0.01 compared with the control and ‖‖P<0.001 compared with Ad-GFP. Original magnification, ×200.
Effect of Clusterin on Renal Fibrosis Is Unlikely Caused by Endocrine Responses
To exclude the possibility that excessive secretion of clusterin by Ad-clusterin may influence renal fibrosis, the direct effect of secreted form of clusterin was examined. Incubation of NRK-52E cells with a conditioned medium containing secreted clusterin, but not control medium, resulted in a strong inhibition of TGF-β–stimulated PAI-1, type I collagen, and fibronectin expression (Supplementary Figure 6). These observations suggest that both clusterin overexpression and secreted clusterin have an antifibrotic effect on renal tubular epithelium-like cells through inhibition of TGF-β signaling.
Finally, to determine whether the antifibrotic effects of clusterin on the UUO kidney stemmed from an endocrine response, the effect of clusterin on UUO-induced renal fibrosis after hepatic clusterin overexpression was examined. Successful delivery of Ad-clusterin into the liver by tail-vein injection was confirmed by immunohistochemical staining for GFP (Supplemental Figure 7A). Hepatic overexpression of clusterin increased plasma and renal clusterin levels (Supplemental Figure 7, B and C), but had no effect on UUO-induced renal damage or renal fibrosis (Supplemental Figure 7A). Moreover, immunohistochemical staining showed that UUO kidneys did not decrease the expression of PAI-1, type I collagen, and fibronectin by hepatic clusterin overexpression (Supplemental Figure 7A). These data suggested that the antifibrotic effects of clusterin on kidney are unlikely to stem from its endocrine effects.
DISCUSSION
This study was undertaken to address whether clusterin inhibits renal fibrosis in obstructive nephropathy and to elucidate the underlying mechanisms. Here, we show that the genetic deficiency of clusterin significantly increases the expression of PAI-1 and matrix proteins and accelerates renal fibrosis and damage after UUO. Furthermore, the upregulation of clusterin by intrarenal infusion of Ad-clusterin decreased UUO-induced PAI-1 and matrix protein expression. Clusterin appears to downregulate PAI-1 and ECM protein expression via the downregulation of TGF-β/Smad3 activity.
Increasing evidence indicates that clusterin is upregulated in several renal diseases. Thus, clusterin is considered to be a useful marker for predicting nephrotoxicity.24–28 Clusterin is expressed particularly in the renal tubular epithelium after renal injury and UUO.27 Clusterin has been shown to be protective against gentamicin-induced renal tubular cell injury29 and to protect against oxidative stress.30 Based on these data, we reasoned that, in experimental renal fibrosis models, the upregulation of clusterin would function as a defense mechanism to prevent renal fibrosis. As predicted, comparison of Clu−/− mice with wild-type mice demonstrated that the loss of clusterin caused accelerated renal expression of PAI-1 and ECM accumulation, as well as renal fibrosis and damage after UUO. Furthermore, intrarenal delivery of Ad-clusterin into UUO kidneys markedly inhibited UUO-induced PAI-1 and ECM accumulation.
TGF-β expression is increased in human and animal experimental renal fibrosis models and has been implicated as a major mediator of ECM protein accumulation in diabetic nephropathy and tubulointerstitial fibrosis.2,5–8 To elucidate the mechanism by which clusterin inhibits PAI-1 and matrix protein expression, we examined whether clusterin inhibits TGF-β–mediated Smad3 activity. TGF-β receptor, a transmembrane Ser/Thr kinase receptor, phosphorylates receptor-regulated Smads, such as Smad2/3. Phosphorylated Smads enter the nucleus, where they activate the expression of target genes, including PAI-1 and matrix proteins, and subsequently contribute to tubulointerstitial fibrosis.34,35 Many studies have investigated the inhibition of TGF-β signaling as a means to alleviate renal fibrosis.13 In this study, we found that Ad-mediated overexpression of clusterin successfully inhibited TGF-β–stimulated target gene expression, including PAI-1 and matrix proteins, in cultured renal cell lines. Initiation and progression of renal fibrosis in an animal model of ureteric obstruction are accompanied by readily quantifiable molecular events such as apoptosis,9,36,37 and inhibition of tubular cell apoptosis protects against the development of fibrosis following ureteric obstruction.36,38 In addition to apoptosis, an initial phase of tubular epithelium-like cell proliferation during the course of UUO plays an important role in the development of renal fibrosis. A decline in this proliferative phase, with the subsequent apoptotic loss of tubular cells, results in interstitial fibrosis.9 Therefore, enhanced proliferation and reduced apoptosis of tubular cells can protect against chronic tubulointerstitial fibrosis.39 However, the present study showed that clusterin did not affect UUO-induced apoptosis or the proliferation of tubular epithelium-like cells, suggesting that the antifibrotic effects of clusterin are primarily mediated by the suppression of TGF-β signaling rather than by the apoptosis of renal tubular epithelium-like cells.
