Abstract
Background
Plasma factor XIII (FXIII) is responsible for stabilization of fibrin clot at the final stage of blood coagulation. Since FXIII has also been shown to modulate inflammation, endothelial permeability, as well as diminish multiple organ dysfunction (MOD) after gut ischemia-reperfusion injury, we hypothesized that FXIII would reduce MOD caused by trauma-hemorrhagic shock (THS).
Materials and methods
Rats were subjected to a 90 min THS or trauma sham shock (TSS) and treated with either recombinant human FXIII A2 subunit (rFXIII) or placebo immediately after resuscitation with shed blood or at the end of the TSS period. Lung permeability, lung and gut myeloperoxidase (MPO) activity, gut histology, neutrophil respiratory burst, microvascular blood flow in the liver and muscles, and cytokine levels were measured 3 h after the THS or TSS. FXIII levels were measured before THS or TSS and after the 3-h post-shock period.
Results
THS-induced lung permeability as well as lung and gut MPO activity was significantly lower in rFXIII-treated than in placebo-treated animals. Similarly, rFXIII-treated rats had lower neutrophil respiratory burst activity and less ileal mucosal injury. rFXIII-treated rats also had a higher liver microvascular blood flow compared with the placebo group. Cytokine response was more favorable in rFXIII-treated animals. Trauma-hemorrhagic shock did not cause a drop in FXIII activity during the study period.
Conclusions
Administration of rFXIII diminishes THS-induced MOD in rats, presumably by preservation of the gut barrier function, limitation of polymorphonuclear leukocyte (PMN) activation, and modulation of the cytokine response.
Keywords: fibrin stabilizing factor, trauma, hemorrhagic shock, oxidative stress, lung injury, microcirculatory disorders
INTRODUCTION
Hemorrhagic shock, a frequent and dangerous complication of trauma, is still associated with high mortality and morbidity. Trauma-hemorrhagic shock (THS) is defined as an inadequate capillary perfusion in vital organs and tissues that leads to numerous adverse consequences. The reduction of circulatory blood volume results in deterioration of organ microcirculation with subsequent tissue hypoxia. In turn, ischemia-reperfusion-induced tissue injury causes the release of numerous proinflammatory mediators such as cytokines and nitric oxide. Hemodynamic and inflammatory alterations result in multiple organ dysfunction (MOD) that is a leading cause of death in patients who survive the initial hemorrhagic event. It has been shown that toxic factors produced by the ischemic gut play an important role in the development of THS-induced MOD. Gut-derived factors contained in intestinal lymph coming from the ischemic gut have been shown to be key factors in the pathogenesis of acute lung injury, bone marrow dysfunction, red blood cell alterations, endothelial cell injury, upregulation of endothelial cell adhesion molecule expression, and neutrophil activation [1–5].
The systemic inflammatory response syndrome plays an important role in the pathogenesis of THS-induced MOD. Increased proinflammatory cytokine and chemokine release after THS has been described in animal models as well as in clinical studies as contributing to augmented inflammatory response. For example, elevated plasma levels of IL-6 and TNF-α have been documented after experimental THS [6–8]. Similar cytokine patterns have been described in trauma patients, and the majority of investigators agree that trauma insult results in an early increase in IL-6 plasma levels [9–12]. Moreover, there is a strong correlation between IL-6 levels and the severity of injury as well as hospital mortality [9, 10, 12].
Agents that can reduce the severity of gut damage and the inflammatory response following THS would be potentially useful for prevention and treatment of subsequent MOD. Previously, we have shown that recombinant factor XIII (rFXIII) limits MOD in an experimental model of isolated gut ischemia-reperfusion injury (superior mesenteric artery occlusion) [13]. FXIII or fibrin stabilizing factor is a transglutaminase involved in the final stage of blood coagulation. In addition to plasma, FXIII is present in platelets, monocytes, and macrophages. Plasma FXIII is a heterotetramer that consists of two catalytic A subunits and two noncatalytic B subunits (A2B2). The FXIII-A subunits possess the catalytic site of the FXIII enzyme, while the FXIII-B subunits function as carrier molecules. Cellular FVIII is a homodimer consisting of two A subunits (A2). FXIII circulates in plasma as an inactive precursor and is activated by thrombin. Activated FXIII stabilizes fibrin clots by cross-linking fibrin monomers with covalent bonds, which increase the mechanical strength of the clot, retard fibrinolysis, and enhance platelet adhesion to the injured tissue [14]. The rationale for use of FXIII in critical conditions is that in addition to its role in hemostasis, activated FXIII has been shown to stabilize endothelial barrier function and reduce endothelial permeability [15, 16]. Additionally, there is evidence that FXIII modulates the inflammatory response by retardation of macrophage migration [17]. Having previously demonstrated that treatment with rFXIII diminishes superior mesenteric artery occlusion-induced MODS [13], the present study aims to test the protective role of rFXIII in a more relevant clinical model of THS.
