Abstract
Purpose
Many Childhood Cancer Survivor Study (CCSS) participants are at increased risk for obesity. The etiology of their obesity is likely multifactorial but not well understood.
Patients and Methods
We evaluated the potential contribution of demographic, lifestyle, treatment, and intrapersonal factors and self-reported pharmaceutical use to obesity (body mass index ≥ 30 kg/m2) among 9,284 adult (> 18 years of age) CCSS participants. Independent predictors were identified using multivariable regression models. Interrelationships were determined using structural equation modeling (SEM).
Results
Independent risk factors for obesity included cancer diagnosed at 5 to 9 years of age (relative risk [RR], 1.12; 95% CI, 1.01 to 1.24; P = .03), abnormal Short Form–36 physical function (RR, 1.19; 95% CI, 1.06 to 1.33; P < .001), hypothalamic/pituitary radiation doses of 20 to 30 Gy (RR, 1.17; 95% CI, 1.05 to 1.30; P = .01), and paroxetine use (RR, 1.29; 95% CI, 1.08 to 1.54; P = .01). Meeting US Centers for Disease Control and Prevention guidelines for vigorous physical activity (RR, 0.90; 95% CI, 0.82 to 0.97; P = .01) and a medium amount of anxiety (RR, 0.86; 95% CI, 0.75 to 0.99; P = .04) reduced the risk of obesity. Results of SEM (N = 8,244; comparative fit index = 0.999; Tucker Lewis index = 0.999; root mean square error of approximation = 0.014; weighted root mean square residual = 0.749) described the hierarchical impact of the direct predictors, moderators, and mediators of obesity.
Conclusion
Treatment, lifestyle, and intrapersonal factors, as well as the use of specific antidepressants, may contribute to obesity among survivors. A multifaceted intervention, including alternative drug and other therapies for depression and anxiety, may be required to reduce risk.
INTRODUCTION
Survivors of childhood cancer are at risk for treatment-related sequelae that place them at an increased risk for being obese. Compared with US normative data from the 1995 National Health Interview Survey, risk of obesity (body mass index [BMI] ≥ 30 kg/m2) was increased 50% among adult female and 20% among adult male leukemia survivors in the Childhood Cancer Survivor Study (CCSS).1 Among CCSS male survivors, Hispanic race/ethnicity and brain radiation were associated with an increased risk of obesity, whereas age at diagnosis of 5 to 9 years, black, non-Hispanic race/ethnicity, brain radiation, and treatment with an anthracycline and an alkylating agent increased the risk of obesity among CCSS female survivors.2
Survivors treated for acute lymphoblastic leukemia (ALL), Hodgkin's lymphoma (HL), and non-Hodgkin's lymphoma (NHL) were more likely to report symptomatic levels on the Brief Symptom Inventory−18 (BSI) depression subscale (ALL, 5.4%; HL, 5.5%; and NHL, 4.4%) than were siblings (3.4%). Female ALL and HL survivors were approximately twice as likely to report symptomatic levels for depression as compared with male survivors.3 CCSS participants with solid tumors had significantly higher scores on the BSI depression, somatic distress, and anxiety subscales than did CCSS sibling participants.4
Weight gain is a frequent adverse effect of the use of some antidepressants, including paroxetine (Paxil; GlaxoSmithKline, Research Triangle Park, NC)5,6 and, in one study, sertraline (Zoloft; Pfizer Inc, New York, NY),6 as well as antipsychotic drugs such as clozapine (Clozaril; Novartis Pharmaceuticals, East Hanover, NJ), olanzapine (Zyprexa; Eli Lilly, Indianapolis, IN), and risperidone (Risperdal; Janssen, Division of Ortho-Mcneil-Janssen Pharmaceuticals Inc, Titusville, NJ)7–18and some drugs used for seizure control and/or mood stabilization, such as sodium valproate (Depakote; Abbott Laboratories, Abbott Park, IL).19–21
In addition to treatment, lifestyle, and intrapersonal factors, this study sought to determine the contribution, if any, of the use of specific pharmaceuticals for depression, anxiety, or mood stabilization to the risk of obesity among adult survivors of childhood cancer. Additionally, we used structural equation modeling (SEM) to identify factors that directly predict, moderate, or mediate obesity to inform interventions for long-term health management.
PATIENTS AND METHODS
A cohort of 20,720 previously untreated patients who were less than 21 years of age at diagnosis, survived for at least 5 years after the date of diagnosis, and were diagnosed with an eligible cancer between January 1, 1970, and December 31, 1986, was identified at the 26 participating institutions of the CCSS. This study was approved by the institutional review board at each participating institution. The study design, cohort characteristics, and baseline and follow-up data collection are presented in detail elsewhere.22–24 Data from survivors who completed the baseline and follow-up 2003 questionnaires and who were older than 18 years at the time of the follow-up 2003 questionnaire were eligible for this analysis. A total of 1,842 of those who were sent the follow-up 2003 questionnaire received a shortened version of the questionnaire from which the four pages of psychological outcome measures had been deleted.
