Abstract
Purpose
A phase I/II study of cixutumumab (IMC-A12) in children with refractory solid tumors was conducted. This study was designed to assess the toxicities, pharmacokinetics, and pharmacodynamics of cixutumumab in children to determine a recommended phase II dose and to assess antitumor activity in Ewing sarcoma (ES).
Patients and Methods
Pediatric patients with relapsed or refractory solid tumors were treated with cixutumumab as a 1-hour intravenous infusion once per week. Two dose levels—6 and 9 mg/kg—were evaluated using a standard three-plus-three cohort design. Patients with refractory ES were treated in an expanded phase II cohort at each dose level.
Results
Forty-seven eligible patients with a median age of 15 years (range, 4 to 28 years) were enrolled. Twelve patients were treated in the dose-finding phase. Hematologic and nonhematologic toxicities were generally mild and infrequent. Dose-limiting toxicities included grade 4 thrombocytopenia at 6 mg/kg and grade 3 dehydration at 9 mg/kg. Mean trough concentration (± standard deviation) at 9 mg/kg was 106 ± 57 μg/mL, which exceeded the effective trough concentration of 60 μg/mL observed in xenograft models. Three patients with ES had confirmed partial responses: one of 10 at 6 mg/kg and two of 20 at 9 mg/kg. Serum insulin-like growth factor I (IGF-I) levels consistently increased after one dose of cixutumumab. Tumor IGF-I receptor expression by immunohistochemistry did not correlate with response in patients with ES.
Conclusion
Cixutumumab is well tolerated in children with refractory solid tumors. The recommended phase II dose is 9 mg/kg. Limited single-agent activity of cixutumumab was seen in ES.
INTRODUCTION
The insulin-like growth factor I receptor (IGF-IR) plays a role in the initiation and progression of a variety of cancers, including pediatric malignancies.1–9 Preclinical data suggest that inhibition of the IGF-IR may constitute an important therapeutic target in a variety of pediatric solid tumors.10–15
Cixutumumab (IMC-A12; ImClone Systems, Branchburg, NJ), a human immunoglobulin G 1/λ monoclonal antibody against the IGF-IR, binds to the IGF-IR with high affinity, decreases cell-surface IGF-IR expression, and blocks interactions with IGF-I and IGF-II ligands.16–18 In preclinical cancer models, cixutumumab has single-agent activity and also potentiates the effect of concomitantly administered cytotoxic therapy.19–22 When evaluated by the Pediatric Preclinical Testing Program, cixutumumab demonstrated single-agent activity in osteosarcoma, Ewing sarcoma (ES), neuroblastoma, glioblastoma, and rhabdomyosarcoma models.23
In a single-agent phase I study in adults, cixutumumab was well tolerated at doses from 3 to 15 mg/kg per week; a maximum-tolerated dose was not defined.24,25 On the basis of pharmacokinetic (PK) data, the recommended phase II dose in adults is 6 mg/kg when administered weekly.24
We report the results of a Children's Oncology Group (COG) phase I trial (ADVL0712) of cixutumumab in pediatric patients with refractory non-CNS solid tumors that included a phase II expansion cohort for relapsed/refractory ES. We also include data from the ES cohort of a COG phase II trial of cixutumumab (ADVL0821).
PATIENTS AND METHODS
Study Population
Patients age 1 to 21 years with relapsed/refractory solid tumors with measurable or evaluable disease and those younger than 30 years of age with measurable disease were eligible for the phase I and II portions of the study, respectively. Additional requirements included: Karnofsky or Lansky performance score of 50 or greater; more than 3 weeks since myelosuppressive chemotherapy; 7 or more days since any antineoplastic biologic agent and 6 or more weeks since therapy with a monoclonal antibody; 2 or more weeks since local palliative radiation, 3 months since total body or craniospinal radiation or 50% or greater irradiation of pelvis, and 6 weeks since other substantial bone marrow radiation; 2 or more months since stem-cell transplantation; 7 or more days since completion of hematopoietic growth factor treatment; absolute neutrophil count of 1000/μL or greater, transfusion-independent platelet count of 100,000/μL or greater (≥ 75,000/μL for ADVL0821), and hemoglobin of 8.0 g/dL or greater; creatinine clearance of 70 mL/min/1.73 m2 or greater or normal serum creatinine for age; total bilirubin of 1.5× upper limit of normal or less and ALT of 110 U/L or less; serum albumin of 2.0 g/dL or greater; and normal serum glucose.
Patients with known diabetes mellitus or uncontrolled infections and pregnant or breast-feeding women were excluded. Treatment with other anticancer agents, insulin, or growth hormone was not allowed.
These trials were sponsored by the National Cancer Institute (NCI), and IMC-A12 was supplied by the NCI through a clinical trial agreement with ImClone Systems. The trials were approved by the institutional review board of each participating institution, and informed consent from the patient or parent/guardian and assent as appropriate were obtained before enrollment.