MATERIALS AND METHODS
Study Design
Male Sprague-Dawley rats weighing between 250 and 300 g received standard rat chow and water ad libitum, and were allowed an acclimatization period of at least 1 wk prior to the experiment. Animals were subjected to a 12 h light/12 h dark cycle, controlled humidity, and room temperature between 18 and 22°C. Animal study protocols were approved by the Novo Nordisk Ethical Review Committee and the University of Medicine and Dentistry – New Jersey Medical School Animal Care and Use Committee. Experiments were performed in compliance with the requirements of the Danish Animal Experiments Council, the Danish Ministry of Justice, and the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Rats subjected to THS or trauma-sham shock (TSS) were treated in blinded fashion with a placebo or a recombinant human FXIII A2 subunit (rFXIII) (Novo Nordisk A/S, Denmark). Animals were randomly divided into four groups (eight animals each): group 1: THS + plus vehicle treatment; group 2: THS + rFXIII treatment; group 3: TSS + vehicle treatment; and group 4: TSS + rFXIII treatment. The vehicle represented a buffer consisting of 40 mM histidine, 8.5% sucrose, and 0.02% Tween 20 at pH 8.0. Lyophilized rFXIII was resuspended in the same buffer to achieve a final concentration of 1 mg/mL. The vehicle (1.0 mL/kg) or rFXIII (1.0 mg/kg) was given intravenously after 90 min of THS and re-infusion of shed blood (in THS groups) or after 90 min of TSS (in sham groups). The chosen dose of rFXIII was in alignment with the available literature data and our previous studies [13, 18].
The majority of the end-point parameters (microvascular blood flow in the muscle and liver, muscle PO2, lung permeability, lung and gut myeloperoxidase activity, neutrophil respiratory burst, and gut histology) were measured 3 h after the 90-min THS or TSS periods. FXIII activity in rat plasma was measured prior to THS or TSS and 3 h after the THS or TSS period.
Study Product
rFXIII is a recombinant, human FXIII-A2 homodimer composed of two FXIII-A subunits only. It is similar in structure and function to the cellular form of human FXIII A subunit. The trial product is manufactured as an intracellular, soluble protein in a yeast (Saccharomyces cerevisiae) production strain containing the episomal expression vector, pD16. It is subsequently isolated by homogenization of cells and purification by several chromatography steps.
Trauma-Hemorrhagic Shock and Trauma-Sham Shock Protocol
THS animals underwent blood withdrawal according to a standard protocol [1]. Rats were anesthetized with sodium pentobarbital (50 mg/kg) injected intraperitoneally and the right femoral artery was isolated by minimal dissection and aseptically cannulated with polyethylene (PE-50) tubing containing 0.1 mL of heparinized saline. The catheter was connected in-line to a blood pressure recorder and polygraph (Model 79E Data Recorder, Grass, Quincy, MA), to allow continuous blood pressure monitoring. Using an aseptic technique, the right external jugular vein was cannulated with a 50-gauge silicone catheter containing 0.1 mL of heparinized saline. After a traumatic injury (laparotomy) was performed, the abdomen was subsequently closed in two layers using running 4-0 silk sutures. To induce shock, blood was withdrawn from the jugular vein into a syringe containing 10 units of heparin suspended in 0.3 mL of 0.9% normal saline to prevent clotting. The mean arterial pressure was reduced to 30 mm Hg and maintained at the level of 30–40 mm Hg for 90 min by the careful withdrawal or re-infusion of shed blood (kept at 37°C) as needed. After 90 min of shock, animals were resuscitated with their shed blood.
TSS control rats had a laparotomy and their blood vessels cannulated, but no blood was withdrawn or given. Blood pressure and heart rate were recorded during 90 min of THS or TSS and the 3-h post-shock period. At the end of the third hour after resuscitation or TSS, the animals were sacrificed by intravenous injection of pentobarbital.
Tissue Perfusion and Oxygenation Measurements
Tissue perfusion and oxygenation were investigated using a combination of OxyFlo and OxyLite monitors (Oxford Optotronix Ltd., Abingdon, UK). OxyFlo is a multichannel system for measuring tissue blood flow (perfusion), which combines laser Doppler technology and digital signal processing. OxyLite operates according to the principle of oxygen quenching of fluorescence and utilizes a small optical sensor featuring zero oxygen consumption for monitoring rapid temporal oxygen changes in a given tissue micro-region. Precalibrated probes (sensor) are able to provide both spatial and continuous real-time measurement of dissolved oxygen (tissue PO2) and temperature.
Blood flow and PO2 in muscles of the medial surface of the thigh was measured using an NP/O/E/4 needle-encased sensor inserted percutaneously via an 18-gauge guiding cannula. Liver blood flow was measured by a reusable MSP300XP miniature surface sensor. Blood flows are expressed in conditional units. The Win Daq software package (Dataq Instruments, Inc., Akron, OH) was used to record and display tissue blood flow, PO2 and temperature in real time on a computer screen as well to save these data for further analysis.
Lung Permeability Assay
Lung permeability was measured by the Evans blue dye (EBD) technique. Two hours and 40 min after THS or TSS, rats are injected with 1 mL (10 mg) of 1% EBD through the internal jugular catheter. After 5 min, to allow for complete circulation of the dye, a blood sample (1.0 mL) was withdrawn from the femoral artery catheter and centrifuged at 1500 rpm at 4°C for 20 min. The plasma was used to determine the plasma EBD concentration. Twenty min after injection of the dye, the rats were sacrificed and the tracheobronchial tree and lungs were harvested as a unit. Bronchoalveolar lavage was performed by lavaging the lungs three times with 5 mL of normal saline. The recovered bronchoalveolar lavage fluid (BALF) was then centrifuged at 1500 rpm at 4°C for 20 min to remove any cells. The supernatant fluid was then assayed spectrophotometrically at 620 nm to measure the concentration of the EBD in the BALF. The concentration of EBD in the BALF was then expressed as the percentage of that present in the plasma.