The current report is based on data from both the baseline (used to determine only baseline frequency of aerobic exercise, defined as the number of days [0 to 7] on which exercise sufficient to induce sweating or breathing hard, lasting ≥ 20 minutes, was performed25) and the 2003 follow-up questionnaire (used for ascertainment of all other data used in these analyses). Two previous CCSS reports on obesity used only data from the CCSS baseline questionnaire that were obtained between 1995 and 1996.1,26 A more recent CCSS publication, restricted to CCSS participants diagnosed with acute lymphoblastic leukemia, used the follow-up 2003 data but evaluated only different categories of cranial irradiation and treatment that included chemotherapy as a dichotomous variable.2
Methods
The primary outcome of interest was BMI, which was calculated using the standard formula—weight (kg)/(height[m])2—based on self-reported weight and height in the follow-up 2003 survey. Individuals were classified as obese if their BMI was ≥ 30 kg/m2.27 Self-reported body weight was adjusted for those with amputated extremities by the following percentages: amputation of foot, −1.5%; below-the-knee amputation, −3.7%; knee disarticulation, −5.7%; Van Ness rotationplasty, −7.2%; above-the-knee amputation, −11.0%; hip disarticulation or hemipelvectomy, −16.0%.28
Pharmaceutical use was assessed by the participant's response to the question, “Please indicate all medicines/drugs you took regularly during the two-year period between September 1, 2000 and September 1, 2002. We are only asking about medicines/drugs which you took consistently for more than one month, or for 30 days or more in a year ” in several categories, including, “Antidepressants or other prescribed drugs for depression or other mood disorders such as Elavil, Prozac, Paxil, Zoloft, Navane, Ritalin or others,” and “Other prescribed drugs.” The specific pharmaceuticals evaluated included sertraline, paroxetine, fluoxetine (Prozac; Eli Lilly), citalopram (Celexa; Forest Laboratories, New York, NY), escitalopram (Lexapro; Forest Laboratories), bupropion (Wellbutrin; GlaxoSmithKline), venlafaxine (Effexor; Pfizer Inc), amitriptyline (Elavil; AstraZeneca UK Limited, London, United Kingdom), risperidone, and sodium valproate. Drugs used by fewer than 30 patients including quetiapine (Seroquel; AstraZeneca Pharmaceuticals LP, Wilmington, DE), clozapine (Clozaril; Novartis Pharmaceuticals), desipramine (Norpramin; sanofi-aventis US LLC, Bridgewater, NJ), nortriptyline (Pamelor; Mallinckrodt Inc, St Louis, MO), ziprasidone (Geodon; Pfizer), thioridazine (Mellaril; Novartis Pharmaceuticals), aripiprazole (Abilify; Otsuka America Pharmaceutical Inc, Rockville, MD), olanzapine, doxepin (Sinequan; Pfizer), imipramine (Tofranil; Ciba-Geigy AG, Basel, Switzerland), and nefazodone (Serzone; Bristol-Myers Squibb, Princeton, NJ) were not included in the univariable or multivariable regression analyses or the SEM analysis.
Additional independent variables included demographics, treatment exposures, baseline frequency of aerobic exercise,25 physical activity, physical function, intrapersonal factors, cancer-related pain, and cancer-related anxiety/fears. Radiation dose to the hypothalamic/pituitary region was estimated for each patient29,30 as previously described by Stovall et al.31,32
Patients were classified as physically active if they indicated that they satisfied the US Centers for Disease Control and Prevention (CDC) guidelines for physical activity (30 minutes of moderate-intensity physical activity on ≥ 5 days of the week or 20 minutes of vigorous intensity physical activity on ≥ 3 days of the week).33 Patients were classified as inactive if they reported no participation in any leisure-time physical activity over the past month (1 = active; 0 = inactive). Physical function was categorized on the basis of participant scores on the physical function subscale of the Short Form–36 (SF-36), with a score ≤ 40 indicating abnormal physical function.34,35
Intrapersonal factors were quantified using the scores on the BSI subscales of depression, somatic distress, and anxiety. Cancer-related pain was quantified using a 5-point scale (1 = no pain; 5 = very bad, excruciating pain). Cancer-related anxiety/fears was quantified using a 5-point scale (1 = no anxiety/fears; 5 = very many, extreme anxiety/fears).
Statistical Analysis
Univariate log-binomial regression analysis was applied to evaluate the effect of demographic, treatment, lifestyle, intrapersonal, and pharmaceutical usage variables on the relative risk of obesity.36,37 Covariates with P < .1 in the univariate analysis were selected for the multivariable model and were further reduced on the basis of the likelihood ratio statistics for type III contrasts.38 Age at questionnaire, sex, and race/ethnicity were forced into both univariate and multivariable models. The data analysis was performed on SAS 9.1 (SAS Institute, Cary, NC).
SEM Measures
Observed and latent variables were modeled in SEM. Factorial validity of the latent variables was established through exploratory and confirmatory factor analyses. The latent variables in the SEM included depression (defined by four of six items from the BSI39,40: lonely, blue, no interest, hopeless) and physical function (defined by five of 10 items from the physical function subscale of the SF-3641,42: climb several stairs, climb one flight of stairs, walk several miles, walk several blocks, walk one block). The conceptually sound, best-fitting model was based on established SEM fit criteria (a root mean square error of approximation [RMSEA] ≤ 0.05,43,44 comparative fit index [CFI] and Tucker Lewis index [TLI] ≥ 0.90,45 and a weighted root mean square residual [WRMR] less than 0.9046 when the outcome variable was binary).
SEM was analyzed using Mplus 6.1 software.47 To model the mediators and moderators in SEM, a sub-program, INDIRECT, was used. The significance of the mediator or moderator was determined by the strength of the estimate, divided by the SE.
RESULTS
Study Population
Nine thousand two hundred eighty-four survivors who were ≥ 18 years of age at the time of completion of the follow-up 2003 questionnaire were included in these analyses (Table 1). Slightly more than half of the study population was male, and participants were predominantly white. Most had at least a high school diploma or equivalent. More than 60% were diagnosed when younger than age 10 years, and nearly 30% were older than age 35 years at the time of evaluation. Approximately 13% of survivors had annual household incomes of less than $25,000 per year. Almost 90% had health insurance or were Canadian residents. Radiation to the hypothalamic-pituitary axis was part of treatment for more than 60% of survivors.
Table 1.