Study Design
Cixutumumab was administered as a 1-hour intravenous infusion once per week in 28-day cycles. Cycles were repeated without interruption if the patient did not have progressive disease and had recovered from the prior course with an absolute neutrophil count of 750/μL or greater, platelet count of 75,000/μL or greater (≥ 50,000/μL during phase II portion), and other laboratory parameters meeting eligibility criteria. Patients who experienced hyperglycemia continued to receive protocol therapy if asymptomatic and if serum glucose was maintained at less than 250 mg/dL (≤ grade 2) with or without the use of insulin or an oral hyperglycemic agent. In the event of reversible dose-limiting toxicity (DLT), patients could remain on protocol therapy with one dose reduction.
In the phase I study, patients were treated in cohorts of three to six at each dose level starting at 6 mg/kg using a standard three-plus-three cohort design. When a dose cohort was suspended for toxicity evaluation, enrollment was open to 10 patients in the ES expansion cohort.
Once the recommended phase II dose was defined, accrual continued as part of COG protocol ADVL0821. We describe the results for patients with ES enrolled onto ADVL0821, which employed the Simon optimal two-stage design.26 Patients who demonstrated a complete or partial response (PR) confirmed by central review were considered responders for study analysis. All other patients were considered nonresponders. If two or fewer responses of 20 evaluable patients were observed, the agent would not be considered sufficiently active for further study. With this design, cixutumumab would be considered sufficiently active with probability of 0.07 when the true response rate was 5% (type I error). If cixutumumab had a true response rate of 25%, the agent would be considered active with probability of 0.88 (P = .25).
Adverse events were graded according to the NCI Common Toxicity Criteria (version 3.0). Nonhematologic DLT was defined as any grade 4 toxicity attributable to cixutumumab and any attributable grade 3 toxicity excluding nausea or vomiting; hepatic transaminase elevation that returned to grade 1 or lower before the next treatment course; fever or infection; hypophosphatemia, hypokalemia, hypocalcemia, or hypomagnesemia responsive to oral supplementation; hyperglycemia that returned to baseline or lower than grade 2 within 7 days of the next scheduled dose; and grade 3 diabetes controlled with insulin or an oral hypoglycemic agent. Hematologic DLTs included grade 4 neutropenia or thrombocytopenia or grade 3 neutropenia or thrombocytopenia continuing more than 7 days past the next scheduled dose.
Patient Evaluation
History, performance status, physical examination, and serum electrolytes were obtained at baseline, weekly throughout cycle one, and before each subsequent cycle. Complete blood counts and serum/urine glucose were obtained weekly throughout treatment. Disease evaluations were performed after cycle one and after each subsequent odd-numbered cycle using RECIST (Response Evaluation Criteria in Solid Tumors).27
Patients enrolled onto the phase I trial were eligible for an optional study to evaluate response by [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET). FDG PET scans (± standard deviation) were performed at baseline and day 15 (± 1) of the first cycle. Central review of responses was performed, and response was designated based on qualitative change in FDG uptake relative to baseline.
PK Studies
Blood samples for PK analysis were obtained on days 1, 8, 15, 22, and 28 of cycle one and days 15 and 28 of cycle two for all patients. If patients consented to additional optional PK sampling, samples (± standard deviation) were also obtained before and at the end of drug infusion and at 1, 3, 6, 24 (± 2), and 72 (± 24) hours after completion of infusion. Samples were collected in tubes without anticoagulant, allowed to clot at room temperature, centrifuged at 1,500 rpm for 15 minutes, and stored at less than −20°C. A validated ELISA (enzyme-linked immunosorbent assay) was used to quantify levels of cixutumumab. Briefly, recombinant human IGF-IR was immobilized to a 96-well microtiter plate by incubating in 1× phosphate-buffered saline. Plates were blocked with 10% horse serum in 1× phosphate-buffered saline–0.1% polysorbate 20 (Tween-20; ICI Americas, Wilmington, DE) and washed, and cixutumumab standard, controls, and test samples were incubated in the wells. Cixutumumab bound to IGF-IR was detected and quantified by addition of horseradish peroxidase conjugated to antihuman λ-light chain antibody using a tetramethylbenzidine colorimetric readout.
