Abstract
Purpose
Cisplatin-induced neurotoxicity and ototoxicity (NTX) are important adverse effects after chemotherapy for testicular cancer (TC). Although serum platinum is measurable years after therapy, its impact on NTX has not been evaluated.
Patients and Methods
In all, 169 cisplatin-treated survivors of TC provided blood samples at Survey I and reported NTX during Survey I (1998-2002) and Survey II (2007-2008). Serum platinum was quantified by inductively coupled plasma mass spectrometry. Patient-reported outcomes were evaluated with the Scale for Chemotherapy-Induced Neurotoxicity (SCIN), regarding the extent of symptom bother as 0, “not at all”; 1, “a little”; 2, “quite a bit”; or 3, “very much.” Summing the six symptom scores yielded a total SCIN score of 0 to 18. Categorizing total SCIN scores into quartiles yielded similar-sized groups with increasing symptoms. Multivariate ordinal logistic regression analyses evaluated associations between NTX and long-term serum platinum levels, adjusting for cisplatin dose, dosing schedule, and age.
Results
At Survey I, a significant four- to five-fold association with total SCIN score emerged for the highest serum platinum quartile (odds ratio [OR], 4.69; 95% CI, 1.82 to 12.08). Paresthesias and Raynaud's syndrome (hands and feet) showed significant two- to four-fold increased risks with the highest platinum quartile. At Survey II, total SCIN score remained significantly associated with the highest platinum quartile (OR, 4.28; 95% CI, 1.36 to 13.48). Paresthesias (hands and feet) and tinnitus showed significant three- to four-fold increased risks for the highest platinum quartile. Cumulative cisplatin dose was not associated with total SCIN score or individual SCIN symptoms in multivariate analyses.
Conclusion
Here we document a significant relationship between increasing levels of residual serum platinum and NTX severity after adjusting for initial cisplatin dose.
INTRODUCTION
Testicular cancer (TC) serves as a model for a curable neoplasm with 5-year survival rates exceeding 97%.1,2 The improved prognosis is primarily due to cisplatin-based chemotherapy, the cornerstone of metastatic TC treatment.3,4 Moreover, platinating agents comprise some of the most widely used cytotoxic drugs worldwide.
There is, however, increasing awareness about long-term toxicities following cisplatin-based chemotherapy, including potentially fatal events, such as cardiovascular disease (CVD), pulmonary dysfunction, and secondary neoplasms, years after treatment discontinuation.5–14 Peripheral paresthesias, Raynaud's phenomenon, hearing impairment, and tinnitus represent platinum-induced toxicities,15–19 with a prevalence of 30% to 40% in TC survivors (TCSs).17,19,20 Both cumulative dose and administration schedule have an impact on development and severity of cisplatin-related neurotoxicity.15 Functional polymorphisms in cisplatin-detoxifying enzymes, such as glutathione S-transferase, are also related to the severity of neurotoxicity.21,22 Reasons for the large interindividual variation in long-term complications remain largely obscure. In the absence of effective remedies for these sequelae, identifying contributing factors for risk prediction and prevention is important.11
Total serum platinum level may represent a biomarker of late toxicity and body burden, since it can be measured years after application, with concentrations up to 1,000 times higher in cisplatin-treated patients than in unexposed controls.23–26 Moreover, up to 10% of circulating platinum remains reactive.23 Whether long-term total serum platinum is associated with development and severity of various cisplatin-related toxicities has not been assessed and was recommended a priority research item during an expert consensus conference.11
Our aim was to examine the association between long-term total serum platinum and the prevalence or severity of peripheral paresthesias, Raynaud's phenomenon, and ototoxicity in a well-characterized cohort of TCSs, taking into account cumulative cisplatin dose, time since treatment, and other variables. We hypothesized that residual long-term serum platinum levels would be positively associated with these sequelae, even when adjusted for initially administered cumulative cisplatin dose.