Characteristics of CCSS Survivors
| Variable | Adult Survivors(N = 9,284) |
|
|---|---|---|
| No. | % | |
| Sex | ||
| Male | 4,707 | 50.70 |
| Female | 4,577 | 49.30 |
| Race/ethnicity | ||
| Non-Hispanic white | 8,262 | 89.33 |
| Hispanic | 332 | 3.59 |
| Non-Hispanic black | 394 | 4.26 |
| Other | 261 | 2.82 |
| Missing | 35 | |
| Education level | ||
| No high school or GED | 430 | 4.68 |
| High school or GED | 2,111 | 22.98 |
| Some college; no bachelor's degree | 2,773 | 30.18 |
| Bachelor's degree or higher | 3,873 | 42.16 |
| Missing | 97 | |
| Age at diagnosis, years | ||
| 0-4 | 3,769 | 41.38 |
| 5-9 | 2,071 | 22.74 |
| 10-14 | 1,866 | 20.49 |
| 15-20 | 1,403 | 15.40 |
| Missing | 175 | |
| Age at questionnaire, years | ||
| 18-25 | 2,479 | 26.70 |
| 26-35 | 4,070 | 43.84 |
| 36-45 | 2,404 | 25.89 |
| 46-55 | 331 | 3.57 |
| > 55 | ||
| Family income | ||
| < $20,000/year | 1,056 | 13.30 |
| ≥ $20,000 and < $40,000/year | 1,870 | 23.55 |
| ≥ $40,000/year | 5,015 | 63.15 |
| Missing | 1,343 | |
| Health insurance | ||
| Yes or Canadian | 8,090 | 87.88 |
| No | 1,116 | 12.12 |
| Missing | 78 | |
| Baseline frequency of aerobic exercise, days/wk | ||
| 0 | 2,786 | 31.13 |
| 1 | 1045 | 11.67 |
| 2 | 1,273 | 14.22 |
| 3 | 1,326 | 14.81 |
| 4 | 807 | 9.02 |
| 5 | 854 | 9.54 |
| 6 | 295 | 3.30 |
| 7 | 565 | 6.31 |
| Missing | 333 | |
| Physical activity | ||
| No | 6,572 | 72.01 |
| Yes | 2,555 | 27.99 |
| Missing | 157 | |
| Inactive lifestyle | ||
| No | 7,140 | 77.21 |
| Yes | 2,107 | 22.79 |
| Missing | 37 | |
| Hypothalamic/pituitary radiation dose | ||
| None | 2,916 | 35.00 |
| < 20 Gy | 3,547 | 42.58 |
| ≥ 20 to ≤ 30 Gy | 1,111 | 13.34 |
| > 30 Gy | 757 | 9.09 |
| Missing | 953 | |
| BSI-18 Depression Score* | ||
| < 63 | 6,805 | 88.11 |
| ≥ 63 | 918 | 11.89 |
| Missing | 1,561 | |
| BSI-18 Somatic Distress Score* | ||
| < 63 | 6,644 | 86.06 |
| ≥ 63 | 1,076 | 13.94 |
| Missing | 1,564 | |
| BSI-18 Anxiety Score* | ||
| < 63 | 7,120 | 92.20 |
| ≥ 63 | 602 | 7.80 |
| Missing | 1,562 | |
| Cancer-related anxiety | ||
| No anxiety/fears | 4,751 | 61.60 |
| Small amount of anxiety/fears | 2,089 | 27.08 |
| Medium amount of anxiety/fears | 607 | 7.87 |
| A lot of anxiety/fears | 199 | 2.58 |
| Very many, extreme anxiety/fears | 67 | 0.87 |
| Missing | 1,571 | |
| Cancer-related pain | ||
| No pain | 5,928 | 77.06 |
| Small amount of pain | 962 | 12.50 |
| Medium amount of pain | 526 | 6.84 |
| A lot of pain | 204 | 2.65 |
| Very bad, excruciating pain | 73 | 0.95 |
| Missing | 1,591 | |
| SF-36 Physical Function | ||
| > 40 | 8,210 | 88.88 |
| ≤ 40 | 1,027 | 11.12 |
| Missing | 47 | |
| Fluoxetine | ||
| No | 8,939 | 97.63 |
| Yes | 217 | 2.37 |
| Missing | 128 | |
| Sertraline | ||
| No | 8,840 | 96.55 |
| Yes | 316 | 3.45 |
| Missing | 128 | |
| Paroxetine | ||
| No | 8,899 | 97.19 |
| Yes | 257 | 2.81 |
| Missing | 128 | |
| Citalopram | ||
| No | 8,975 | 98.02 |
| Yes | 181 | 1.98 |
| Missing | 128 | |
| Escitalopram | ||
| No | 9,065 | 99.01 |
| Yes | 91 | 0.99 |
| Missing | 128 | |
| No | 8,987 | 98.15 |
| Yes | 169 | 1.85 |
| Missing | 128 | |
| Nefazodone | ||
| No | 9,135 | 99.77 |
| Yes | 21 | 0.23 |
| Missing | 128 | |
| Venlafaxine | ||
| No | 8,991 | 98.20 |
| Yes | 165 | 1.80 |
| Missing | 128 | |
| Amitriptyline | ||
| No | 9,125 | 99.66 |
| Yes | 31 | 0.34 |
| Missing | 128 | |
| Imipramine | ||
| No | 9,155 | 99.99 |
| Yes | 1 | 0.01 |
| Missing | 128 | |
| Desipramine | ||
| No | 9,153 | 99.97 |
| Yes | 3 | 0.03 |
| Missing | 128 | |
| Nortriptyline | ||
| No | 9,149 | 99.92 |
| Yes | 7 | 0.08 |
| Missing | 128 | |
| Olanzapine | ||
| No | 9,134 | 99.76 |
| Yes | 22 | 0.24 |
| Missing | 128 | |
| Aripiprazole | ||
| No | 9,150 | 99.93 |
| Yes | 6 | 0.07 |
| Missing | 128 | |
| Thioridazine | ||
| No | 9,155 | 99.99 |
| Yes | 1 | 0.01 |
| Missing | 128 | |
| Quetiapine | ||
| No | 9,134 | 99.76 |
| Yes | 22 | 0.24 |
| Missing | 128 | |
| Clozapine | ||
| No | 9,154 | 99.98 |
| Yes | 2 | 0.02 |
| Missing | 128 | |
| Risperidone | ||
| No | 9,115 | 99.55 |
| Yes | 41 | 0.45 |
| Missing | 128 | |
| Valproate | ||
| No | 9,061 | 98.96 |
| Yes | 95 | 1.04 |
| Missing | 128 | |
Abbreviations: BSI-18, Brief Symptom Inventory–18; CCSS, Childhood Cancer Survivor Study; GED, general education degree; SF-36, Short Form–36.
A BSI-18 scale score of T ≥ 63 reflects a level of emotional symptoms reported by ≤ 10% of the most distressed subjects in the normative standardization sample.
Antidepressant use was reported by 13.8% of survivors overall. Of those who used an antidepressant, 77.4% reported the use of only a single antidepressant during the 2-year period. Fluoxetine, sertraline, and paroxetine were the most commonly used of this class of drugs. Poor physical function was present in 11.12% of survivors. Only 27.99% met the CDC guidelines for physical activity; 22.79% reported no physical activity over the past month. Using the BSI outcome, nearly 12% of survivors were depressed, 13.94% had somatic distress, and 7.80% had anxiety. More than 38% of the survivors reported some degree of cancer-related anxiety, and almost 23% reported some cancer-related pain.