PAI-1 is considered to play a critical role in the development of renal fibrosis.17,19,35,40–42 Oda et al. have demonstrated that PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction.17 PAI-1 expression is tightly regulated at the transcriptional level.14 On the basis of these data, we examined whether clusterin is involved in the regulation of PAI-1. PAI-1 expression was significantly increased in Clu−/− mice, and intrarenal delivery of Ad-clusterin into UUO kidneys significantly inhibited PAI-1 expression.
The present study showed that clusterin decreases not only p-Smad3 but also inhibits the trapping of p-Smad3 in the nucleus. There are two possible mechanisms by which clusterin could inhibit TGF-β/Smad3 signaling. One mechanism, as suggested by previous studies, is that clusterin inhibits Smad3 phosphorylation by directly interacting with the TGF-β receptor.43 The second mechanism is that clusterin inhibits the nuclear translocation of p-Smad3. Interestingly, transient transfection studies showed that clusterin inhibited Smad3/4-stimulated PAI-1 promoter activity in the absence of ALK5 stimulation, suggesting that clusterin acts at the lower level of TGF-β receptor. Collectively, these data suggest clusterin inhibits both receptor-mediated Smad3 phosphorylation and receptor-independent Smad3 activity, such as the inhibition of nuclear localization of p-Smad3. We are currently unable to provide further data regarding the mechanism by which clusterin inhibits p-Smad3 trapping in the nucleus. Further studies are necessary to elucidate how clusterin inhibits the TGF-β/Smad3 pathway.
The clusterin gene encodes two isoforms. It has been reported that the secreted form is cytoprotective,44,45 whereas the nuclear form is proapoptotic.46 Recently, Lee et al. reported that truncated clusterin increases TGF-β/Smad signaling by modulating Smad2/3 stability.47 In the present study, full-length clusterin was used, which could theoretically result in the expression of secreted and nuclear forms of clusterin. Although the nuclear form of clusterin increases Smad 3 activity, nuclear clusterin cannot influence Smad3 activity when clusterin is present in the cytoplasm. This is because cytosolic clusterin blocks Smad3 phosphorylation and nuclear translocation by trapping p-Smad3 before nuclear clusterin can affect Smad3 activity. Moreover, in the present study, despite using a full-length vector, Ad-mediated delivery of clusterin into the kidney mainly caused the expression of the secreted form. The discrepancy with previous published studies showing that nuclear clusterin enhances Smad3 activity could be explained by the use of truncated clusterin in previous studies, such as in the study by Lee et al. Because the antifibrotic effects of clusterin on UUO kidney are attributed mainly to the secretory form of clusterin, we examined whether the systemic secretion of clusterin could prevent renal fibrosis. However, systemic secretion of clusterin by hepatic overexpression of clusterin did not attenuate UUO-induced renal fibrosis, suggesting that the antifibrotic effects of clusterin are unlikely to be associated with its endocrine effects. However, the data showing inhibition of fibrogenic gene expression in TGF-β–stimulated NRK-52E cells following treatment with secreted clusterin suggest that the paracrine effect of clusteirn might explain its antifibrotic effect. Therefore, further work will be required to show that it is the paracrine effects of clusterin that are responsible for the antifibrotic outcome.
In conclusion, the results suggest that the upregulation of clusterin during renal injury may be a protective response against, rather than a causative response to, the development of renal fibrosis. Therefore, the discovery of an increase in clusterin during other pathologic conditions would be informative for this promising strategy for the prevention of renal fibrosis.