PATIENTS AND METHODS
Study Population and Design
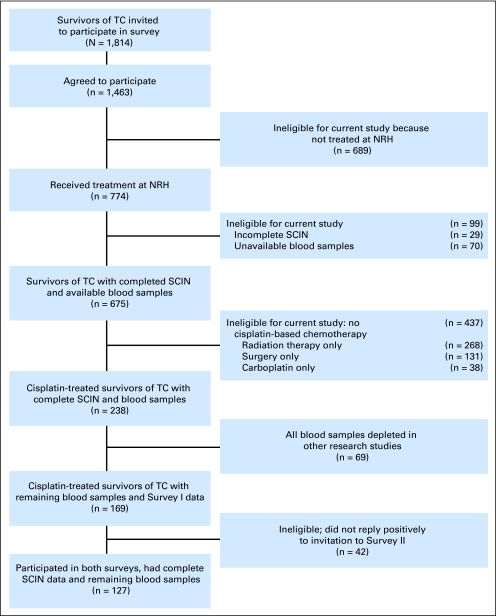
From January 1998 through April 2002, a national follow-up survey (Survey I) was performed at all five Norwegian University hospitals to assess physical and psychosocial morbidity in long-term TCSs. All men treated for unilateral germ cell TC from January 1980 through December 1994 at age 18 to 75 years were identified through the Cancer Registry of Norway and regional university hospitals and were invited to participate (n = 1,814; Fig 1). A total of 81% of eligible men consented, completed a 219-item questionnaire, and underwent clinical examination at outpatient clinics. At the Norwegian Radium Hospital (NRH) only, blood samples were drawn during Survey I and stored at −70°C. Therefore, this study is restricted to TCSs treated at NRH. Blood samples had also been used in prior research projects,21,22,27 but sufficient material remained for 169 previously cisplatin-exposed TCSs.
Fig 1.
Overview of survivors of testicular cancer (TC) included in Survey I (2000), Survey II (2007), and this study (Patients and Methods). NRH, Norwegian Radium Hospital; SCIN, Scale for Chemotherapy-Induced Neuropathy.
Questionnaire-based Survey II (2007) was performed a median 8 years (range, 7 to 9 years) after Survey I. Of 169 TCSs with blood samples in Survey I, 127 (75.1%) participated in Survey II. Both surveys were approved by the Norwegian Committee for Medical Research Ethics of the Southern Health Region.
Standards of Treatment, 1980 to 1994
Orchiectomy was typically the first step of treatment, with staging according to the Royal Marsden Hospital System.28 From 1980 to 1994, most TCSs included in this study were treated according to protocols of the European Organisation for Research and Treatment for Cancer, the Medical Research Council, or the Swedish-Norwegian Testicular Cancer Project.29–32
Typically cisplatin was combined with bleomycin and either etoposide (BEP) or vinblastine (CVB). Thirty-one TCSs were randomly assigned to dose-intensive regimens in which cisplatin was applied over 2 or 3 days instead of 5 days or particularly high cumulative doses were given (Appendix, online only). Two patients received carboplatin-based chemotherapy and cisplatin-based regimens. The therapeutic dose of the two drugs has generally been described as a ratio of 4:1, based on clinical studies in ovarian cancer in which these doses achieved a relative equivalency.33–35 On the basis of these results and procedures in a prior report by Travis et al,36we chose to calculate corresponding cisplatin doses by dividing carboplatin by four. The remaining patients received other types of cisplatin-based regimens.29,31,37 Bleomycin was administered to 160 (94.7%) of 169 patients (median cumulative dose: 300,000 IU; range, 90,000 to 390,000 IU) and exceeded a total dose of 300,000 IU for only 32 patients, because most protocols specified 300,000 IU as the dose limit.38 All patients received rigorous hydration regimens.
Questionnaire
A 219-item questionnaire39 that included the validated six-item Scale for Chemotherapy-Induced Neurotoxicity (SCIN) was used in Survey I.40 SCIN assessed neuropathy in hands (fingers) and feet (toes), Raynaud's phenomenon in hands (fingers) and feet (toes), tinnitus, and impaired hearing. For simplicity, we subsequently use the terms hands and feet. Item scores ranged from 0, “not at all”; 1, “a little”; and 2, “quite a bit;” to 3, “very much” (Appendix Table A1, online only). Summation of the six item scores yields a total score with a range of 0 to 18. Categorization of the total SCIN score into quartiles yielded four groups of similar size.
Survey II27 consisted of an 83-item questionnaire including SCIN and also addressed tobacco use, comorbidities, medication use, and alcohol use (only Survey I).15 Numbers with regard to alcohol abuse and diabetes were considered too small for statistical analyses.