Univariate and Multivariable Analyses
The results of univariate analyses to identify factors associated with obesity are shown in Table 2. Factors associated with an increased risk of obesity (BMI > 30 kg/m2) included Hispanic or non-Hispanic; black race/ethnicity; age at questionnaire of older than 25 years; hypothalamic/pituitary radiation dose exceeding 20 Gy; BSI-18 somatic distress score ≥ 63; a lot of or very bad, excruciating cancer-related pain; poor physical function based on the SF-36 score ≤ 40; and treatment with sertraline, paroxetine, risperidone, or valproate. Factors that decreased the risk of obesity included a bachelor's degree or higher educational attainment, family income ≥ $ 40,000/year, baseline frequency of aerobic exercise, meeting the CDC guidelines for physical activity, participation in any leisure-time physical activity over the past month, and a small amount of cancer-related anxiety/fears.
Table 2.
Relative Risk of Obesity: Univariate Analyses
| Variable | No. of Obese Participants | RR | 95% CI |
|---|---|---|---|
| Sex | |||
| Male | 935 | 1.00 | |
| Female | 972 | 1.07 | 0.99 to 1.16 |
| Race/ethnicity | |||
| Non-Hispanic white | 1,662 | 1.00 | |
| Non-Hispanic black | 86 | 1.35 | 1.12 to 1.62 |
| Hispanic | 105 | 1.33 | 1.12 to 1.57 |
| Other | 48 | 0.94 | 0.73 to 1.21 |
| Age at questionnaire, years | |||
| 18-25 | 418 | 1.00 | |
| 26-35 | 884 | 1.29 | 1.16 to 1.43 |
| 36-45 | 533 | 1.31 | 1.17 to 1.47 |
| 46-55 | 72 | 1.29 | 1.03 to 1.61 |
| Education level | |||
| No high school or GED | 98 | 1.00 | |
| High school or GED | 547 | 1.07 | 0.89 to 1.29 |
| Some college no bachelor's degree | 621 | 0.92 | 0.77 to 1.11 |
| Bachelor's degree or higher | 626 | 0.61 | 0.51 to 0.74 |
| Age at diagnosis, years | |||
| 0-4 | 706 | 1.00 | |
| 5-9 | 473 | 1.09 | 0.97 to 1.22 |
| 10-14 | 404 | 0.97 | 0.85 to 1.10 |
| 15-20 | 278 | 0.85 | 0.72 to 1.01 |
| Family income | |||
| < $20,000/year | 265 | 1.00 | |
| ≥ $20,000, < $40,000/year | 450 | 0.93 | 0.82 to 1.06 |
| ≥ $40,000/year | 910 | 0.68 | 0.60 to 0.77 |
| Health insurance | |||
| No | 237 | 1.00 | |
| Yes or Canadian | 1,654 | 0.93 | 0.83 to 1.05 |
| Baseline frequency of aerobic exercise* | 1,754 | 0.95 | 0.93 to 0.96 |
| Physical activity | |||
| No | 1,502 | 1.00 | |
| Yes | 377 | 0.65 | 0.59 to 0.72 |
| Inactive lifestyle | |||
| Yes | 533 | 1.00 | |
| No | 1,371 | 0.76 | 0.70 to 0.83 |
| Hypothalamic/pituitary radiation | |||
| None | 554 | 1.00 | |
| < 20 Gy | 652 | 0.94 | 0.84 to 1.04 |
| 20-30 Gy | 318 | 1.48 | 1.31 to 1.66 |
| > 30 Gy | 181 | 1.28 | 1.11 to 1.49 |
| BSI-18 Depression Score | |||
| < 63 | 1,383 | 1.00 | |
| ≥ 63 | 206 | 1.13 | 0.99 to 1.28 |
| BSI-18 Somatic Distress Score | |||
| < 63 | 1,308 | 1.00 | |
| ≥ 63 | 279 | 1.30 | 1.16 to 1.45 |
| BSI-18 Anxiety Score | |||
| < 63 | 1,462 | 1.00 | |
| ≥ 63 | 126 | 1.02 | 0.87 to 1.20 |
| Cancer-related anxiety | |||
| 1: None | 1,023 | 1.00 | |
| 2: Small amount | 388 | 0.85 | 0.76 to 0.94 |
| 3: Medium amount | 123 | 0.91 | 0.77 to 1.08 |
| 4: A lot | 41 | 0.94 | 0.71 to 1.23 |
| 5: Very many, extreme | 14 | 0.97 | 0.61 to 1.54 |
| Cancer-related pain | |||
| 1: None | 1,173 | 1.00 | |
| 2: Small amount | 202 | 1.03 | 0.90 to 1.17 |
| 3: Medium amount | 118 | 1.11 | 0.94 to 1.31 |
| 4: A lot | 58 | 1.42 | 1.14 to 1.77 |
| 5: Very bad, excruciating | 25 | 1.65 | 1.20 to 2.26 |
| SF-36 Physical Function | |||
| > 40 | 8,210 | 1.00 | |
| ≤ 40 | 1,027 | 1.53 | 1.38 to 1.70 |
| Fluoxetine | |||
| No | 1,824 | 1.00 | |
| Yes | 52 | 1.12 | 0.88 to 1.42 |
| Sertraline | |||
| No | 1,794 | 1.00 | |
| Yes | 82 | 1.26 | 1.04 to 1.52 |
| Paroxetine | |||
| No | 1,804 | 1.00 | |
| Yes | 72 | 1.40 | 1.15 to 1.71 |
| Citalopram | |||
| No | 1,848 | 1.00 | |
| Yes | 28 | 0.76 | 0.54 to 1.07 |
| Escitalopram | |||
| No | 1,857 | 1.00 | |
| Yes | 19 | 1.05 | 0.71 to 1.57 |
| Bupropion | |||
| No | 1,832 | 1.00 | |
| Yes | 44 | 1.29 | 1.00 to 1.66 |
| Venlafaxine | |||
| No | 1,836 | 1.00 | |
| Yes | 40 | 1.17 | 0.89 to 1.55 |
| Amitriptyline | |||
| No | 1,870 | 1.00 | |
| Yes | 6 | 0.93 | 0.45 to 1.89 |
| Risperidone | |||
| No | 1,860 | 1.00 | |
| Yes | 16 | 1.98 | 1.36 to 2.89 |
| Valproate | |||
| No | 1,849 | 1.00 | |
| Yes | 27 | 1.44 | 1.05 to 1.97 |
NOTE. Boldface indicates decreased risk of obesity. Italics indicates increased risk of obesity.