CONCISE METHODS
Reagents and Plasmids
Recombinant human TGF-β was purchased from R&D systems (Minneapolis, MN). Anti–ED-1 was purchased from Serotec (Oxford, UK). Anti–PAI-1, antifibronectin, anti-PCNA, and anti–E-cadherin antibodies were purchased from BD Biosciences (San Jose, CA). Anti–collagen type I, anti-F4/80, and anti-GFP antibodies were purchased from Abcam (Cambridge, UK). Anti-actin antibody and anti–α-SMA antibody were purchased from Sigma (St. Louis, MO). Anti–phospho-Smad3 (ser423/425) and anti-Smad3 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Anti-clusterin, anti–NF-κB, and p-smad2/3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The cDNA encoding rat clusterin was purchased from Benebiosis (Seoul, Korea).48 The pcDNA3HA-ALK5TD plasmids were kind gifts from Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Sweden). The pRK5-Smad3 and pRK5-Smad4 plasmids were kind gifts from Rik Derynck (University of California, San Francisco, CA).
Animals
Male 8-week-old Sprague–Dawley (SD) rats weighing 250 g and male 8-week-old C57BL/6 mice were purchased from Samtako (Osan, Korea). To generate Clu−/− knockout mice on a C57BL/6 genetic background, clusterin-deficient mice originally generated using a Swiss black genetic background49 were backcrossed onto the C57BL/6 strain for at least seven generations. All procedures were performed in accordance with the institutional guidelines for animal research.
Cell Culture
The NRK-52E rat renal proximal tubular epithelium-like cell line was purchased from the American Type Culture Collection (Manassas, VA). NRK-52E cells were cultured in 5% CO2 at 37°C in DMEM containing 4 mmol/L l-glutamine and 25 mmol/L glucose. The medium was supplemented with 5% FBS. Equal numbers of NRK-52E cells were seeded onto tissue culture plates. NRK-52E cells were rendered quiescent by incubation for 24 hours in medium containing 0.5% FBS. Cells were infected with Ad-clusterin in serum-free medium for 2 hours, and this was changed to medium containing 0.5% FBS with or without TGF-β (2 ng/ml, 3 hours) for 24 hours. Cells were subsequently processed for the isolation of RNA and protein as described below.
Experimental UUO Animal Model and In Vivo Infection
UUO surgery was performed as previously described.50 Briefly, after a midabdominal incision under anesthesia using pentobarbital (50 mg/kg), the left ureter of the rat was ligated with 5-0 silk suture at two separate points and was cut between the two ligation points. A flexible cannula was inserted into the left renal artery via the branch of the femoral artery, and the distal segment of the renal artery was transiently ligated. Ad encoding rat clusterin or GFP (3×109 plaque-forming units [pfu]/kidney, n=5/group) was infused into the left kidney and incubated for 5 minutes. Upon completion, the infusion catheter was removed, blood flow to the kidney was restored by the release of ligatures, and the wound was closed. Sham-operated rats were used as controls. Seven days after UUO and Ad infection, the rats were euthanized, and their left kidneys were removed, cut in thirds, fixed for 20 hours in 4% paraformaldehyde, and either embedded in paraffin for histologic examination or frozen in liquid nitrogen for the isolation of protein or RNA. In Clu KO (Clu−/−) and wild-type (Clu+/+) mice, after a midabdominal incision under anesthesia using pentobarbital (50 mg/kg), the left ureter was ligated with 5-0 silk suture at two separate points. On days 7 and 14 after UUO, mice were euthanized, and left kidneys were removed, fixed for 20 hours in 4% paraformaldehyde, and either embedded in paraffin for histologic examination or frozen in liquid nitrogen for the isolation of protein or RNA.
Generation of Recombinant Ad
The cDNA encoding rat full-length clusterin was inserted into the BglII/XhoI sites of the pAd-Track-CMV shuttle vector. The vector was then electroporated into BJ5183 cells that contained the adenoviral vector, Adeasy, to generate a recombinant adenoviral plasmid. Recombinants were amplified in the human embryonic kidney cell line HEK-293 and purified by CsCl (Sigma) gradient centrifugation. Viral preparations were collected and desalted, and titers were determined using Adeno-X rapid titer (BD Bioscience) according to the manufacturer’s instructions. The efficiency of adenoviral infection was assessed using a recombinant Ad encoding clusterin fused to GFP (data not shown).