Pharmacodynamic Studies
Serum IGF-I, IGF-II, IGF binding protein 2 (IGFBP-2), and IGFBP-3 concentrations were measured from blood samples obtained on days 1 and 8 of cycle one using commercial ELISA kits from Diagnostic Systems Laboratories (Webster, TX). Change in peripheral blood mononuclear cell (PBMC) IGF-IR expression after cixutumumab was quantified by Western blotting. Whole blood samples were collected, and the PBMC layer was removed; cells were lysed using standard methods. Equal amounts of protein were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted with anti–IGF-IR (Santa Cruz Biotechnology, Santa Cruz, CA) and Lck (Millipore, Billerica, MA). Blots were hybridized with primary antibody at 1:1000, washed, and then hybridized with antirabbit horseradish peroxidase (GE Healthcare, Piscataway, NJ) at 1:5000 and visualized with enhanced chemiluminescence (Perkin Elmer, Waltham, MA) on a Fluorchem HD2 imager (Alpha Innotech, San Leandro, CA). Levels of IGF-IR were normalized for levels of Lck.
Immunohistochemistry
Unstained sections of formalin-fixed, paraffin-embedded tumor on glass slides were de-waxed and rehydrated using standard methods; for antigen retrieval, slides were prepared as previously described.28–30 Antibody dilutions were as follows: anti–IGF-I (rabbit polyclonal, catalogue No. 07-1411; Millipore, Temecula, CA) 1:800, anti–IGF-IR (mouse monoclonal, clone 24-31 catalogue No. MAB1120; Millipore) 1:100, and anti–IGF-II, (rabbit polyclonal, catalogue No. 25,071; Abbiotec, San Diego, CA) 1:200.
Digital images of slides were obtained at 20× magnification (resolution of 0.58 μmol/L2 per raw image pixel) using a whole slide scanner (ScanScope CS; Aperio, Vista, CA) fitted with a 20×/0.75 Plan Apo objective lens (Olympus, Center Valley, PA) and saved and retrieved using a software interface (Spectrum; Aperio). Ten digital annotation regions in representative areas of each tumor section were applied using a pen tablet screen (Cintiq 21UX; Wacom, Tokyo, Japan). Within annotated regions, pixels that exceeded three threshold limits (ie, weak, moderate, and strong intensity) in the brown colorimetric channel were quantified using image analysis software (Positive Pixel Count v9; Aperio). Immunohistochemistry (IHC) score was defined as [(1× No. of weak-intensity pixels) + (2× No. of moderate-intensity pixels) + (3× No. of strong-intensity pixels)]/total No. of pixels. The average IHC score across 10 annotated regions on each tumor section was calculated using Excel (Microsoft, Redmond, WA).
RESULTS
Forty-eight patients (47 eligible) were enrolled onto the study: 12 (all eligible) onto the dose-finding study and 36 (35 eligible) onto the ES expansion/phase II cohort. One patient with ES was not eligible, because required baseline imaging was not obtained before enrollment. Of the 47 eligible patients, three were not fully evaluable for toxicity for the following reasons: first, removal from protocol therapy before completion of the first cycle because of noncompliance; second, withdrawal of consent before completion of the first cycle; and third, disease progression within the first cycle. Forty-five patients were evaluable for response, and 20 patients with ES treated at the recommended phase II dose of 9 mg/kg had measurable disease. Characteristics of all eligible patients in the dose-finding and ES cohorts are summarized in Table 1.
Table 1.
Eligible Patient Demographics and Clinical Characteristics (n = 47)
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 15 | |
| Range | 4-28 | |
| Sex | ||
| Male | 24 | 51 |
| Female | 23 | 49 |
| Race | ||
| White | 39 | 83 |
| African American | 3 | 6 |
| Native American | 1 | 2 |
| Asian | 1 | 2 |
| Other/unknown | 3 | 6 |
| Diagnosis | ||
| Ewing sarcoma/peripheral PNET | 35 | 76 |
| Osteosarcoma | 3 | 6 |
| Rhabdomyosarcoma | 2 | 4 |
| Wilms tumor | 2 | 4 |
| Alveolar soft part sarcoma | 1 | 2 |
| Clear cell sarcoma | 1 | 2 |
| Epithelioid sarcoma | 1 | 2 |
| Fibrosarcoma | 1 | 2 |
| Spindle cell sarcoma | 1 | 2 |
| Prior therapy | ||
| No. of chemotherapy regimens | ||
| Median | 1 | |
| Range | 1-10 | |
| Radiation therapy | 38 | 81 |
Abbreviation: PNET, primitive neuroectodermal tumor.
Toxicity
Observed DLTs included one instance of grade 4 thrombocytopenia in a patient with rhabdomyosarcoma treated at 6 mg/kg and one instance of grade 3 dehydration in a patient with ES treated at 9 mg/kg. Grade 2 and higher toxicities are listed in Table 2. Toxicities (≥ grade 2) observed in more than one patient in cycle one were anemia (n = 7), neutropenia (n = 3), lymphopenia (n = 2), thrombocytopenia (n = 3), hyperglycemia (n = 4), fatigue (n = 3), elevated ALT/AST (n = 3), anorexia (n = 2), and vomiting (n = 2). Mild hyperglycemia (grade 1) was observed in 10 patients. The quantity and severity of toxicities were similar at both dose levels.