Abbreviations: BSI-18, Brief Symptom Inventory–18; GED, general education degree; RR, relative risk; SF-36, Short Form–36.
Baseline frequency of aerobic exercise included as a continuous variable.
Factors that remained significant in the multivariable model are shown in Table 3. The risk of obesity was increased among those 5 to 9 years of age at diagnosis (RR = 1.12; 95% CI, 1.01 to 1.24; P = .03), those who received 20 to 30 Gy of hypothalamic/pituitary radiation dose (RR = 1.17; 95% CI, 1.05 to 1.30; P = .01), and those with abnormal SF-36 physical function (RR = 1.19; 95% CI, 1.06 to 1.33; P < .001). The risk of obesity was decreased among those who met the CDC guidelines for physical activity (RR = 0.90; 95% CI, 0.82 to 0. 97; P = .01) and among those with a medium amount of cancer-related anxiety (RR = 0.86; 95% CI, 0.75 to 0.99; P = .04). Of the pharmaceuticals evaluated, only paroxetine was independently associated with an increased risk for obesity (RR = 1.29; 95% CI, 1.08 to 1.54; P = .01).
Table 3.
Relative Risk of Obesity: Multivariate Analyses
| Variable | RR | 95% CI |
|---|---|---|
| Sex | ||
| Male | 1.00 | |
| Female | 1.02 | 0.95 to 1.09 |
| Race/ethnicity | ||
| Non-Hispanic white | 1.00 | |
| Non-Hispanic black | 1.10 | 0.88 to 1.36 |
| Hispanic | 1.12 | 0.93 to 1.33 |
| Other | 0.84 | 0.65 to 1.10 |
| Age at questionnaire, years | ||
| 18-25 | 1.00 | |
| 26-35 | 1.11 | 1.00 to 1.24 |
| 36-45 | 1.13 | 0.98 to 1.30 |
| 46-55 | 1.19 | 0.95 to 1.51 |
| Education level | ||
| No high school or GED | 1.00 | |
| High school or GED | 1.03 | 0.85 to 1.26 |
| Some college no bachelor's degree | 1.00 | 0.82 to 1.21 |
| Bachelor's degree or higher | 0.85 | 0.70 to 1.03 |
| Age at diagnosis, years | ||
| 0-4 | 1.00 | |
| 5-9 | 1.12 | 1.01 to 1.24 |
| 10-14 | 1.06 | 0.94 to 1.19 |
| 15-20 | 1.04 | 0.90 to 1.20 |
| Family income | ||
| < $20,000/year | 1.00 | |
| ≥ $20,000, < $40,000/year | 1.08 | 0.96 to 1.22 |
| ≥ $40,000/year | 0.95 | 0.85 to 1.07 |
| Baseline frequency of aerobic exercise* | 0.99 | 0.97 to 1.00 |
| Physical activity | ||
| No | 1.00 | |
| Yes | 0.90 | 0.82 to 0.97 |
| Hypothalamic/pituitary radiation | ||
| None | 1.00 | |
| < 20 Gy | 0.94 | 0.86 to 1.02 |
| 20-30 Gy | 1.17 | 1.05 to 1.30 |
| > 30 Gy | 1.00 | 0.87 to 1.15 |
| BSI-18 Somatic Distress Score | ||
| < 63 | 1.00 | |
| ≥ 63 | 1.04 | 0.94 to 1.16 |
| Cancer-related anxiety | ||
| None | 1.00 | |
| Small amount | 0.94 | 0.87 to 1.02 |
| Medium amount | 0.86 | 0.75 to 0.99 |
| A lot | 0.85 | 0.67 to 1.08 |
| Very many, extreme | 0.76 | 0.50 to 1.17 |
| SF-36 Physical Function | ||
| > 40 | 1.00 | |
| ≤ 40 | 1.19 | 1.06 to 1.33 |
| Paroxetine | ||
| No | 1.00 | |
| Yes | 1.29 | 1.08 to 1.54 |
| Bupropion | ||
| No | 1.00 | |
| Yes | 1.15 | 0.91 to 1.47 |
| Risperidone | ||
| No | 1.00 | |
| Yes | 1.32 | 0.88 to 1.98 |
| Sertraline | ||
| No | 1.00 | |
| Yes | 1.08 | 0.91 to 1.30 |
NOTE. Boldface indicates decreased risk of obesity. Italics indicates increased risk of obesity.
Abbreviations: BSI-18, Brief Symptom Inventory–18; GED, general education degree; RR, relative risk; SF-36, Short Form–36.
Baseline frequency of aerobic exercise included as a continuous variable.
SEM Analysis
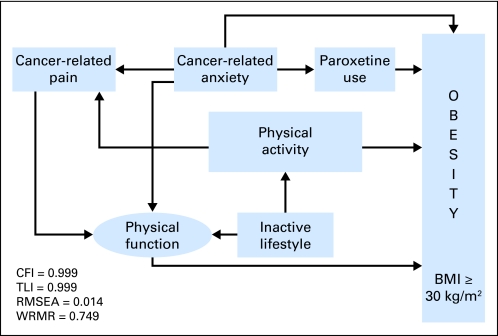
All of the significant variables and their contributions to the model are shown in Table 4. A simplified graphic version of the complete SEM results is shown in Figure 1. A well-fitting model (N = 8,244; CFI = 0.999; TLI = 0.999; RMSEA = 0.014; WRMR = 0.749) identified the complex interrelationships among the directly observed and latent variables that influence obesity in adult survivors of childhood cancer.
Table 4.