Quantitative Real-Time RT-PCR
Total RNA was obtained from NRK-52E cells and rat or mouse kidneys using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was synthesized using a first-strand cDNA kit (Fermentas, Hanover, MD). Quantitative real-time RT-PCR was performed using the SYBR Green PCR Master Mix Kit (Applied Biosystems, Warrington, UK) on the StepOnePlus Real-Time PCR System (Applied Biosystems) under the following conditions: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Primer sets were designed using AB StepOne software v2.1 based on the sequences from GenBank and were as follows: rat PAI-1 (sense: 5′-CACCCCTTCCAGAGTCCCATA-3′ and antisense: 5′-GCTGAAACACTTTTACTCCGAAGTT-3′); rat type 1 collagen (sense: 5′-GTGCGATGGCGTGCTATG-3′ and antisense: 5′-TCGCCCTCCCGTTTTTG-3′); rat fibronectin (sense: 5′-ACCTGCAAGCCAATAGCTGAGA-3′ and antisense: 5′-CCAGCCTTGGTAGGGCTTTT-3′); rat GAPDH (sense: 5′-TGCCGCCTGGAGAAACC-3′ and antisense: 5′-AGCCCAAGGATGCCCTTTAGT-3′); mouse PAI-1 (sense: 5′-AATCCCACACAGCCCATCA-3′ and antisense: 5′-GGACCACCTGCTGAAACACTTT-3′); mouse type 1 collagen (sense: 5′-GCCTTGGAGGAAACTTTGCTT-3′ and antisense: 5′-GCACGGAAACTCCAGCTGAT-3′); mouse fibronectin (sense: 5′-GATATCACCGCCAACTCATTCA-3′ and antisense: 5′-CAGAATGCTCGGCGTGATG-3′); mouse GAPDH (sense: 5′-GAAGGGTGGAGCCAAAAG-3′ and antisense: 5′-GCTGACAATCTTGAGTGAGT-3′); and rat and mouse clusterin (sense: 5′-TGGACACAGTGGCGGAGAA-3′ and antisense: 5′-CATTCCGCAGGCTTTTC-3′). Reaction specificity was confirmed by melting curve analysis. The housekeeping gene GAPDH was used as an internal standard.
Immunofluorescence Staining
Cells were plated on cover glasses in culture medium. After culturing with Ad-clusterin, the cells were fixed in 2% paraformaldehyde for 15 minutes and then permeabilized with 0.2% Triton X-100 for 15 minutes at room temperature. Cells were incubated with p-Smad3 antibody and Alexa Fluor 568 anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR) and counterstained with 4′-6-diamidino-2-phenylindole. The cells were observed through an inverted MRc5 Carl Zeiss fluorescence microscope (Thornwood, NY).
Western Blot Analysis
Cells were washed twice with PBS and suspended in RIPA buffer. Cells were lysed on ice for 30 minutes. The cell lysate was collected after centrifugation at 15,000 × g for 10 minutes. Protein quantification was performed using a Bio-Rad Protein Assay system (Bio-Rad, Richmond, CA). Cell lysates of 30 μg were electrophoresed by SDS-PAGE and electrotransferred to polyvinylidine fluoride membranes (Millipore Corporation, Bedford, MA). After blocking with 5% skimmed milk in Tris-buffered saline-Tween 20 (0.1%) for 1 hour, the membrane was incubated with anti-clusterin (1:3000), Smad3 (1:1000), and phospho-smad3 (1:1000) polyclonal antibodies at 4°C with gentle shaking overnight and washed three times for 10 minutes in washing buffer (Tris-buffered saline containing 0.1% Tween 20). Antibodies were detected by horseradish peroxidase–linked secondary antibody using the enhanced chemiluminescence Western blotting detection system, as specified by the manufacturer (Amersham, Buckinghamshire, UK). The membrane was reblotted with anti-actin antibody to verify equal loading of the protein in each lane. Densitometric measurements of the bands were made using the digitalized scientific program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT).