Myeloperoxidase Assay
Myeloperoxidase (MPO) activity was measured in the ileum and lung. Harvested tissue samples were homogenized for 30 s in 4 mL of 20 mmol/L potassium phosphate buffer (pH 7.4) and centrifuged (40,000 × g) at 4°C. The pellet was resuspended in 4 mL of 50 mmol/L potassium phosphate buffer (pH 6) containing 0.5 g/dL hexadecyltrimethylammonium bromide. Samples were sonicated for 90 s, incubated in a 60°C water bath for 2 h, and centrifuged. The supernatant in the amount of 0.1 mL was added to 2.9 mL of 50 mmol/mL potassium phosphate buffer (pH 6) containing 0.167 mg/mL o-dianisidine and 0.0005% hydrogen peroxide. Absorbance at 460 nm of visible light (A460) was measured for 3 min. MPO activity was calculated using the following formula:
MPO activity (units/g tissue) = (δ A460 × 13.5)/weight (g), where δ A460 equals the rate of change in absorbance at 460 nm between 1 and 3 min. The coefficient 13.5 was empirically determined such that one unit of MPO activity is the amount of enzyme that will reduce 1 μmol peroxide per min [19].
Neutrophil Respiratory Burst Assay
Flow cytometry was used to assess neutrophil respiratory burst. Heparinized whole blood samples (100 μL) were placed into 5 mL poly-styrene round bottom tubes containing an equal volume of Dulbecco’s modified Eagle’s medium, and the red blood cells were lysed by means of 1% Pharm Lyse solution (BD Biosciences, San Jose, CA, USA). The tubes were spun at 1135 rpm for 5 min at 25°C. The supernatants were discarded and the cells were washed twice with Hank’s balanced salt solution (HBBS). After the white blood cell pellets were resuspended in 400 μL of HBBS, 15 ng/mL of dihydrorhodamine (DHR) was added to the tubes. Five minutes after DHR was added, polymorphonuclear neutrophils (PMNs) were stimulated with phorbol myristal acetate. After a 15-min incubation at 37°C, the PMN respiratory burst was measured by flow cytometry.
Measurement of Plasma Cytokine and Chemokine Levels
The levels of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-10, and macrophage inflammatory protein-2 (MIP-2) in plasma were determined by commercial enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions. The absorbance at 450 mm was read using Spectra Max Plus Microplate Spectrophotometer (Molecular Devices Corporation, Sunnyvale, CA). Cytokine and the chemokine levels were calculated from a standard curve and expressed in pico-grams per milliliter.
Histologic Examination
After the rats were sacrificed, a segment of the terminal ileum was excised and fixed in 10% buffered formalin. After processing, semi-thin (2–4 μm) sections were cut and stained with 1% hematoxylin-eosin. Five random fields with 100–250 villi were analyzed in a blinded fashion by means of light microscopy at ×100 magnification. The overall percentage of ileal villous damage was determined by dividing the number of injured villi by the total number of villi examined. Illeal mucosal damage score was calculated as described by Chiu et al. [20]. The authors suggest the following scoring system for the gut injury: grade 0: normal mucosal villi; grade 1: development of subepithelial space, usually at the apex of the villus; grade 2: extension of the subepithelial space with moderate lifting of epithelial layer from the lamina propria; grade 3: massive epithelial lifting down the sides of villi, a few tips may be denuded; grade 4: denuded villi with lamina propria and dilated capillaries; and grade 5: digestion and disintegration of lamina propria; hemorrhage and ulceration.
FXIII Activity Assay
We measured the activity of the FXIII A subunit (FXIIIA). The photometric FXIII activity assay (BerichromF XIII, Dade Behring, Newark, DE) was performed on rat plasma samples according to the manufacturer’s instructions but adapted to a microtiter plate format read on a SpectraMax (Molecular Devices) at 340 nm in the kinetic mode. Plasma samples were diluted 1:3 in 20 mM Hepes, 150 mM NaCl, pH 7.4 and rFXIII standards were diluted in FXIII- deficient plasma (George King BioMedical, Inc., Overland Park, KS).
Statistics
Data were analyzed using SPSS 9.0 for Windows (SPSS, Chicago, IL), and presented as the mean ± standard deviation. Mean values were compared by analysis of variance (ANOVA) followed by a Tukey test. The level of statistical significance was set at P < 0.05.
RESULTS
In this study, the effects of rFXIII on the parameters that result in MOD under conditions of THS are assayed. These parameters include hemodynamic and inflammatory influences, as well as gut integrity, with the latter further contributing to inflammatory, toxic and oxidative stress conditions that lead to MOD.
Rats subjected to THS and treated with rFXIII demonstrated more stable hemodynamics 3 h post-shock than placebo-treated animals. They had higher levels of mean blood pressure and lower heart rates, which did not differ from sham values (Table 1). Treatment with rFXIII improved regional microvascular blood flow and oxygenation after THS. After 3 h of THS, muscle PO2 as well as muscle and liver blood flows were significantly higher in rFXIII-treated rats than in placebo-treated animals but lower than in sham groups (Table 2).
TABLE 1.