SEM Results: Impact of Survivor, Treatment, and Lifestyle Factors on Obesity in Childhood Cancer Survivors
| Factor | Estimate | SE | Estimate/SE | P |
|---|---|---|---|---|
| Obesity | ||||
| Physical function* | –0.098 | 0.013 | –7.407 | < .001 |
| Cancer-related anxiety | –0.127 | 0.027 | –4.748 | < .001 |
| Education level | –0.068 | 0.020 | –3.336 | .001 |
| Physical activity | –0.067 | 0.024 | –2.842 | .004 |
| Age at questionnaire | 0.007 | 0.002 | 2.791 | .005 |
| Hypothalamic/pituitary radiation | 0.049 | 0.018 | 2.707 | .007 |
| Family income | –0.055 | 0.022 | –2.490 | .013 |
| Paroxetine | 0.081 | 0.041 | 1.972 | .049 |
| Physical function* | ||||
| Cancer-related pain | –0.964 | 0.063 | –15.253 | < .001 |
| Gender | –0.711 | 0.073 | –9.683 | < .001 |
| Inactive lifestyle | 0.925 | 0.096 | 9.613 | < .001 |
| Education level | 0.376 | 0.041 | 9.246 | < .001 |
| Age at questionnaire | -0.037 | 0.005 | –8.001 | < .001 |
| Hypothalamic/pituitary radiation | –0.171 | 0.035 | –4.878 | < .001 |
| Cancer-related anxiety | –0.136 | 0.042 | –3.227 | .001 |
| Cancer-related anxiety | ||||
| Sex | 0.238 | 0.029 | 8.164 | < .001 |
| Age at questionnaire | 0.008 | 0.002 | 4.198 | < .001 |
| Cancer-related pain | ||||
| Cancer-related anxiety | 0.465 | 0.021 | 22.629 | < .001 |
| Age at questionnaire | 0.021 | 0.002 | 8.911 | < .001 |
| Education level | –0.144 | 0.021 | –6.993 | < .001 |
| Physical activity | –0.083 | 0.023 | –3.607 | < .001 |
| Baseline frequency of aerobic exercise | –0.062 | 0.020 | –3.115 | .002 |
| Baseline frequency of aerobic exercise | ||||
| Age at questionnaire | –0.031 | 0.002 | –20.136 | < .001 |
| Sex | –0.287 | 0.023 | –12.218 | < .001 |
| Hypothalamic/pituitary radiation | –0.089 | 0.013 | –6.850 | < .001 |
| Education level | 0.065 | 0.013 | 4.946 | < .001 |
| Inactive lifestyle | ||||
| Education level | 0.070 | 0.008 | 9.145 | < .001 |
| Baseline frequency of aerobic exercise | 0.148 | 0.021 | 6.950 | < .001 |
| Hypothalamic/pituitary radiation | –0.013 | 0.005 | –2.513 | .012 |
| Physical activity | ||||
| Baseline frequency of aerobic exercise | 0.288 | 0.017 | 17.206 | <0.001 |
| Education level | 0.138 | 0.018 | 7.704 | < .001 |
| Sex | 0.223 | 0.031 | 7.283 | < .001 |
| Hypothalamic/pituitary radiation | –0.092 | 0.018 | –5.125 | < .001 |
| Age at questionnaire | –0.005 | 0.002 | –2.579 | .010 |
| Family income | ||||
| Education level | 0.285 | 0.017 | 16.632 | < .001 |
| Age at questionnaire | 0.026 | 0.002 | 12.521 | < .001 |
| Hypothalamic/pituitary radiation | –0.146 | 0.016 | –9.110 | < .001 |
| Cancer-related pain | –0.124 | 0.019 | –6.509 | < .001 |
| Sex | –0.125 | 0.030 | –4.113 | < .001 |
| Inactive lifestyle | 0.161 | 0.042 | 3.782 | < .001 |
| Physical activity | 0.067 | 0.023 | 2.957 | .003 |
| Paroxetine | ||||
| Cancer-related anxiety | 0.303 | 0.036 | 8.440 | < .001 |
| Sex | 0.253 | 0.062 | 4.105 | < .001 |
| Education level | –0.128 | 0.032 | –3.955 | < .001 |
NOTE. Boldface variables represent the multiple outcome measures in the model. The unbolded variables reflect the predictors or antecedents of that outcome. The first column is the unstandardized estimate (EST), followed by the SE of that estimate, followed by the estimate divided by the SE (EST/SE). The final column represents the P value associated with the strength of the path from the predictor/antecedent to the outcome variable.
Abbreviation: SEM, structural equation modeling.
Physical Function in the SEM is the five items retained from the SF-36 Physical Function scale by the analytical method (Methods).
Fig 1.
Direct and mediating influences on survivor obesity. Latent variables are illustrated as ellipses, and directly observed variables are illustrated as rectangles. BMI, body mass index; CFI, comparative fit index; TLI, Tucker Lewis index; RMSEA, root mean square error of approximation; WRMR, weighted root mean square residual.
Poor physical function was the strongest direct predictor of obesity, followed by lower self-reported cancer-related anxiety, less education, not meeting CDC guidelines for physical activity, older age at questionnaire, hypothalamic/pituitary radiation exposure, lower family income, and paroxetine use (Table 4). Analysis of potential moderators and mediators of obesity demonstrated significance for cancer-related pain through physical function (EST/SE = 7.714, P ≤ .001), cancer-related anxiety through physical function (EST/SE = 2.986, P = .003), and cancer-related anxiety through cancer-related pain and physical function (EST/SE = 7.279, P ≤ .001). Not meeting CDC guidelines for recommended physical activity mediated obesity through cancer-related pain and physical function (EST/SE = −3.411, P = .001).
DISCUSSION
In the general population, obesity is associated with increased morbidity and mortality.48 The adverse health implications of obesity may be greater among childhood cancer survivors whose exposures place them at an increased risk for severe and life-threatening chronic health conditions.49 Understanding the factors that contribute to obesity in childhood cancer survivors, either directly or as mediators and moderators, can facilitate clinical management. Greater insight into the predictors of obesity will facilitate design and evaluation of innovative intervention/prevention strategies targeting childhood cancer survivors.
Using two different, but complementary, analytic approaches, this study evaluated the risk factors associated with obesity among adult survivors of childhood cancer who participated in the CCSS. The results of the multivariable model demonstrated that impaired physical function, hypothalamic-pituitary radiation, use of paroxetine, and younger age at cancer diagnosis were statistically significant independent predictors for a BMI ≥ 30 kg/m2. Meeting CDC guidelines for physical activity and a moderate amount of anxiety decreased the risk for a BMI ≥ 30 kg/m2.
SEM provided similar findings, while indicating that the impact of physical function on obesity was mediated by cancer-related anxiety, cancer-related pain, and an inactive lifestyle. The primary differences in findings between the two approaches relate to education level, age at questionnaire, and family income, where SEM identified a direct association with obesity, but no statistically significant associations were evident in the multivariable model. It is not immediately apparent why these differences exist. Possible explanations include the formulation of some variables in the SEM as continuous variables (eg, age at questionnaire) that were analyzed as categorical variables in the multivariable model. In addition, latent variables derived in the SEM (eg, physical function), though based on the SF-36, are not the same variable as the dichotomized variable used in the multivariable analysis that was based on the entire SF-36 Physical Function score.