In Vitro Transient Transfection and Reporter Assay
NRK-52E cells were plated at a density of 1×105 cells per well in a 12-well plate and cultured for 1 day in culture medium. Cells were transiently transfected at a concentration of 300 ng/well with p800neo-luc (expression vector containing the −800 bp PAI-1 promoter fragment51), rat full length clusterin, pRK5-Smad3, pRK5-Smad4, and pcDNA3HA-ALK5 plasmids using Lipofectamine 2000 transfection reagent (Invitrogen). Cells were cotransfected with a plasmid encoding β-galactosidase as an internal control. Cells were transfected for 4 hours, washed to remove plasmids, and then cultured in medium containing 0.5% FBS. Cells were harvested approximately 24 hours after transfection. For luciferase and β-galactosidase assays, 20 μl of cell lysate was analyzed using a luciferase assay system according to the manufacturer’s instructions (Promega, Madison, WI). Luciferase activity was detected using a SIRUS luminometer (Berthold, Germany), and luciferase activity was normalized to β-galactosidase activity.
Histologic Analysis
Kidneys were fixed with PBS containing 4% paraformaldehyde and embedded in paraffin, and 4-μm sections were cut. Histochemical staining was performed with H&E and Sirius red staining. Kidney sections were deparaffinized in xylene and rehydrated through graded ethanol concentrations. For Sirius red staining, to evaluate interstitial collagen deposition, slides were immersed for 18 hours in saturated picric acid with 0.1% Sirius red F3BA (Aldrich Chemicals). Slides were then washed in 0.01 N hydrochloric acid for 2 minutes and rapidly dehydrated through graded alcohol concentrations, starting at 70%. The slides were transferred to xylene, and the coverslip was mounted with Permount (Fisher Scientific, Edmonton, Alberta, Canada). Fields (×200) were captured, and collagen deposition was expressed as the proportion of the renal cortex stained with Sirius red. For immunohistochemical staining with ED-1 (1:100), kidney sections were treated with 0.3% H2O2 to quench the endogenous peroxidase activity, treated with 0.1% trypsin (Sigma), and incubated at 4°C overnight. Immunohistochemical staining was also performed using anti-GFP (1:250), clusterin (1:100), α-SMA (1:250), PAI-1 (1:250), type I collagen (1:250), fibronectin (1:250), F4/80 (1:50), NF-κB (1:100), PCNA (1:100), and E-cadherin (1:200) primary antibodies followed by horseradish peroxidase–conjugated anti-mouse, anti-rat, or anti-rabbit IgG secondary antibodies (Dako, Glostrup, Denmark), according to the manufacturer’s instructions. Renal fibrotic areas were quantified by morphometric analysis using a light microscope equipped with an imaging system comprising an MRc5 Carl Zeiss microscope and iSolution DT Ver7.7 software (IMT i-Solution, Coquitlam, Canada). Sirius red–stained areas (fibrotic areas) and areas of positive immunostaining with PAI-1, type I collagen, and fibronectin in the renal fibrotic regions (brown) were quantified by computer-based morphometric analysis. Areas of positive immunostaining with α-SMA, F4/80, and ED-1 were quantified by computer-based morphometric analysis. In situ detection of DNA fragmentation was performed by TUNEL using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) according to the manufacturer’s instructions. The number of PCNA-positive, NF-kB–positive, and apoptotic cells was determined by counting positively stained tubular epithelium-like cells of renal tubules in five random fields of each kidney from five different animals under high-power magnification. The number of atrophic tubules was determined by measurement of the abnormal irregular and dilated tubular basement membrane in the fields of five random H&E-stained sections from each kidney of five different animals under high-power magnification. All data were normalized to the control (=1) and in all bar graphs were expressed as fold increase relative to the control.
Statistical Analyses
Data were evaluated using ANOVA followed by a post hoc least significant difference test and expressed as means ± SEM. Values of P<0.05 were considered statistically significant. All experiments were performed at least three times.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A08-4335-AA2004-08N1-00020B), a National Research Foundation grant funded by the Korean Government (MEST) (2010-0008023), a grant from the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Anti-Aging and Well-Being Research Center), and a World Class University program funded by the Ministry of Education, Science and Technology through the National Research Foundation of Korea (R32-10064).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011010048/-/DCSupplemental.