Quantification of Serum Platinum Levels
In 2009, by using well-established methods,41–43 serum samples from 169 TCSs were analyzed for total platinum blinded to the demographics or treatment of TCSs at the University of Massachusetts in Boston. The deep-frozen samples were shipped on dry ice, equilibrated at room temperature for 4 hours, and homogenized with a GlobalSpec laboratory slow shaker (GlobalSpec, East Greenbush, NY) for 30 minutes. Approximately 0.1 mL of serum was pipetted into a trace-metal-clean test tube and verified gravimetrically to ± 0.001 g. Samples were diluted by using 18.2 MΩ/cm−1 resistance water and acidified by using ultra-pure (12.4 mol/L−1) hydrochloric acid. Known concentrations of indium, bismuth, and iridium were added to the samples and were used to monitor instrument drift.42,43 Serum platinum concentrations were quantified by using a Perkin-Elmer DRC II inductively coupled plasma mass spectrometer (Perkin-Elmer, Norwalk, CT) by using external calibration procedures previously developed for other biologic tissues.41–43a Platinum detection was performed according to Brouwers et al.43,44 Limits of platinum detection (0.010 pmol/g), quantification (0.035 pmol/g), and method detection (0.097 pmol/g) were calculated according to Long and Winefordner.45
Statistical Methods
Continuous variables were described with median and range, and categorical variables were described with counts and proportions. Crude associations between pairs of continuous variables were assessed by using Pearson correlation. The χ2 test of trend was used to assess crude associations between pairs of ordered categorical variables. Associations between the SCIN scores and continuous variables were analyzed with ordinal logistic regression (OLR).
OLR was used to model total SCIN score and ordered individual SCIN symptoms as a function of administered cisplatin dose, dose-intensive versus standard chemotherapy, age at survey, and quartiles of serum platinum levels. Variables of high clinical relevance or those reaching P < .2 were included in OLR. Odds ratios (ORs) for any dichotomization of the four ordered outcome levels were reported for each independent predictor, along with 95% CIs. Adjusted tests of trend for serum platinum quartiles were based on refitting OLR models with serum platinum quartiles coded as 1, 2, 3, or 4 and modeled linearly in logit. A test of parallel lines checked the proportional odds assumption that the OR for each predictor was similar for all three possible dichotomizations of the outcome. ORs represent a comparison of higher to lower grouped ordered outcomes (ie, symptoms of “a little” or more v “not at all”; “very much” or “quite a bit” v “a little” or “not at all”; and “very much” v “quite a bit” or less). For tinnitus and hearing impairment, the two highest toxicity groups were pooled to remedy violations of this assumption; however, results were nearly identical without pooling. All tests were two-sided and were conducted at the 0.05 level of significance. Statistical analyses were performed by using SPSS 17.0 (SPSS, Chicago, IL).
RESULTS
Median age at TC diagnosis and at Survey I (n = 169 patients) was 28.7 years and 40.8 years, respectively (Table 1). Most TCSs presented with nonseminomatous tumors (81.1%) and metastatic disease (82.8%). Primary chemotherapy consisted of CVB (40.8%) and standard BEP (39.1%). Median cumulative dose of cisplatin was 401 mg/m2 (range, 191 to 1,565 mg/m2). Median serum platinum concentration was 0.769 nmol/L (range, 0.031 to 12.710 nmol/L) at a median of 12 years after treatment. Serum platinum was positively correlated with cumulative cisplatin dose (Pearson correlation, P < .001) and inversely correlated with time since treatment (Pearson correlation, P < .001). Serum platinum was significantly higher after administration of dose-intensive chemotherapy compared with standard chemotherapy (χ2 trend, P < .001).
Table 1.