Hemodynamics Before and After THS or TSS
| THS + placebo | THS + rFXIII | TSS + placebo | TSS + rFXIII | |
|---|---|---|---|---|
| Before THS or TSS | ||||
| MBP (mm Hg) | 96.7 ± 14.2 | 86.9 ± 9.2 | 87.9 ± 9.4 | 88.9 ± 7.5 |
| SBP (mm Hg) | 122.4 ± 18.2 | 111.5 ± 13.2 | 108.0 ± 11.0 | 106.6 ± 12.2 |
| HR (beat/min) | 319.2 ± 60.9 | 305.7 ± 47.1 | 313.6 ± 20.1 | 308.0 ± 24.9 |
| At the end of resuscitation after the 90 min THS or TSS period | ||||
| MBP (mm Hg) | 94.2 ± 21.1 | 91.5 ± 10.3 | 89.4 ± 6.2 | 90.3 ± 8.4 |
| SBP (mm Hg) | 137.6 ± 13.3*** | 124.1 ± 14.0*** | 107.6 ± 8.3 | 108.0 ± 13.9 |
| HR (beat/min) | 363.5 ± 54.1*** | 355.9 ± 42.5*** | 316.3 ± 26.5 | 310.5 ± 17.7 |
| 3 h after the end of resuscitation or TSS period | ||||
| MBP (mm Hg) | 75.1 ± 8.8* | 91.7 ± 7.1 | 87.3 ± 9.7 | 93.0 ± 7.2 |
| SBP (mm Hg) | 106.7 ± 8.3** | 111.5 ± 10.1 | 105.4 ± 8.2 | 112.0 ± 13.4 |
| HR (beat/min) | 388.0 ± 37.2* | 346.0 ± 48.6 | 319.8 ± 28.2 | 317.8 ± 23.6 |
MBP = mean blood pressure; SBP = systolic blood pressure; HR = heart rate.
P < 0.05 versus before THS and versus all other groups after THS and resuscitation.
P < 0.05 versus after THS and resuscitation.
P < 0.05 versus both shams.
TABLE 2.
rFXIII Improves Tissue Oxygenation and Perfusion After THS
| Groups of animals | Muscle PO2 (mm Hg) | Muscle blood flow (conditional units) | Liver blood flow (conditional units) |
|---|---|---|---|
| THS + placebo | 46.8 ± 3.1* | 173.0 ± 49.6* | 470.6 ± 63.8* |
| THS + rFXIII | 54.3 ± 4.1** | 277.2 ± 52.4** | 763.4 ± 100.8** |
| TSS + placebo | 60.2 ± 3.0 | 348.2 ± 83.9 | 1101.2 ± 222.5 |
| TSS + rFXIII | 59.9 ± 2.3 | 328.5 ± 44.0 | 1145.4 ± 96.2 |
P < 0.05 versus all other groups.
P < 0.05 versus both shams.
Treatment with rFXIII also modulated the inflammatory parameters as assessed by the levels of plasma cytokine and chemokine levels. TNF-α levels after THS in rFXIII-treated rats did not differ from sham animals. Rats subjected to THS and treated with placebo had higher TNF-α levels than sham animals (Table 3). However, the difference in TNF-α levels between rats subjected to THS followed by placebo or rFXIII treatment did not reach statistical significance. MIP-2 levels after THS in rFXIII-treated rats were lower than in placebo-treated rats but higher than in sham animals. Animals subjected to THS had elevated levels of IL-6 compared with sham rats, regardless of rFXIII or placebo treatment. Trauma-hemorrhagic shock caused an increase in levels of IL-10, an anti-inflammatory cytokine, both in rFXIII and placebo treatment groups. However, these levels were significantly higher in rats subjected to THS followed by rFXIII treatment. Thus, rFXIII treatment shifted the balance of pro- and anti-inflammatory cytokines in favor of an anti-inflammatory response.
TABLE 3.
Cytokine Levels after THS or TSS
| Groups of animals | TNF-α (pg/mL) | MIP-2 (pg/mL) | IL-6 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|
| THS + placebo | 22.3 ± 10.6*** | 208.7 ± 42.5* | 529.3 ± 218.9** | 50.5 ± 15.6* |
| THS + rFXIII | 17.8 ± 4.5 | 150.1 ± 23.8*** | 462.7 ± 276.4** | 141.7 ± 37.0** |
| TSS + placebo | 13.4 ± 3.4 | 121.8 ± 37.8 | 99.8 ± 125.1 | 10.4 ± 7.2 |
| TSS + rFXIII | 16.4 ± 5.2 | 96.9 ± 19.6 | 51.7 ± 77.3 | 11.7 ± 5.0 |
P < 0.05 versus all other groups.
P < 0.05 versus both shams.
P < 0.05 versus corresponding sham.