REFERENCES
- 1.Ha H, Lee HB: Reactive oxygen species and matrix remodeling in diabetic kidney. J Am Soc Nephrol 14[Suppl 3]: S246–S249, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Jim B, Ziyadeh FN: Diabetic nephropathy and transforming growth factor-beta: Transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 23: 532–543, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Verrecchia F, Mauviel AJ: Transforming growth factor-beta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol 118: 211–215, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Ihn H: Pathogenesis of fibrosis: Role of TGF-beta and CTGF. Curr Opin Rheumatol 14: 681–685, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Hills CE, Squires PE: TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol 31: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Hill C, Flyvbjerg A, Grønbaek H, Petrik J, Hill DJ, Thomas CR, Sheppard MC, Logan A: The renal expression of transforming growth factor-beta isoforms and their receptors in acute and chronic experimental diabetes in rats. Endocrinology 141: 1196–1208, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN: Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol 158: 1653–1663, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Grande JP: Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med (Maywood) 227: 943–956, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Morrissey J: Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chevalier RL, Forbes MS, Thornhill BA: Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Krag S, Danielsen CC, Carmeliet P, Nyengaard J, Wogensen L: Plasminogen activator inhibitor-1 gene deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int 68: 2651–2666, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zhou A, Ueno H, Shimomura M, Tanaka R, Shirakawa T, Nakamura H, Matsuo M, Iijima K: Blockade of TGF-beta action ameliorates renal dysfunction and histologic progression in anti-GBM nephritis. Kidney Int 64: 92–101, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hwang M, Kim HJ, Noh HJ, Chang YC, Chae YM, Kim KH, Jeon JP, Lee TS, Oh HK, Lee YS, Park KK: TGF-beta 1 siRNA suppresses the tubulointerstitial fibrosis in the kidney of ureteral obstruction. Exp Mol Pathol 81: 48–54, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Binder BR, Christ G, Gruber F, Grubic N, Hufnagl P, Krebs M, Mihaly J, Prager GW: Plasminogen activator inhibitor 1: Physiological and pathophysiological roles. News Physiol Sci 17: 56–61, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Ma LJ, Fogo AB: PAI-1 and kidney fibrosis. Front Biosci 14: 2028–2041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA: A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest 112: 379–388, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda T, Jung YO, Kim HS, Cai X, López-Guisa JM, Ikeda Y, Eddy AA: PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, López-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA: Multifunctionality of PAI-1 in fibrogenesis: Evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ghosh AK, Vaughan DE: PAI-1 in tissue fibrosis [published online ahead of print April 4, 2011]. J Cell Physiol doi: 10.1002/jcp.22783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calero M, Rostagno A, Frangione B, Ghiso J: Clusterin and Alzheimer’s disease. Subcell Biochem 38: 273–298, 2005 [PubMed] [Google Scholar]
- 21.Trougakos IP, Gonos ES: Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol 34: 1430–1448, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Trougakos IP, Gonos ES: Chapter 9: Oxidative stress in malignant progression: The role of Clusterin, a sensitive cellular biosensor of free radicals. Adv Cancer Res 104: 171–210, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Pucci S, Bonanno E, Pichiorri F, Angeloni C, Spagnoli LG: Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene 23: 2298–2304, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Gautier JC, Riefke B, Walter J, Kurth P, Mylecraine L, Guilpin V, Barlow N, Gury T, Hoffman D, Ennulat D, Schuster K, Harpur E, Pettit S: Evaluation of novel biomarkers of nephrotoxicity in two strains of rat treated with Cisplatin. Toxicol Pathol 38: 943–956, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Davis JW, 2nd, Goodsaid FM, Bral CM, Obert LA, Mandakas G, Garner CE, 2nd, Collins ND, Smith RJ, Rosenblum IY: Quantitative gene expression analysis in a nonhuman primate model of antibiotic-induced nephrotoxicity. Toxicol Appl Pharmacol 200: 16–26, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Huang Q, Dunn RT, 2nd, Jayadev S, DiSorbo O, Pack FD, Farr SB, Stoll RE, Blanchard KT: Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci 63: 196–207, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ishii A, Sakai Y, Nakamura A: Molecular pathological evaluation of clusterin in a rat model of unilateral ureteral obstruction as a possible biomarker of nephrotoxicity. Toxicol Pathol 35: 