Demographics, Type of Cisplatin-Based Chemotherapy Regimen, and Lifestyle Factors for Cisplatin-Treated Survivors of TC* Participating in Surveys I and II
| Characteristic | Survey I (2000) |
Survey II (2007) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 169 | 127/169 | ||
| Age at TC diagnosis, years | ||||
| Median | 28.7 | 29.1 | ||
| Range | 14.8-58.5 | 14.8-58.5 | ||
| Histology | ||||
| Seminoma | 32 | 18.9 | 24 | 18.9 |
| Nonseminoma | 137 | 81.1 | 103 | 81.1 |
| Age at survey, years | ||||
| Median | 40.8 | 50.5 | ||
| Range | 23.0-73.0 | 33.0-81.0 | ||
| Time between TC diagnosis and survey, years | ||||
| Median | 12.0 | 20.0 | ||
| Range | 4.0-19.0 | 13.0-27.0 | ||
| Royal Marsden stage | ||||
| I | 29 | 17.2 | 21 | 16.5 |
| IM | 5 | 3.0 | 5 | 3.9 |
| II | 79 | 46.7 | 57 | 44.9 |
| III | 10 | 5.9 | 7 | 5.5 |
| IV | 46 | 27.2 | 37 | 29.1 |
| Cisplatin-based regimens† | ||||
| Standard-dose regimens | ||||
| BEP 20 | 66 | 39.1 | 50 | 39.4 |
| CVB | 69 | 40.8 | 52 | 40.9 |
| Others | 3 | 1.8 | 2 | 1.6 |
| Dose-intensive regimens | ||||
| BOP/VIP | 9 | 5.3 | 8 | 6.3 |
| BEP 40, 50, and 60 | 13 | 7.7 | 8 | 6.3 |
| Others | 9 | 5.3 | 7 | 5.5 |
| Smoking status at survey‡ | ||||
| Yes (current/former) | 70 | 41.4 | 70 | 55.1 |
| No (never/rare) | 93 | 55.0 | 56 | 44.1 |
| Missing | 6 | 3.6 | 1 | 0.8 |
NOTE. Number of schedules could vary according to randomization in protocols, primary disease stage, and response during chemotherapy. Dose-intensive regimens are described in Appendix Table A1 (online only).
Abbreviations: BEP, cisplatin, etoposide and bleomycin; BOP, cisplatin, vincristine, and bleomycin; CVB, cisplatin, vinblastine, and bleomycin; TC, testicular cancer; VIP, cisplatin, etoposide, and ifosfamide.
All 169 men initially underwent orchiectomy for TC, with staging uniformly performed according to the Royal Marsden Hospital Staging System.
Standard cisplatin-based regimens. BEP 20: cisplatin 20 mg/m2 days 1 through 5, etoposide 100 mg/m2 on days 1 through 5, and bleomycin 30,000 IU on days 2, 5, and 15. CVB: cisplatin 20 mg/m2 on days 1 through 5, vinblastine 6 mg/m2 on days 1 and 2, and bleomycin 30,000 IU on days 2, 5, and 15. CEB: carboplatin at a formula of (5 × glomerular filtration rate + 125) on day 1, etoposide 120 mg/m2 on days 1 through 3, and bleomycin at 30,000 IU on days 1, 8, and 15. EP: cisplatin 20 mg/m2 on days 1 through 5, etoposide 100 mg/m2 on days 1 through 5. HOP: cisplatin 20 mg/m2 on days 1 through 5, ifosfamide 1,200 mg/m2 on days 1 through 5, and vincristine 2 mg on day 1.
Smoking: current and former “regular smokers” were registered as smokers (ie, “yes”). Never smokers or those who “only smoked a few times” (ie, rarely) were grouped into the “no” category.
Since 127 (75.1%) of the 169 TCSs from Survey I also participated in Survey II, longitudinal neurotoxicity data were available for the majority of patients at a median of 20.0 years (range, 13.0 to 27.0 years) since the end of cisplatin-based treatment. Distribution of histology, stage, and cisplatin-based protocol for the 127 TCSs who participated in Survey II did not differ from that for the 169 participants in Survey I (Table 1).
Serum Platinum and Neurotoxicities in Survey I
Univariate analyses.
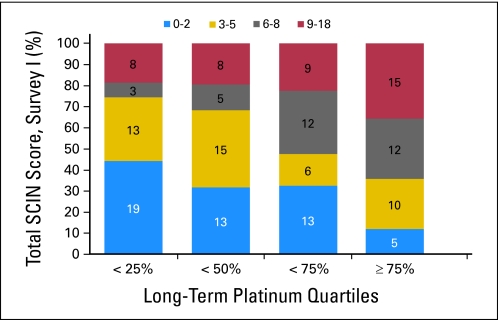
Increasing serum platinum quartiles were significantly associated with increasing total SCIN score (Fig 2; χ2 trend, P < .001) and Raynaud's phenomenon in hands and feet (χ2 trend, P = .004 and P = .001, respectively; data not shown). Serum platinum levels were significantly higher after administration of dose-intensive chemotherapy compared with standard chemotherapy (χ2 trend, P < .001). Ototoxicity (ie, tinnitus and hearing impairment) was also increased at Survey I for the dose-intensive group (χ2 trend, P = .002 and P = .001, respectively). There were no statistical differences in symptom severity between men who received the CVB or the BEP regimen as their initial treatment (data not shown). Total SCIN score was positively associated with administered cisplatin dose, dose-intensive therapy, and age at survey (χ2 trend, P = .016, P = .032, and P = .035, respectively). These variables were included in multivariate analyses for both surveys.