Table 2.
Grade 2 and Greater Toxicities Related to Protocol Therapy
| Toxicity Type | Maximum Grade of Toxicity |
|||||
|---|---|---|---|---|---|---|
| Course One (total, 44 courses) |
Courses Two to 11 (total, 80 courses) |
|||||
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Hematologic | ||||||
| Hemoglobin | 4 | 3 | 2 | 1 | ||
| Leukopenia | 1 | 1 | ||||
| Lymphopenia | 1 | 1 | ||||
| Neutrophils/granulocytes | 1 | 2 | 1 | |||
| Platelets | 2 | 1 | 1 | |||
| Nonhematologic | ||||||
| Fatigue (asthenia, lethargy, malaise) | 3 | 2 | ||||
| Fever (without neutropenia) | 1 | |||||
| Weight loss | 1 | 2 | ||||
| Flushing | 1 | |||||
| Alopecia | 1 | |||||
| Pruritus | 1 | |||||
| Hot flashes/flushes | 1 | |||||
| Elevated insulin | 1 | |||||
| Anorexia | 2 | 1 | ||||
| Dehydration | 1 | 1 | ||||
| Diarrhea | 1 | |||||
| Oral mucositis/stomatitis | 1 | |||||
| Vomiting | 2 | 1 | ||||
| Opportunistic infection | 1 | |||||
| Hypoalbuminemia | 1 | |||||
| Alkaline phosphatase | 1 | |||||
| ALT | 2 | 1 | 1 | |||
| AST | 2 | 1 | 1 | |||
| Hyperglycemia | 4 | 1 | ||||
| Hypophosphatemia | 1 | 1 | ||||
| Proteinuria | 1 | |||||
| Hypertriglyceridemia | 1 | |||||
| Mood alteration/depression | 1 | |||||
| Headache | 1 | |||||
| Pain/oral cavity | 1 | |||||
Antitumor Activity
There were no complete or partial responses in the dose-finding cohort. One patient with alveolar soft part sarcoma treated at 6 mg/kg had stable disease (SD) for 11 cycles, and one patient with fibrosarcoma treated at 9 mg/kg had SD for seven cycles. In the ES cohort at 6 mg/kg, one patient had a confirmed PR, which was sustained for seven cycles, and four patients had SD for three or more cycles (range, three to 11 cycles). In the ES cohort at 9 mg/kg, PRs were observed in two of 20 patients with measurable disease, and an additional patient had SD for five cycles. Median progression-free survival for this cohort was 44 days (95% CI, 28 to 96).
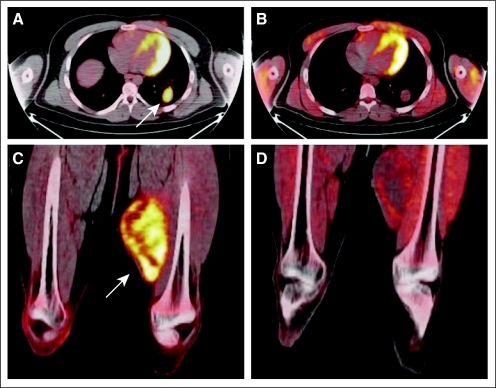
Antitumor activity as assessed by FDG PET at day 15 in the first cycle was evaluated in 10 patients who participated in this optional companion study. An FDG PET response designation was assigned after central review of the scans based on qualitative change in FDG uptake from baseline (Table 3). There were insufficient data to calculate quantitative standardized uptake value measurements. One patient with ES treated at 6 mg/kg who had a PR by RECIST had resolution of FDG PET activity at day 15. Two patients (one with ES, one with fibrosarcoma) with SD by RECIST had tumor FDG PET activity that decreased markedly but did not resolve by day 15 (representative images in Figs 1A to 1D). Six patients who had progressive disease by RECIST had a corresponding increase in FDG PET activity at day 15.
Table 3.
Response by FDG PET
| Diagnosis | Dose (mg/kg) | RECIST Response |
FDG PET Response* | |
|---|---|---|---|---|
| Course One | Overall | |||
| Ewing sarcoma | 6 | SD† | SD† | PR† |
| Ewing sarcoma | 6 | PR | PR | CR§ |
| Ewing sarcoma | 6 | PD | PD | PD‖ |
| Ewing sarcoma | 6 | SD† | PD | PD |
| Osteosarcoma | 6 | SD† | PD | PD |
| Osteosarcoma | 9 | PD | PD | PD |
| Fibrosarcoma | 9 | SD† | SD† | PR† |
| Ewing sarcoma | 9 | PD | PD | PD |
| Ewing sarcoma | 9 | SD† | SD† | SD¶ |
| Ewing sarcoma | 9 | PD | PD | PD |
Abbreviations: CR, complete response; FDG, [18F]fluorodeoxyglucose; PET, positron emission tomography; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
At day 15 of cycle one.