The association between obesity, the use of specific pharmaceuticals, and their relationship with cancer-related anxiety, cancer-related pain, physical activity, and physical function are novel findings. The use of antidepressants has increased dramatically in the 18- to 44-year age group during the period 1992 to 2002, along with a significant shift from prescribing tricyclic antidepressants to selective serotonin reuptake inhibitors.50 Weight gain is a frequent adverse effect of the use of some antidepressant and antipsychotic drugs.5–18 Among the drugs used for seizure control, weight gain is increased among patients treated with sodium valproate compared with carbamazepine (Tegretol; Novartis Pharmaceuticals).19–21
We identified the use of a specific antidepressant, paroxetine (Paxil), as a risk factor associated with obesity in adult survivors of childhood cancer in the multivariable model and as a direct predictor of obesity in the SEM. We lack longitudinal data, particularly BMI data, before the initiation of antidepressant therapy. Therefore, we cannot determine whether obesity, possibly caused by prior treatment, such as cranial irradiation, resulted in depression that was then treated with an antidepressant or whether depression in a nonobese CCSS participant treated with an antidepressant resulted in the development of obesity. A longitudinal study is needed to address these questions. In addition, we lack data on calorie intake and therefore cannot evaluate the relationship of this important determinant of energy balance to the risk of obesity in our population.
An additional unique finding of this analysis was poor physical function as a direct predictor of obesity. Poor physical function was predicted by female sex, older current age, having less education, having been exposed to hypothalamic/pituitary radiation, increased cancer-related pain and anxiety, and leisure-time physical inactivity. Increased physical performance limitations and decreased ability to do routine activities have been documented in adult childhood cancer survivors,51 but their link to obesity has not been established. Diminished functional performance and disability have been linked to obesity, however, in the general population.52–54
Increased cancer-related anxiety/fears predicted nonobesity in the present study; previous studies have documented that underweight survivors were more likely to report adverse health and major medical conditions.1 Correspondingly, those who are most worried about their cancer are those who also report more late effects and related symptoms.25 Cancer-related anxiety was also antecedent to paroxetine use; paroxetine is commonly prescribed for the treatment of anxiety.
Although the single item addressing cancer-related fears/anxiety was significant in the SEM, the BSI anxiety subscale was not. The BSI anxiety subscale assesses symptoms present over the past 7 days and likely reflects generalized acute or “state” anxiety; it does not measure nonpathologic specific anxiety/worry as does the single-item cancer-related anxiety measure. Indeed, specific anxiety contributes to greater generalized anxiety,55 and we have illustrated this relationship in previous reports.25 Cancer-specific anxiety may well exacerbate state anxiety symptoms, but is conceptually and, in this case analytically, distinct from the BSI.
Cranial radiation is a well-established risk factor for obesity among adult survivors of ALL.2,26 Cranial radiation ≥ 10 Gy was associated with a statistically significant mean BMI increase of 0.41 kg/m2/year among female survivors and 0.29 kg/m2/year among male survivors, in comparison with siblings.2 In addition to the direct effect of hypothalamic/pituitary radiation exposure on obesity, the SEM identified radiation of hypothalamic/pituitary axis as a moderator of obesity through its negative impact on physical function, baseline exercise frequency, and leisure time physical activity.
Meeting CDC guidelines for regular physical activity was associated with a lower risk of obesity in both the multivariable analysis and in the SEM. Previous data from the CCSS indicated that male and female survivors with all diagnoses were more likely to lead an inactive lifestyle compared with CCSS sibling participants. Only male survivors with the diagnoses of other CNS tumor or HL and female survivors with the diagnoses of acute myeloid leukemia, other or unspecified leukemia, HL, kidney tumor, or Ewing sarcoma met the CDC physical activity guidelines.56
In conclusion, this study identified previously unreported factors that are associated with obesity in adult survivors of childhood cancer. The use of specific pharmaceuticals to address anxiety and depression and their relationship with cancer-related pain, decreased physical activity, and physical function have not been reported previously. Important mediators and moderators of obesity help to identify more accurately those who are at risk for obesity and potentially suggest novel strategies (eg, distance-delivered interventions that specifically target anxiety, motivation, and strategies for behavior change) that may be investigated in patients during and after pediatric cancer therapy to diminish their risk for post-therapy obesity.
Footnotes
Supported by the National Cancer Institute (Grants No. CA-55727, L.L. Robison, principal investigator, and CA-21765, M.B. Kastan, principal investigator) of the National Institutes of Health. Support provided to the University of Minnesota Cancer Center from the Children's Cancer Research Fund and to St Jude Children's Research Hospital by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Leslie L. Robison, Eli Lilly (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Daniel M. Green, Sarah S. Donaldson, Gregory T. Armstrong, Leslie L. Robison
Financial support: Leslie L. Robison
Provision of study materials or patients: Marilyn Stovall
Collection and assembly of data: Marilyn Stovall, Kirsten K. Ness,Leslie L. Robison
Data analysis and interpretation: Daniel M. Green, Cheryl L. Cox, Liang Zhu, Kevin R. Krull, Deo Kumar Srivastava, Vikki G. Nolan, Kirsten K. Ness, Kevin C. Oeffinger, Lillian R. Meacham, Charles A. Sklar, Leslie L. Robison
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: A report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 2.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zebrack BJ, Zeltzer LK, Whitton J, et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin's disease, and non-Hodgkin's lymphoma: A report from the Childhood Cancer Survivor Study. Pediatrics. 2002;110:42–52. doi: 10.1542/peds.110.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Zebrack BJ, Zevon MA, Turk N, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2007;49:47–51. doi: 10.1002/pbc.20914. [DOI] [PubMed] [Google Scholar]
- 5.Fava M, Judge R, Hoog SL, et al. Fluoxetine versus sertraline and paroxetine in major depressive disorder: Changes in weight with long-term treatment. J Clin Psychiatry. 2000;61:863–867. doi: 10.4088/jcp.v61n1109. [DOI] [PubMed] [Google Scholar]