Fig 2.
Number of survivors of testicular cancer according to quartile of serum platinum level and total Scale for Chemotherapy-Induced Neuropathy (SCIN) score at Survey I (2000).
Multivariate analyses.
Cumulative dose of cisplatin was not associated with either total SCIN score (OR, 1.10; 95% CI, 0.88 to 1.39) or any of the individual symptoms (Table 2). In contrast, the highest compared with the lowest serum platinum quartile was significantly associated with total SCIN score (OR, 4.69; 95% CI, 1.82 to 12.08). Furthermore, risk of experiencing individual symptoms was increased two- to four-fold for TCSs in the highest compared with the lowest platinum quartile for paresthesias in hands (OR, 2.87; 95% CI, 1.08 to 7.62) and feet (OR, 2.83; 95% CI, 1.09 to 7.40) and Raynaud's phenomenon in hands (OR, 4.15; 95% CI, 1.60 to 10.76) and feet (OR, 4.46; 95% CI, 1.70 to 11.71).
Table 2.
Adjusted Associations of SCIN Symptoms at Survey I With Serum Platinum Quartiles, Administered Cisplatin Dose, Dose-Intensive Therapy, and Agea
| Symptom | Survey I (2000) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Administered Cisplatin Doseb |
Dose-Intensive Therapy (yes/no)c |
Age at Surveyd |
Quartile of Serum Platinum Levele |
Adjusted P for Trendf | |||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | 25 to 50 OR | 95% CI | 50 to 75 OR | 95% CI | > 75 OR | 95% CI | ||
| Total score | 1.10 | 0.88 to 1.39 | 1.35 | 0.57 to 3.23 | 1.71c | 1.25 to 2.32 | 1.26 | 0.57 to 2.80 | 1.79 | 0.79 to 4.04 | 4.69g | 1.82 to 12.08 | .002g |
| Paresthesias hands | 0.90 | 0.71 to 1.15 | 1.12 | 0.46 to 2.78 | 1.44g | 1.06 to 1.96 | 1.08 | 0.36 to 2.18 | 1.31 | 0.55 to 3.13 | 2.87g | 1.08 to 7.62 | .044g |
| Paresthesias feet | 1.15 | 0.92 to 1.45 | 0.75 | 0.31 to 1.82 | 1.86g | 1.36 to 2.54 | 1.15 | 0.50 to 2.65 | 1.27 | 0.54 to 2.97 | 2.83g | 1.09 to 7.40 | .055 |
| Raynaud's phenomenon hands | 0.94 | 0.75 to 1.17 | 1.22 | 0.51 to 2.86 | 1.26 | 0.94 to 1.71 | 1.07 | 0.46 to 2.46 | 1.56 | 0.67 to 3.60 | 4.15g | 1.60 to 10.76 | .003g |
| Raynaud's phenomenon feet | 1.10 | 0.88 to 1.38 | 0.74 | 0.31 to 1.79 | 1.31 | 0.97 to 1.78 | 1.18 | 0.50 to 2.77 | 1.83 | 0.78 to 4.31 | 4.46g | 1.70 to 11.71 | .002g |
| Tinnitush | 1.14 | 0.89 to 1.44 | 2.63g | 1.08 to 6.25 | 1.44g | 1.05 to 1.98 | 1.38 | 0.58 to 3.32 | 1.22 | 0.50 to 3.02 | 1.56 | 0.57 to 4.26 | .457 |
| Hearing impairmenth | 1.16 | 0.90 to 1.48 | 4.00g | 1.61 to 10.00 | 1.89g | 1.35 to 2.64 | 1.46 | 0.60 to 3.57 | 1.99 | 0.81 to 4.93 | 1.80 | 0.64 to 5.07 | .174 |
Abbreviations: OR, odds ratio; SCIN, Scale for Chemotherapy-Induced Neurotoxicity.
Associations were assessed with multivariable ordinal logistic regression analyses and are presented with ORs and 95% CIs. ORs represent a comparison of higher with lower grouped and ordered patient outcomes (ie, symptoms of “a little” or more v “not at all”; “very much” or “quite a bit” v “a little” or “not at all”; and “very much” v “quite a bit” or less, assuming all three dichotomizations have the same OR) per decade of age, per 100 mg/m2 increase of administered cisplatin, and for each quartile of serum platinum compared with the lowest quartile (referent group).