SD ≥ three cycles.
Qualitative decrease in FDG uptake.
Resolution of FDG uptake.
Increased FDG uptake or new focus of FDG activity.
No significant interval change.
Fig 1.
Reduction in [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) activity with cixutumumab. Fused axial PET/computed tomography of chest (A, B) and lower extremities (C,D) showing increased uptake at baseline in metastatic left lower lobe lung nodule (A, arrow) and intense baseline uptake in primary left midthigh mass at baseline (C, arrow) in patient with metastatic fibrosarcoma. After 2 weeks of cixutumumab therapy, although lung nodule had not changed in size, there was no significant residual FDG uptake remaining (B). Similarly, there was significant reduction in FDG uptake at primary left thigh site with no apparent change in size of tumor (D) after 2 weeks of therapy.
PKs
Results of noncompartmental PK analysis are presented in Table 4. Mean trough concentrations (Cmin; ± standard deviation) achieved after the first infusions at 6 and 9 mg/kg were 59 ± 31 μg/mL and 106 ± 57 μg/mL, respectively. A Cmin of 60 μg/mL or greater has resulted in significant tumor growth inhibition in xenograft models of colon and pancreatic cancers.18 This threshold Cmin was exceeded after the first dose in 14 of 17 patients treated at 9 mg/kg and eight of 17 patients treated at 6 mg/kg. For patients treated at 9 mg/kg, there was a significant correlation between age and Cmin (Spearman rank correlation, 0.5; P = .04), with Cmin (± standard deviation) of 75.8 ± 30.3 for patients age 13.5 years or younger versus 139.0 ± 62.1 for older patients (P = .03).
Table 4.
Pharmacokinetic Parameters (mean ± standard deviation)After First Infusion
| Dose Level (mg/kg) | Cmin (μg/mL)* | Cmax (μg/mL) | Clearance (mL/h/kg) | AUC0-∞ (hr × mg/mL) | Half-Life (days) |
|---|---|---|---|---|---|
| 6 | 59 ± 31 | 252 ± 95 | 0.25 ± 0.12 | 32.6 ± 21.1 | 4.2 ± 1.3 |
| No. of patients | 17 | 7 | 7 | 7 | 7 |
| 9 | 106 ± 57 | 400 ± 141 | 0.22 ± 0.08 | 46.3 ± 20.8 | 4.4 ± 1.1 |
| No. of patients | 17 | 14 | 9 | 9 | 9 |
Abbreviations: AUC0-∞, area under concentration versus time curve extrapolated to infinity; Cmax, peak concentration; Cmin, trough concentration.
Trough concentration 7 days after initial infusion.
Pharmacodynamics
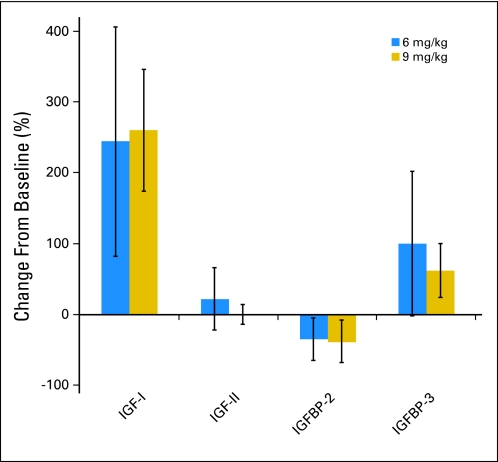
A marked increase in mean serum IGF-I and moderate increase in serum IGFBP-3 were observed after one dose of cixutumumab; this relative change was similar for each dose level (Fig 2A). Serum IGF-II and IGFBP-2 concentrations did not seem to change from baseline.
Fig 2.
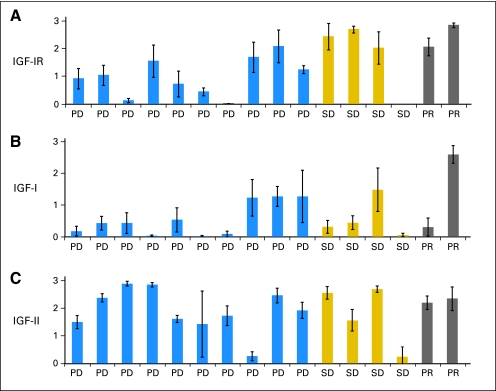
Ewing sarcoma tumor expression of (A) insulin-like growth factor I receptor (IGF-IR), (B) IGF-I, and (C) IGF-II by immunohistochemistry (IHC). Each bar represents the mean IHC score (± standard deviation) calculated from 10 separate representative sections from tumor tissue for a single patient. PD, progressive disease; PR, partial response; SD, stable disease ≥ 3 cycles.