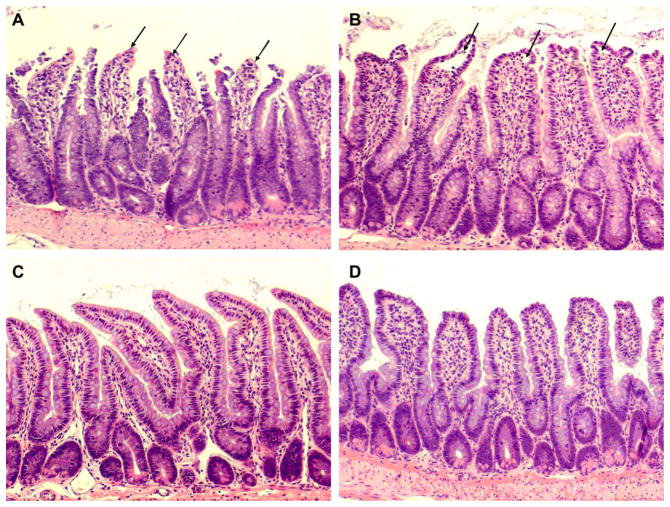
The severity of gut injury in rFXIII-treated animals was reduced compared with placebo-treated animals as indicated by the lower ileal mucosal injury score and the reduced number of injured ileal villi (Table 4). Moreover, ileal mucosal injury score after THS in rFXIII-treated rats did not differ from sham values. Rats subjected to THS and placebo treatment demonstrated severe disruption of villi tips (Fig. 1A). Ileal mucosal injury was less profound after THS and rFXIII treatments. However, extension of subepithelial space as well as villi edema was recorded (Fig. 1B). Ileal damage was minimal in both sham groups (Fig. 1C and D).
TABLE 4.
rFXIII Reduces the Severity of Ileal Mucosal Damage After THS
| Groups of animals | Villi examined | Injured villi (%) | Injury scores |
|---|---|---|---|
| THS + placebo | 205.9 ± 8.8 | 40.9 ± 8.6* | 2.99 ± 0.12* |
| THS + rFXIII | 228.0 ± 19.8 | 28.1 ± 7.8** | 1.37 ± 0.74 |
| Sham + placebo | 229.8 ± 20.4 | 11.1 ± 3.7 | 1.00 ± 0.05 |
| Sham + rFXIII | 229.5 ± 11.5 | 9.2 ± 3.1 | 1.00 ± 0.05 |
P < 0.01 versus all other groups.
P < 0.01 versus both shams.
FIG. 1.
rFXIII reduces the severity of ileal mucosal damage after THS. Hematoxylin-eosin staining. Magnification ×100. (A) Rat is subjected to THS and placebo treatment. Villi tips are denuded (arrows). Red blood cell congestion is seen. Gut injury level is classified as grade 3 mucosal damage score. (B) Rat is subjected to THS and rFXIII treatment. Extension of the subepithelial space as well as villi edema (arrows) is seen. Gut injury level is classified as grade 2 mucosal damage score. (C) Rat is subjected to TSS and placebo treatment. Almost normal villi are seen (grades 0–1 mucosal damage score). (D) Rat is subjected to TSS and rFXIII treatment. Almost normal villi are seen (grades 0–1 mucosal damage score).
Acute lung injury, as measured by lung permeability in rats subjected to THS and treated with rFXIII, was significantly lower than in placebo-treated animals but higher than in sham groups (Fig. 2).
FIG. 2.
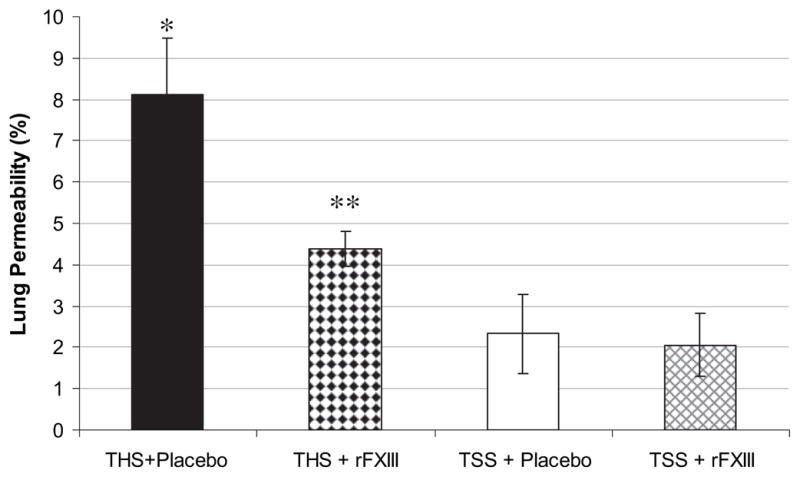
rFXIII diminishes lung permeability alterations after THS. Lung permeability is evaluated by measuring concentration of EBD in bronchoalveolar lavage fluid and expressed as the percentage of that present in the plasma. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 versus all other groups; **P < 0.05 versus both shams.
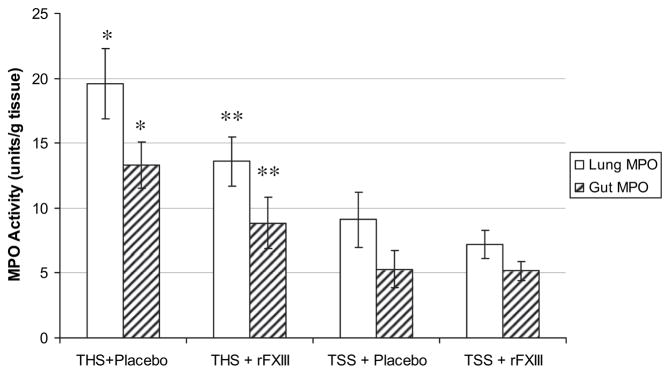
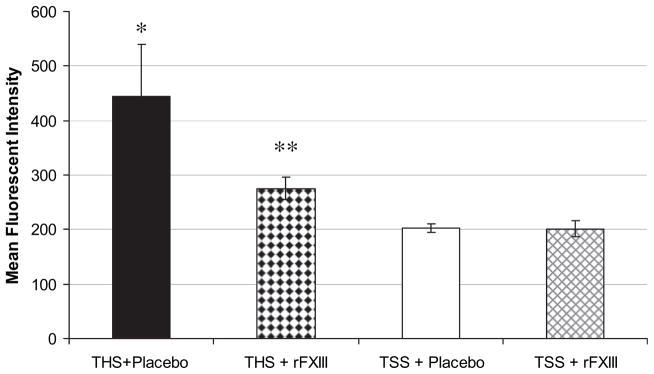
Neutrophil accumulation, as indicated by lung and gut MPO activity after THS was also significantly lower in rFXIII-treated rats, compared with placebo-treated animals but higher than in sham groups (Fig. 3). Neutrophil respiratory burst (rapid release of reactive oxygen species by neutrophils) in rats subjected to THS and treated with rFXIII, was significantly lower than in placebo-treated animals but higher than sham values (Fig. 4).