376–382, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G: Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol 28: 463–469, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Girton RA, Sundin DP, Rosenberg ME: Clusterin protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am J Physiol Renal Physiol 282: F703–F709, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Schwochau GB, Nath KA, Rosenberg ME: Clusterin protects against oxidative stress in vitro through aggregative and nonaggregative properties. Kidney Int 53: 1647–1653, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg ME, Girton R, Finkel D, Chmielewski D, Barrie A, 3rd, Witte DP, Zhu G, Bissler JJ, Harmony JA, Aronow BJ: Apolipoprotein J/clusterin prevents a progressive glomerulopathy of aging. Mol Cell Biol 22: 1893–1902, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada K, Hori Y, Hanafusa N, Okuda T, Nagano N, Choi-Miura NH, Couser WG, Miyata T, Kurokawa K, Fujita T, Nangaku M: Clusterin is up-regulated in glomerular mesangial cells in complement-mediated injury. Kidney Int 59: 137–146, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Javelaud D, Mauviel A: Mammalian transforming growth factor-betas: Smad signaling and physio-pathological roles. Int J Biochem Cell Biol 36: 1161–1165, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Noble NA: PAI-1 as a target in kidney disease. Curr Drug Targets 8: 1007–1015, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP: Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney Int 58: 1841–1850, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Docherty NG, O’Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW: Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Misseri R, Meldrum KK: Mediators of fibrosis and apoptosis in obstructive uropathies. Curr Urol Rep 6: 140–145, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X: HSP 72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 295: F202–F214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barreira AL, Takiya CM, Castiglione RC, Maron-Gutierrez T, Barbosa CM, Ornellas DS, Verdoorn KS, Pascarelli BM, Borojevic R, Einicker-Lamas M, Leite M, Jr, Morales MM, Vieyra A: Bone marrow mononuclear cells attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells after unilateral ureteral obstruction. Cell Physiol Biochem 24: 585–594, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA: A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. J Am Soc Nephrol 19: 329–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang G, Kernan KA, Collins SJ, Cai X, López-Guisa JM, Degen JL, Shvil Y, Eddy AA: Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: Role of plasmin-activated signals. J Am Soc Nephrol 18: 846–859, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA: Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int 67: 1297–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Reddy KB, Karode MC, Harmony AK, Howe PH: Interaction of transforming growth factor beta receptors with apolipoprotein J/clusterin. Biochemistry 35: 309–314, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Zhong B, Sallman DA, Gilvary DL, Pernazza D, Sahakian E, Fritz D, Cheng JQ, Trougakos I, Wei S, Djeu JY: Induction of clusterin by AKT—Role in cytoprotection against docetaxel in prostate tumor cells. Mol Cancer Ther 9: 1831–1841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jun HO, Kim DH, Lee SW, Lee HS, Seo JH, Kim JH, Kim JH, Yu YS, Min BH, Kim KW: Clusterin protects H9c2 cardiomyocytes from oxidative stress-induced apoptosis via Akt/GSK-3β signaling pathway. Exp Mol Med 43: 53–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA: Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem 278: 11590–11600, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Lee KB, Jeon JH, Choi I, Kwon OY, Yu K, You KH: Clusterin, a novel modulator of TGF-beta signaling, is involved in Smad2/3 stability. Biochem Biophys Res Commun 366: 905–909, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Yoo EK, Kim JY, Choi YK, Lee HJ, Kim JK, Jeoung NH, Lee KU, Park IS, Min BH, Park KG, Lee CH, Aronow BJ, Sata M, Lee IK: Protective role of clusterin/apolipoprotein J against neointimal hyperplasia via antiproliferative effect on vascular smooth muscle cells and cytoprotective effect on endothelial cells. Arterioscler Thromb Vasc Biol 29: 1558–1564, 2009 [DOI] [PubMed] [Google Scholar]
- 49.McLaughlin L, Zhu G, Mistry M, Ley-Ebert C, Stuart WD, Florio CJ, Groen PA, Witt SA, Kimball TR, Witte DP, Harmony JA, Aronow BJ: Apolipoprotein J/clusterin limits the severity of murine autoimmune myocarditis. J Clin Invest 106: 1105–1113, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita S, Maeshima A, Kojima I, Nojima Y: Activin A is a potent activator of renal interstitial fibroblasts. J Am Soc Nephrol 15: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Keeton MR, Curriden SA, van Zonneveld AJ, Loskutoff DJ: Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J Biol Chem 266: 23048–23052, 1991 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.