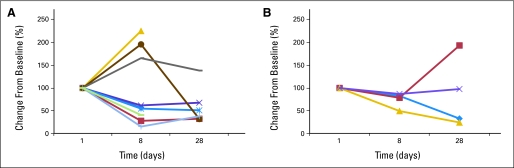
Sufficient material was available from eight patients treated at 6 mg/kg and four patients treated at 9 mg/kg to analyze IGF-IR expression by PBMCs before and after treatment with cixutumumab (Fig 2B). Varying degrees of change in IGF-IR expression were observed after one dose of cixutumumab (at day 8 of cycle one) at both dose levels. Correlation between PBMC IGF-IR expression and response was not feasible because of the small sample size.
Tumor IGF-I, IGF-II, and IGF-IR Expression
Archival tissue samples from patients with ES (original diagnosis or recurrence) were obtained for IHC evaluation (Fig 2C). IGF-IR expression was evident in a majority of tumors; however, the degree of expression varied. There was not an apparent correlation between response to cixutumumab and tumor expression of IGF-I, IGF-II, or IGF-IR.
DISCUSSION
Cixutumumab administered intravenously once per week was well tolerated in pediatric patients at the two dose levels evaluated. No DLTs in have been reported phase I trials of cixutumumab administered on schedules of once per week and every other week in adults at doses up to 15 mg/kg.24,25 Adverse events common to the various anti–IGF-IR monoclonal antibodies in early-phase testing include hyperglycemia, mild skin toxicities, and fatigue.31 Consistent with data from adult trials, mild (≤ grade 2) hyperglycemia occurred relatively frequently in our study (14 of 44 evaluable patients). However, no patient had grade 3 or greater hyperglycemia or required treatment for hyperglycemia. The incidence and severity of other adverse events were similar to those seen in adult trials, and no hypersensitivity reactions were observed.
Apart from dose-limiting thrombocytopenia in one patient, hematologic toxicities were mild. Thrombocytopenia was not reported on adult phase I trials of cixutumumab, but it has been observed in early-phase trials of other anti–IGF-IR antibodies.24,25,32 The mechanism of thrombocytopenia is unclear, but temporally, it is not consistent with bone marrow suppression. The dose-limiting thrombocytopenia in our study occurred in a patient who had baseline grade 2 thrombocytopenia, had previously undergone autologous stem-cell transplantation, and had intercurrent pneumonia.
Noncompartmental PK analysis showed lower mean trough concentration (± standard deviation; 59 ± 31 μg/mL) in pediatric patients treated at 6 mg/kg than in adults treated at the same dose (87 ± 7 μg/mL).24 Data from colon and pancreatic cancer xenograft models suggest that antitumor activity is best achieved at steady-state cixutumumab concentrations above 60 μg/mL—a concentration that was achieved at a dose of 6 mg/kg per week in adults.18,24 Because this concentration was achieved in a majority of patients at 9 mg/kg in this study, and no additional toxicity was observed, the recommended phase II dose of cixutumumab in children is 9 mg/kg intravenously once per week.
An abundance of preclinical data illustrate the role of IGF-IR in childhood cancer and suggest that inhibition of the IGF-IR is a potentially important strategy for the treatment of pediatric solid tumors.10,12,13,33–35 In addition, data from early-phase trials in adults have generated enthusiasm for evaluation of IGF-IR antibodies in patients with ES.32,36 In this study, the number of responses observed did not meet the statistical criteria for single-agent activity in ES, with two PRs among 20 patients at the recommended phase II dose. The COG phase II trial (ADVL0821) of cixutumumab at 9 mg/kg once per week in a variety of other pediatric solid tumors is ongoing.
Despite the small number of responses in ES, further evaluation of cixutumumab in pediatric cancers may be warranted. The response rate of 10% in patients with refractory ES is similar to what has been observed for ES in early-phase studies with other IGF-IR antibodies. Responses were observed in 12% of patients with ES in a phase I study of figitumumab (Pfizer, New York, NY) and in 14% of patients with ES in a phase II study of R1507 (Roche, Nutley, NJ).37,38 Further development of cixutumumab and other IGF-IR antibodies will require discovery of robust biomarkers that can predict response. We did not demonstrate a correlation between IGF-IR expression by IHC and response. However, better methods for detecting and quantifying tumor IGF-IR expression as well as evaluation of mechanisms of resistance may improve our ability to tailor therapy with IGF-IR antibodies by identifying those patients most likely to benefit. Given the relatively good concordance of early FDG PET with RECIST response in our study, this may provide a useful tool to evaluate the activity of IGF-IR antibodies. In addition, the true value of IGF-IR inhibition may be seen with combination strategies to enhance the cytotoxic effect of other agents. COG trials combining cixutumumab with temsirolimus (phase I) or multiagent chemotherapy for metastatic rhabdomyosarcoma (pilot) are underway. There are also numerous ongoing phase I and II studies in adults with a variety of solid tumors evaluating cixutumumab in combination with multiagent chemotherapy or tyrosine kinase inhibitors.