Administered cisplatin per 100 mg/m2. This category includes both standard-dose and dose-intensive regimens.
A yes/no indicator of dose-intensive v standard chemotherapy.
Increase in indicated symptom per decade of patient age.
Serum platinum was quantified in blood samples obtained at Survey I (2000).
Adjusted P value for trend testing the effect of serum platinum level modeled as a continuous function of quartile score (1, 2, 3, 4) in the ordinal logistic regression model, adjusted for age, administered cisplatin dose, and dose-intensive therapy.
P < .05 (two-sided) was considered statistically significant.
For analyses of tinnitus and hearing impairment, the top two toxicity groups were pooled in each survey.
Age had a significant impact on total SCIN score (OR, 1.71; 95% CI, 1.25 to 2.32), paresthesias in hands (OR, 1.44; 95% CI, 1.06 to 1.96) and feet (OR, 1.86; 95% CI, 1.36 to 2.54), tinnitus (OR, 1.44; 95% CI, 1.05 to 1.98), and hearing impairment (OR, 1.89; 95% CI, 1.35 to 2.64). Dose-intensive therapy had a significant impact on tinnitus and hearing impairment (OR, 2.63; 95% CI, 1.08 to 6.25 and OR, 4.00; 95% CI, 1.61 to 10.00, respectively).
Survey II
Univariate analyses.
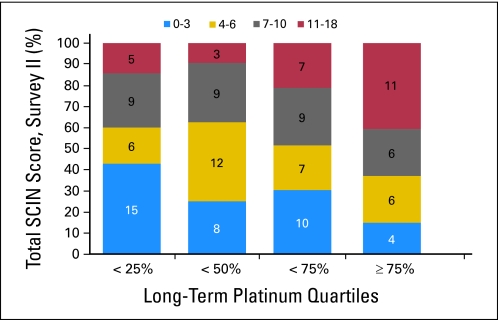
Increasing serum platinum quartiles were significantly associated with total SCIN score (Fig 3; χ2 trend, P = .007). Serum platinum quartiles were significantly associated with five of six self-reported SCIN symptoms; paresthesias in hands and feet, Raynaud's phenomenon in the feet, tinnitus, and impaired hearing (χ2 trend, P values between .003 and .033; data not shown). Hearing impairment was significantly associated with dose-intensive therapy (χ2 trend, P = .002), whereas a borderline significant relationship existed for tinnitus (χ2 trend, P = .094). Cisplatin dose, dose-intensive therapy, and age did not reach significance for total SCIN score, but significant differences emerged for some SCIN symptoms.
Fig 3.
Number of survivors of testicular cancer according to quartile of serum platinum level and total Scale for Chemotherapy-Induced Neuropathy (SCIN) score at Survey II (2007).
Multivariate analyses.
Cumulative dose of cisplatin was not significantly associated with either total SCIN score (OR, 1.01; 95% CI, 0.78 to 1.30) or with any of the individual symptoms (Table 3). Similar to Survey I, a significant four-fold increased association with total SCIN score (OR, 4.28; 95% CI, 1.36 to 13.48) was found for the highest compared with the lowest serum platinum quartile. The highest quartile of serum platinum was also associated with significantly increased four- to five-fold risks of paresthesias in hands (OR, 4.08; 95% CI, 1.29 to 12.93) and feet (OR, 4.63; 95% CI, 1.45 to 14.76). Similarly, increased three-fold risks were observed for Raynaud's phenomenon in hands (OR, 3.11; 95% CI, 0.97 to 9.94) and feet (OR, 2.80; 95% CI, 0.90 to 8.71) and tinnitus (OR, 3.44; 95% CI, 1.03 to 11.54). Age had an impact on the total SCIN score, with an OR per decade of 1.43 (95% CI, 1.03 to 1.99). Further, paresthesias in the feet (OR, 1.66; 95% CI, 1.19 to 2.33) and hearing impairment were significantly associated with increasing age (OR, 1.86; 95% CI, 1.29 to 2.68). We found no significant impact of dose-intensive therapy on tinnitus and hearing impairment at Survey II.
Table 3.