FIG. 3.
rFXIII reduces neutrophil sequestration in lung and gut after THS. The level of neutrophil sequestration is evaluated by measuring myeloperoxidase activity. Data are expressed as means ± SD (n = 8 in each group). *P < 0.01 versus all other groups. **P < 0.01 versus both shams.
FIG. 4.
rFXIII decreases the level of oxidative stress after THS. The level of oxidative stress is assessed by neutrophil respiratory burst activity. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 versus all other groups; **P < 0.05 versus both shams.
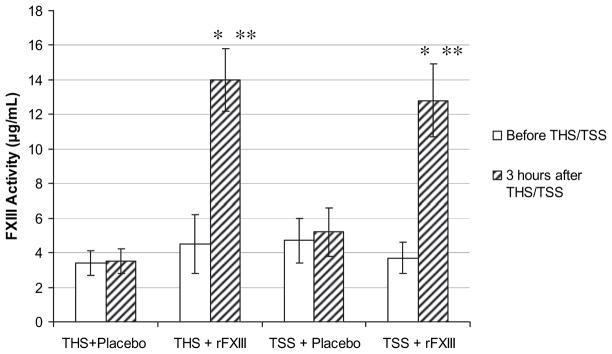
THS did not cause a drop in FXIII activity. FXIII activity in rats subjected to THS and treated with placebo did not differ from sham placebo-treated animals (Fig. 5). Similarly, FXIII activity increased equally in THS and sham animals if they were treated with rFXIII.
FIG. 5.
THS does not decrease FXIII activity. FXIII activity in rat plasma is determined using Berichrom assay before and 3 h after THS or TSS. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 versus before THS/TSS; **P < 0.05 versus groups with placebo treatment.
DISCUSSION
Our data indicate that rFXIII partially protects distant organs after THS as evidenced by the improved perfusion and oxygenation of the muscle and liver tissue, and less severe gut and lung injury. Possibly one or more protective mechanisms come into play. First, the attenuation of THS-induced gut injury may lower the amount of toxic factors penetrating into the intestinal lymph and damaging distant organs. It has been clearly shown that post-hemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury [1, 2, 4] and can also result in abnormal and rigid RBCs, that initiate microcirculatory disorders in distant organs [21, 22]. However, the effectiveness of rFXIII in lowering the intestinal lymph toxicity in animals subjected to THS needs to be confirmed by additional direct studies.
The protective effect of rFXIII in this model of THS can be also explained by a stabilizing effect of rFXIII on the endothelium. Endothelium of vascular beds is extremely sensitive to ischemia. During THS, endothelial cells become edematous and limit microvascular blood flow in vital organs [23]. Reperfusion causes further endothelial dysfunction, aggravating tissue damage by passage of leucocytes into the tissue through the capillary walls. [24, 25]. In vitro experiments have shown that activated FXIII is able to stabilize the endothelial barrier function and decrease endothelial permeability, presumably by interacting with extracellular matrix proteins in the paracellular spaces and/or minimizing disruption of adherens junctions, the protein complexes that occur at cell-cell junctions [13, 15, 16].
The effect of rFXIII on enhancing microvascular blood flow after THS may be attributed to its transglutaminase properties. It has been shown that transglutaminases participate in vascular remodeling [26]. Mice lacking tissue-specific transglutaminase exhibit a delay in arterial remodeling in response to reduced blood flow compared with wild-type mice. The delayed arterial remodeling in turn was shown to be dependent on adventitial monocytes/macrophages which are a source of FXIII [27].
Yet another protective mechanism appears to be the modulation of the cytokine and chemokine response observed after trauma-hemorrhage. Trauma-hemorrhage is associated with increase of the pro-inflammatory cytokine, IL-6 as well as IL-10, an anti-inflammatory cytokine [28–31]. Several studies support protective benefits of IL-10 in a THS setting [32–34]. Administration of rFXIII tended to decrease TNF-α and IL-6 levels, significantly lower MIP-2 and significantly increase IL-10 levels. The rFXIII-mediated modulation of the inflammatory response is likely a consequence of its protective effect on the gut. In response to hypoxia and acidosis ensuing from THS, the gut-mucosa has been demonstrated to be a significant generator of proinflammatory cytokines [7, 35, 36].