In summary, cixutumumab is well tolerated in children as a single agent. The pharmacokinetic profile at 9 mg/kg in pediatric patients is similar to that at 6 mg/kg in adults, and the recommended pediatric phase II dose is 9 mg/kg intravenously once per week. Limited single-agent activity was observed in refractory ES.
Appendix
Fig A1.
Insulin-like growth factor I (IGF-I) and IGF binding protein 3 (IGF-BP3) increase from baseline after treatment with cixutumumab. Mean percent change (± standard deviation) from baseline in serum levels of IGF-I, IGF-II, IGFBP-2, and IGFBP-3 after one dose of cixutumumab at 6 (n = 16) and 9 mg/kg (n = 6).
Fig A2.
Insulin-like growth factor I receptor (IGF-IR) expression in peripheral blood mononuclear cells decreases with exposure to cixutumumab. Percent change from baseline in degree of IGF-IR expression by peripheral blood mononuclear cells for patients treated at (A) 6 mg/kg intravenously once per week (n = 8) and (B) 9 mg/kg (n = 4).
Footnotes
See accompanying article on page 308
Supported by National Cancer Institute Grants No. U01 CA97452 and U10 CA98543 and General Clinical Research Center Grant No. M01-RR00188-46.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Suman Malempati, Helen X. Chen, Peter C. Adamson, Susan M. Blaney
Administrative support: Stephen Schmechel, Helen X. Chen
Provision of study materials or patients: Suman Malempati
Collection and assembly of data: Suman Malempati, Brenda Weigel, Ashish M. Ingle, Julie M. Carroll, Joel M. Reid, Stephen Schmechel, Mark D. Krailo, Susan M. Blaney
Data analysis and interpretation: Suman Malempati, Ashish M. Ingle, Charlotte H. Ahern, Julie M. Carroll, Charles T. Roberts, Joel M. Reid, Stephen Schmechel, Stephan D. Voss, Steve Y. Cho, Helen X. Chen, Peter C. Adamson, Susan M. Blaney
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 2.Ayalon D, Glaser T, Werner H. Transcriptional regulation of IGF-I receptor gene expression by the PAX3-FKHR oncoprotein. Growth Horm IGF Res. 2001;11:289–297. doi: 10.1054/ghir.2001.0244. [DOI] [PubMed] [Google Scholar]
- 3.El-Badry OM, Minniti C, Kohn EC, et al. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1:325–331. [PubMed] [Google Scholar]
- 4.MacEwen EG, Pastor J, Kutzke J, et al. IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma. J Cell Biochem. 2004;92:77–91. doi: 10.1002/jcb.20046. [DOI] [PubMed] [Google Scholar]
- 5.El-Badry OM, Romanus JA, Helman LJ, et al. Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J Clin Invest. 1989;84:829–839. doi: 10.1172/JCI114243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toretsky JA, Kalebic T, Blakesley V, et al. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 7.Werner H, Re GG, Drummond IA, et al. Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993;90:5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeRoith D, Werner H, Beitner-Johnson D, et al. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 9.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 10.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 11.Tanno B, Mancini C, Vitali R, et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–6780. doi: 10.1158/1078-0432.CCR-06-1479. [DOI] [PubMed] [Google Scholar]
- 12.Gansler T, Furlanetto R, Gramling TS, et al. Antibody to type I insulinlike growth factor receptor inhibits growth of Wilms' tumor in culture and in athymic mice. Am J Pathol. 1989;135:961–966. [PMC free article] [PubMed] [Google Scholar]
- 13.Geoerger B, Daudigeous E, Debussche LR, et al. The anti insulin-like growth factor I receptor (IGF1-R) antibody AVE1642 exhibits anti-tumor activity against neuroblastoma cell lines and xenografts. Proc Am Assoc Cancer Res. 2006;47 abstr 1222. [Google Scholar]
- 14.Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073–5083. [PubMed] [Google Scholar]
- 15.Benini S, Manara MC, Baldini N, et al. Inhibition of insulin-like growth factor I receptor increases the antitumor activity of doxorubicin and vincristine against Ewing's sarcoma cells. Clin Cancer Res. 2001;7:1790–1797. [PubMed] [Google Scholar]
- 16.Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 17.Sachdev D, Singh R, Fujita-Yamaguchi Y, et al. Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: Implications for anti-insulin-like growth factor therapy in breast cancer. Cancer Res. 2006;66:2391–2402. doi: 10.1158/0008-5472.CAN-05-3126. [DOI] [PubMed] [Google Scholar]