Adjusted Associations of SCIN Symptoms at Survey II With Serum Platinum Quartiles, Administered Cisplatin Dose, Dose-Intensive Therapy, and Agea
| Symptom | Survey II (2007) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Administered Cisplatin Doseb |
Dose-Intensive Therapy (yes/no)c |
Age at Surveyd |
Quartile of Serum Platinum Levele |
Adjusted P for Trendf | |||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | 25 to 50 OR | 95% CI | 50 to 75 OR | 95% CI | > 75 OR | 95% CI | ||
| Total score | 1.01 | 0.78 to 1.30 | 1.22 | 0.43 to 3.57 | 1.43g | 1.03 to 1.99 | 1.22 | 0.51 to 2.93 | 1.45 | 0.59 to 3.59 | 4.28g | 1.36 to 13.48 | .032g |
| Paresthesias hands | 0.96 | 0.74 to 1.24 | 1.12 | 0.39 to 3.23 | 1.25 | 0.90 to 1.74 | 2.22 | 0.90 to 5.47 | 1.60 | 0.63 to 4.04 | 4.08g | 1.29 to 12.93 | .043g |
| Paresthesias feet | 1.14 | 0.88 to 1.48 | 0.67 | 0.23 to 1.92 | 1.66g | 1.19 to 2.33 | 1.44 | 0.59 to 3.53 | 2.05 | 0.81 to 5.16 | 4.63g | 1.45 to 14.76 | .013g |
| Raynaud's phenomenon hands | 0.89 | 0.67 to 1.17 | 0.63 | 0.22 to 1.85 | 0.97 | 0.70 to 1.35 | 0.91 | 0.38 to 2.19 | 1.04 | 0.42 to 2.59 | 3.11 | 0.97 to 9.94 | .120 |
| Raynaud's phenomenon feet | 1.04 | 0.81 to 1.34 | 0.77 | 0.27 to 2.22 | 1.14 | 0.82 to 1.59 | 0.79 | 0.32 to 1.95 | 1.54 | 0.62 to 3.84 | 2.80 | 0.90 to 8.71 | .072 |
| Tinnitush | 0.98 | 0.75 to 1.28 | 1.43 | 0.47 to 4.35 | 1.18 | 0.84 to 1.66 | 2.54 | 1.00 to 6.52g | 2.05 | 0.78 to 5.43 | 3.44b | 1.03 to 11.54 | .055 |
| Hearing impairmenth | 1.32 | 0.94 to 1.85 | 2.94 | 0.92 to 9.09 | 1.86g | 1.29 to 2.68 | 0.86 | 0.35 to 2.16 | 1.04 | 0.40 to 2.70 | 1.33 | 0.39 to 4.55 | .688 |
Abbreviations: OR, odds ratio; SCIN, Scale for Chemotherapy-Induced Neurotoxicity.
Associations were assessed with multivariable ordinal logistic regression analyses and are presented with ORs and 95% CIs. ORs represent a comparison of higher with lower grouped and ordered patient outcomes (ie, symptoms of “a little” or more v “not at all”; “very much” or “quite a bit” v “a little” or “not at all”; and “very much” v “quite a bit” or less, assuming all three dichotomizations have the same OR) per decade of age, per 100 mg/m2 increase of administered cisplatin, and for each quartile of serum platinum compared with the lowest quartile (referent group).
Administered cisplatin per 100 mg/m2. This category includes both standard-dose and dose-intensive regimens.
A yes/no indicator of dose-intensive v standard chemotherapy.
Increase in indicated symptom per decade of patient age.
Serum platinum was quantified in blood samples obtained at Survey I (2000).
Adjusted P value for trend testing the effect of serum platinum level modeled as a continuous function of quartile score (1, 2, 3, 4) in the ordinal logistic regression model, adjusted for age, administered cisplatin dose, and dose-intensive therapy.
P < .05 (two-sided) was considered statistically significant.
For analyses of tinnitus and hearing impairment, the top two toxicity groups were pooled in each survey.
DISCUSSION
To the best of our knowledge, this is the first study demonstrating that long-term serum platinum levels are significantly associated with the severity of neurotoxicity 5 to 20 years after cisplatin-based chemotherapy. Importantly, the relationship remained significant after adjustment for initial cisplatin dose. Serum platinum quartiles were positively associated with overall neurotoxicity and ototoxicity as assessed by total SCIN scores at Surveys I and II (P trend = .002 and .032, respectively), as were most individual symptoms.
Strengths of our study include a large well-characterized cohort of TCSs with long-term follow-up and detailed information regarding cancer therapy. All men were treated at the same hospital and underwent standardized examinations at Surveys I and II. The high participation rate ensures validity of our findings.