Based on the available literature data [18] as well as our previous experience with the gut ischemia-reperfusion model [13], we used a rFXIII A dose of 1.0 mg/kg or 28 μg/mL of rat plasma. The concentration of FXIII A subunit in human plasma is reported to be 10–15 μg/mL [37]. The mean endogenous level of FXIII A subunit in rat plasma is 7 μg/mL (range 5–12 μg/mL). This implies that supra-physiologic levels of FXIII were used in the study to achieve a protective effect after THS.
There are very limited data regarding the effect of trauma-hemorrhage on FXIII levels. Seekamp et al. measured FXIII A sub-unit levels in 24 severely traumatized patients over a 14-d period [38]. Levels of FXIII A sub-unit decreased to about 40% (normal range, 50%–150%) at hospital admission. During the follow-up period, FXIII A sub-unit levels increased 6 h after admission, and then declined and returned to normal values at d 8. These findings are consistent with the data reported by Tanaka et al., who measured FXIII levels in 30 patients with hemorrhagic shock secondary to severe injury and in 16 patients with septic shock complicated by MOD [39]. Both groups of patients had decreased FXIII levels; however, the drop in FXIII levels was significantly more pronounced in septic patients. In our series of experiments, we did not detect any drop in FXIII activity 3 h following THS. Moreover, rats subjected to either THS or TSS demonstrated an equal increase in FXIII activity when rFXIII was given. However, we cannot exclude the possibility that FXIII levels could drop at a later period if septic complications developed. The increased FXIII levels achieved by rFXIII treatment were sufficient to show a protective effect in rats that were subjected to THS.
The study is not free from limitations. First, the protective effect of rFXIII was demonstrated using the model of controlled THS. It is relevant to establish whether rFXIII is effective in conditions of uncontrolled hemorrhage. Second, we have shown that experimental treatment ameliorated markers of multiple organ dysfunction at 3 h following THS. However, it is remains unclear whether rFXIII can provide long lasting protection after trauma-hemorrhage. Also, similar to other investigators, we used relatively high dose of rFXIII. It is important to investigate if a smaller dosage of rFXIII can mitigate manifestations of multiple organ dysfunction after THS. Lastly, the use of Evans blue dye for the assessment of the lung permeability precluded the examination of coagulation profile changes after experimental treatment. All the aforementioned aspects will be taken into consideration in future studies.
In conclusion, rFXIII ameliorates early markers of multiple organ dysfunction caused by experimental THS in rats. The mechanism of the protective effect of rFXIII might be explained by preservation of the gut endothelial barrier function, preservation of microvascular blood flow in organs and tissues, modulation of the inflammatory response, and amelioration of permeability alterations in distant organs. Further studies are required to determine other mechanisms of rFXIII action.
References
- 1.Deitch EA, Forsythe R, Anjaria D, et al. The role of lymph factors in lung injury, bone marrow suppression and endothelial cell dysfunction in a primate model of trauma-hemorrhagic shock. Shock. 204;22:221. doi: 10.1097/01.shk.0000133592.55400.83. [DOI] [PubMed] [Google Scholar]
- 2.Zallen G, Moore EE, Johnson JL, et al. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 3.Zaets SB, Berezina TL, Caruso J, et al. Mesenteric lymph duct ligation prevents shock-induced red blood cell deformability and shape changes. J Surg Res. 2003;109:51. doi: 10.1016/s0022-4804(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 4.Deitch EA, Shi HP, Lu Q, et al. Mesenteric lymph from burned rats induces endothelial cell injury and activates neutrophils. Crit Care Med. 2004;32:533. doi: 10.1097/01.CCM.0000109773.00644.F4. [DOI] [PubMed] [Google Scholar]
- 5.Xu DZ, Lu Q, Adams CA, et al. Trauma-associated shock-induced upregulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32:760. doi: 10.1097/01.ccm.0000114815.88622.9d. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA, Xu D, Franko L, et al. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Grotz MR, Ding J, Guo W, et al. Comparison of plasma cytokine levels in rats subjected to superior mesenteric artery occlusion or hemorrhagic shock. Shock. 1995;3:362. [PubMed] [Google Scholar]
- 8.Guo W, Ding J, Huang Q, et al. Alterations in intestinal bacterial flora modulates the systemic cytokine response to hemorrhagic shock. Am J Physiol. 1995;269(6 Pt 1):827. doi: 10.1152/ajpgi.1995.269.6.G827. [DOI] [PubMed] [Google Scholar]
- 9.Gabhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135:291. doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 10.Strecker W, Gebhard F, Perl M, et al. Biochemical characterization of individual injury pattern and injury severity. Injury. 2003;34:879. doi: 10.1016/s0020-1383(03)00022-6. [DOI] [PubMed] [Google Scholar]