- 18.Rowinsky EK, Youssoufian H, Tonra JR, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 19.Wu JD, Haugk K, Coleman I, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–6160. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 20.Wu KD, Zhou L, Burtrum D, et al. Antibody targeting of the insulin-like growth factor I receptor enhances the anti-tumor response of multiple myeloma to chemotherapy through inhibition of tumor proliferation and angiogenesis. Cancer Immunol Immunother. 2007;56:343–357. doi: 10.1007/s00262-006-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen GW, Saba C, Armstrong EA, et al. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67:1155–1162. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Chakravarty G, Kim S, et al. Growth-inhibitory effects of human anti-insulin-like growth factor-I receptor antibody (A12) in an orthotopic nude mouse model of anaplastic thyroid carcinoma. Clin Cancer Res. 2006;12:4755–4765. doi: 10.1158/1078-0432.CCR-05-2691. [DOI] [PubMed] [Google Scholar]
- 23.Kolb EA, Morton C, Houghton PJ, et al. Pediatric Preclinical Testing Program (PPTP) evaluation of the fully human anti-IGF-IR antibody IMC-A12. Presented at 20th European Organisation for Research and Treatment of Cancer–National Cancer Institute–American Association for Cancer Research Symposium on Molecular Targets and Cancer Therapeutics; October 21-24, 2008; Geneva, Switzerland. [Google Scholar]
- 24.Higano CS, Yu EY, Whiting SH, et al. A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monocolonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2007;25(suppl; abstr 3505) [Google Scholar]
- 25.Rothenberg ML, Poplin E, Sandler AB, et al. Phase I dose-escalation study of the anti-IGF-IR recombinant human IgG1 monoclonal antibody (Mab) IMC-A12, administered every other week to patients with advanced solid tumors. Presented at the American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics; October 22-26, 2007; San Francisco, CA. [Google Scholar]
- 26.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Weber MM, Fottner C, Liu SB, et al. Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer. 2002;95:2086–2095. doi: 10.1002/cncr.10945. [DOI] [PubMed] [Google Scholar]
- 29.Freier S, Eran M, Reinus C, et al. Relative expression and localization of the insulin-like growth factor system components in the fetal, child and adult intestine. J Pediatr Gastroenterol Nutr. 2005;40:202–209. doi: 10.1097/00005176-200502000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Giani C, Campani D, Rasmussen A, et al. Insulin-like growth factor II (IGF-II) immunohistochemistry in breast cancer: Relationship with the most important morphological and biochemical prognostic parameters. Int J Biol Markers. 2002;17:90–95. doi: 10.1177/172460080201700203. [DOI] [PubMed] [Google Scholar]
- 31.Rodon J, DeSantos V, Ferry RJ, Jr, et al. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: Lessons from the first clinical trials. Mol Cancer Ther. 2008;7:2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolcher AW, Rothenberg ML, Rodon J, et al. A phase I pharmacokinetic and pharmacodynamic study of AMG 479, a fully human monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-1R), in advanced solid tumors. J Clin Oncol. 2007;25(suppl; abstr 3002) [Google Scholar]
- 33.Chou AJ, Merola PR, Sowers R, et al. IGF-I/IGF-I receptor signal transduction in osteosarcoma cell lines. Proc Am Assoc Cancer Res. 2004;45 abstr 5193. [Google Scholar]
- 34.Shang Y, Mao Y, Batson J, et al. Antixenograft tumor activity of a humanized anti-insulin-like growth factor-I receptor monoclonal antibody is associated with decreased AKT activation and glucose uptake. Mol Cancer Ther. 2008;7:2599–2608. doi: 10.1158/1535-7163.MCT-07-2401. [DOI] [PubMed] [Google Scholar]
- 35.Makawita S, Ho M, Durbin AD, et al. Expression of insulin-like growth factor pathway proteins in rhabdomyosarcoma: IGF-2 expression is associated with translocation-negative tumors. Pediatr Dev Pathol. 2009;12:127–135. doi: 10.2350/08-05-0477.1. [DOI] [PubMed] [Google Scholar]
- 36.Leong S, Gore L, Benjamin R, et al. A phase I study of R1507, a human monoclonal antibody IGF-1R (insulin-like growth factor receptor) antagonist given weekly in patients with advanced solid tumors. Presented at the American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics; October 22-26, 2007; San Francisco, CA. [Google Scholar]
- 37.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: A phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pappo AS, Patel S, Crowley J, et al. Activity of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R), in patients (pts) with recurrent or refractory Ewing's sarcoma family of tumors (ESFT): Results of a phase II SARC study. J Clin Oncol. 2010;28(suppl 15; abstr 10000):698s. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]