- 6.Aberg-Wistedt A, Agren H, Ekselius L, et al. Sertraline versus paroxetine in major depression: Clinical outcome after six months of continuous therapy. J Clin Psychopharmacol. 2000;20:645–652. doi: 10.1097/00004714-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Saddichha S, Ameen S, Akhtar S. Predictors of antipsychotic-induced weight gain in first-episode psychosis: Conclusions from a randomized, double-blind, controlled prospective study of olanzapine, risperidone, and haloperidol. J Clin Psychopharmacol. 2008;28:27–31. doi: 10.1097/jcp.0b013e3181602fe6. [DOI] [PubMed] [Google Scholar]
- 8.Safer DJ. A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol. 2004;24:429–436. doi: 10.1097/01.jcp.0000130558.86125.5b. [DOI] [PubMed] [Google Scholar]
- 9.Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17:407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Basson BR, Kinon BJ, Taylor CC, et al. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry. 2001;62:231–238. doi: 10.4088/jcp.v62n0404. [DOI] [PubMed] [Google Scholar]
- 11.Bustillo JR, Buchanan RW, Irish D, et al. Differential effect of clozapine on weight: A controlled study. Am J Psychiatry. 1996;153:817–819. doi: 10.1176/ajp.153.6.817. [DOI] [PubMed] [Google Scholar]
- 12.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 13.McQuade RD, Stock E, Marcus R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: Results from a randomized, double-blind study. J Clin Psychiatry. 2004;65:47–56. [PubMed] [Google Scholar]
- 14.Kinon BJ, Lipkovich I, Edwards SB, et al. A 24-week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. J Clin Psychopharmacol. 2006;26:157–162. doi: 10.1097/01.jcp.0000204137.82298.06. [DOI] [PubMed] [Google Scholar]
- 15.Daniel DG, Zimbroff DL, Potkin SG, et al. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: A 6-week placebo-controlled trial—Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 16.Arato M, O'Connor R, Meltzer HY. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: The Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17:207–215. doi: 10.1097/00004850-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Small JG, Hirsch SR, Arvanitis LA, et al. Quetiapine in patients with schizophrenia. A high- and low-dose double-blind comparison with placebo Seroquel Study Group. Arch Gen Psychiatry. 1997;54:549–557. doi: 10.1001/archpsyc.1997.01830180067009. [DOI] [PubMed] [Google Scholar]
- 18.Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: A comparison with haloperidol and placebo—The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42:233–246. doi: 10.1016/s0006-3223(97)00190-x. [DOI] [PubMed] [Google Scholar]
- 19.Verity CM, Hosking G, Easter DJ. A multicentre comparative trial of sodium valproate and carbamazepine in paediatric epilepsy: The Paediatric EPITEG Collaborative Group. Dev Med Child Neurol. 1995;37:97–108. doi: 10.1111/j.1469-8749.1995.tb11978.x. [DOI] [PubMed] [Google Scholar]
- 20.Richens A, Davidson DL, Cartlidge NE, et al. A multicentre comparative trial of sodium valproate and carbamazepine in adult onset epilepsy: Adult EPITEG Collaborative Group. J Neurol Neurosurg Psychiatry. 1994;57:682–687. doi: 10.1136/jnnp.57.6.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults: The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med. 1992;327:765–771. doi: 10.1056/NEJM199209103271104. [DOI] [PubMed] [Google Scholar]
- 22.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 24.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox CL, Montgomery M, Oeffinger KC, et al. Promoting physical activity in childhood cancer survivors: Results from the Childhood Cancer Survivor Study. Cancer. 2009;115:642–654. doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 27.National Heart, Lung and Blood Institute Expert Panel on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults: Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 1998. [Google Scholar]
- 28.Osterkamp LK. Current perspective on assessment of human body proportions of relevance to amputees. J Am Diet Assoc. 1995;95:215–218. doi: 10.1016/S0002-8223(95)00050-X. [DOI] [PubMed] [Google Scholar]
- 29.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 30.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: Gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–552. doi: 10.1016/j.ijrobp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 33.Physical Activity Guidelines Advisory Committee: Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: Department of Health and Human Services; 2008. [Google Scholar]
- 34.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2008;17:435–446. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality-Metric; 2000. [Google Scholar]
- 36.Wacholder S. Binomial regression in GLIM: Estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 37.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 38.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 39.Derogatis LR. Brief Symptom Inventory (BSI) 18: Administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 40.Recklitis CJ, Parsons SK, Shih MC, et al. Factor structure of the Brief Symptom Inventory-18 in adult survivors of childhood cancer: Results from the Childhood Cancer Survivor Study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 41.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 42.McHorney CA, Ware JEJ, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JA, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 44.Hu L, Bentler P. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 45.Bollen K. Overall fit in covariance structure models: Two types of sample size effects. Psychol Bull. 1990;107:256–259. [Google Scholar]
- 46.Yu CY, Muthe′n BO. Evaluation of model fit indices for latent variable models with categorical and continuous outcomes. Los Angeles, CA: UCLA, Graduate School of Education and Information Studies; 2002. [Google Scholar]
- 47.Muto R, Yamamori S, Ohashi H, et al. Prediction by FISH analysis of the occurrence of Wilms tumor in aniridia patients. Am J Med Genet. 2002;108:285–289. doi: 10.1002/ajmg.10094. [DOI] [PubMed] [Google Scholar]
- 48.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 49.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 50.Woodwell DA, Cherry DK. National Ambulatory Medical Care Survey: 2002 Summary (Report No. 346) Hyattsville, MD: National Center for Health Statistics; 2004. [Google Scholar]
- 51.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143:639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 52.Arena VC, Padiyar KR, Burton WN, et al. The impact of body mass index on short-term disability in the workplace. J Occup Environ Med. 2006;48:1118–1124. doi: 10.1097/01.jom.0000241050.26059.2b. [DOI] [PubMed] [Google Scholar]
- 53.Guallar-Castillón P, Sagardui-Villamor J, Banegas JR, et al. Waist circumference as a predictor disability among older adults. Obesity. 2007;15:233–244. doi: 10.1038/oby.2007.532. [DOI] [PubMed] [Google Scholar]
- 54.Harkonmäki K, Korkeila K, Vahtera J, et al. Childhood adversities as a predictor of disability retirement. J Epidemiol Community Health. 2007;61:479–484. doi: 10.1136/jech.2006.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin KA, Borkovec TD, Sibrava NJ. The effects of worry and rumination on affect states and cognitive activity. Behav Ther. 2007;38:23–38. doi: 10.1016/j.beth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]