- 11.Yadav K, Zehtabchi S, Nemes PC, et al. Early immunologic responses to trauma in the emergency department patients with major injuries. Resuscitation. 2009;80:83. doi: 10.1016/j.resuscitation.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Stensballe J, Christiansen M, Tonnesen E, et al. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53:515. doi: 10.1111/j.1399-6576.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaets SB, Xu D-Z, Lu Q, et al. Recombinant factor XIII diminishes multiple organ dysfunction in rats caused by gut ischemia-reperfusion injury. Shock. 2009;31:621. doi: 10.1097/SHK.0b013e31818bbe21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muszbek L, Adany R, Mikkola H. Novel aspects of blood coagulation factor XIII. Structure, distribution, activation, and function. Crit Rev Lab Sci. 1996;33:357. doi: 10.3109/10408369609084691. [DOI] [PubMed] [Google Scholar]
- 15.Noll T, Wozniak G, McCarson K, et al. Effect of factor XIII on endothelial barrier function. J Exp Med. 1999;189:1373. doi: 10.1084/jem.189.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wozniak G, Noll T, Brunner U, Hehrlein FW. Topical treatment of venous ulcer with fibrin stabilizing factor: Experimental investigation of effects on vascular permeability. Vasa. 1999;28:160. doi: 10.1024/0301-1526.28.3.160. [DOI] [PubMed] [Google Scholar]
- 17.Lanir N, Ciano PS, Van de Water L, et al. Macrophage migration in fibrin gel matrices. II. Effects of clotting factor XIII, fibrinec-tin, and glycosanoglycan content on cell migration. J Immunol. 1988;140:2340. [PubMed] [Google Scholar]
- 18.D’Argenio G, Grossman A, Cosenza V, et al. Recombinant factor XIII improves established experimental colitis in rats. Dig Dis Sci. 2000;45:987. doi: 10.1023/a:1005541512152. [DOI] [PubMed] [Google Scholar]
- 19.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 20.Chiu CJ, McArdle AH, Brown R, et al. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 21.Machiedo G, Kim J, Morgan C, et al. Intestinal lymph-induced damage to red blood cells (RBC) following trauma/hemorrhagic shock (T/HS) is mediated by leukocytes (WBC) Arch Helenic Med. 2003;20(Suppl A):102. [Google Scholar]
- 22.Machiedo GW, Zaets SB, Berezina TL, et al. Trauma-hemorrhagic shock-induced red blood cell damage leads to decreased microcirculatory blood flow. Crit Care Med. 2009;37:1000. doi: 10.1097/CCM.0b013e3181962d39. [DOI] [PubMed] [Google Scholar]
- 23.Korzonek-Szlacheta I, Gwozdz B. Effects of endothelin-1 on prevention of microvascular endothelium injuries in hemorrhagic shock in rats. Pharmacol Rep. 2007;59:98. [PubMed] [Google Scholar]
- 24.Beishuizen A, Girbes AR. Hemorrhagic shock and reperfusion injury: The critical interplay of fibrin fragments, leukocytes, and vascular endothelial-cadherin. Crit Care Med. 2009;37:771. doi: 10.1097/CCM.0b013e318194bd9e. [DOI] [PubMed] [Google Scholar]
- 25.Harlan JM, Winn RK. Leukocyte-endothelial interaction: Clinical trials of anti-adhesion therapy. Crit Care Med. 2002;30 (Suppl):214. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]
- 26.Bakker EN, Buus CL, Spaan JA, et al. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 27.Bakker EN, Pistea A, Spaan JA, et al. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: Possible compensation by macrophage-derived factor XIII. Circ Res. 2006;99:86. doi: 10.1161/01.RES.0000229657.83816.a7. [DOI] [PubMed] [Google Scholar]
- 28.Shih HC, Wei YH, Lee CH. Magnolol alters cytokine response after hemorrhagic shock and increases survival in subsequent intra-abdominal sepsis in rats. Shock. 2003;20:264. doi: 10.1097/00024382-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Schneider CP, Schwacha MG, Chaudry IH. The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim Biophys Acta. 2004;1689:22. doi: 10.1016/j.bbadis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Lee CC, Chang IJ, Yen ZS, et al. Effect of different resuscitation fluids on cytokine response in a rat model of hemorrhagic shock. Shock. 2005;24:177. doi: 10.1097/01.shk.0000171870.42900.15. [DOI] [PubMed] [Google Scholar]
- 31.Kubulus D, Mathes A, Pradarutti S, et al. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock. 2008;29:583. doi: 10.1097/SHK.0b013e318157e526. [DOI] [PubMed] [Google Scholar]
- 32.Karakozis S, Hinds M, Cook JW, et al. The effects of interleukin-10 in hemorrhagic shock. J Surg Res. 2000;90:109. doi: 10.1006/jsre.2000.5860. [DOI] [PubMed] [Google Scholar]
- 33.Kobbe P, Schmidt J, Stoffels B, et al. IL-10 administration attenuates pulmonary neutrophil infiltration and alters pulmonary iNOS activation following hemorrhagic shock. Inflamm Res. 2009;58:170. doi: 10.1007/s00011-009-8116-z. [DOI] [PubMed] [Google Scholar]
- 34.Kobbe P, Stoffels B, Schmidt J, et al. IL-10 deficiency augments acute lung but not liver injury in hemorrhagic shock. Cytokine. 2009;45:26. doi: 10.1016/j.cyto.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Homma H, Hoy E, Xu D-Z, et al. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288:466. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]
- 36.Yang R, Han X, Uchiyama T, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:621. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 37.Cheung PP, Kunapuhi SP, Scott CF, et al. Genetic basis of total kininogen deficiency in Williams’ trait. J Biol Chem. 1993;268:23361. [PubMed] [Google Scholar]
- 38.Seekamp A, Barthels M, Sturm JA. Factor XIIIA2/XIIIB2 ratio in severely traumatized patients with soft tissue trauma. Thromb Res. 1992;65:809. doi: 10.1016/0049-3848(92)90119-u. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka H, Sugimoto H, Yoshioka T, et al. Role of granulocyte elastase in tissue injury in patients with septic shock complicated by multiple-organ failure. Ann Surg. 1991;213:81. doi: 10.1097/00000658-199101000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]