Although our study is derived from the largest cohort of TCSs to date,5,10,15,22,27 numbers of patients available in various subgroup analyses remain small, limiting statistical power. Serum platinum levels were available for Survey I only, precluding calculation of platinum elimination rates. The questionnaires, however, were collected at both Survey I and Survey II. Interpretation of our findings would have been stronger if the symptom measurements and serum platinum levels had been measured in a longitudinal fashion at both surveys, also including objective measurements.
Reliance on self-reported symptoms without objective measurements is a limitation of this study. Ototoxicity as recorded on the SCIN was previously validated among these patients through objective measurements.39 Neurologic tests, however, were not performed in our study. Other studies observed that, compared with self-report, prevalence of adverse effects may be higher when measured objectively.39,46
Most patients received cisplatin in combination with bleomycin, usually regarded as the principal cause of Raynaud's phenomenon. However, cisplatin might also contribute to cold-induced vasospasms. Vogelzang et al47 reported the incidence of Raynaud's phenomenon to be 21% after vinblastine and bleomycin compared with 41% after therapy with CVB. In the comprehensive report by Brydoy et al,15 Raynaud's phenomenon was not associated with bleomycin in contrast to a dose-dependent effect observed for cisplatin and dose-intensive cisplatin-based chemotherapy.
Studies performed within 15 months after cisplatin administration,48–51 show increased platinum levels in most organs. In human autopsies conducted 4 to 867 days after the last cisplatin dose, platinum concentrations were highest in dorsal root ganglia and lowest in tissues protected by the blood-brain barrier, in line with observations of sensory neuropathies and histopathologically documented peripheral nerve damage.48 Subsequent rates of decline in tissue platinum are lowest in sensory ganglia, sural nerves, and liver.48 Previous data have suggested that both the incidence and severity of neurotoxicity are mainly determined by the cumulative cisplatin dose, and are determined for ototoxicity also by the dose-intensive regimen.15,52,53 However, in our study, the highest long-term serum platinum levels were most strongly correlated with the severity of SCIN, even when adjusted for initial cisplatin dose. A possible explanation for this finding is that ongoing exposure to low-level platinum in neural tissue, as well as any residual systemic effects, may limit resolution of the acute and dose-dependent sensory neuropathy, hypothetically also contributing to ongoing damage.54
The biokinetic behavior of platinum closely resembles that of other toxic metals (eg, mercury and chromium55,56) in which serum and urine concentrations also correlate with toxic outcomes.55,57 Importantly, Brouwers et al23 showed that approximately 10% of platinum remains reactive over the long term.
Similar to prior studies,23,24,26 we found a significant relationship between initial dose and platinum levels and an inverse relationship between time since administration and platinum levels. Future efforts evaluating the potential toxic impact of circulating serum platinum would benefit from the prospective collection of larger sample volumes, because available amounts did not permit determination of reactive platinum hypothesized by some to be the pharmacologically and toxicologically active species.23
Several studies of TCSs have demonstrated ongoing endothelial changes for more than 10 years after cisplatin administration, indicating a possible prolonged effect of residual platinum.6,54,58 In particular, Vaughn et al,54 showed endothelial damage in TCSs at a median of 5.1 years after cisplatin-based treatment. These investigators postulated that the increased risk of CVD in TCSs could be due partly to persistent exposure of endothelial cells to circulating platinum. Other studies have shown an increased risk for late CVD in TCSs given cisplatin-based chemotherapy.6,10,12,59 It may also contribute to increased risk of metabolic syndrome in TCSs;5,6 however, results are inconclusive.10
Several studies8,12,13,60 have shown significantly increased risks of solid tumors and leukemia9 among cisplatin-treated TCSs. Whether cisplatin in tissue deposits or in circulating form, alone or with other agents, may serve an initiating or promoting carcinogenic role is not known.
Our results suggest that interventional strategies reducing long-term platinum levels may show promise for lessening long-term neurotoxicity, with the importance of further studies in this area recently underscored by a National Cancer Institute panel.61 Currently, to the best of our knowledge, there are no agents that ameliorate the clinical neurotoxicity of platinating agents. According to a recent Cochrane Database review,62 information on possible neuroprotective agents, such as acetylcysteine, amifostine, calcium and magnesium, diethyldithiocarbamate, glutathione, Org 2766, oxycarbazepine, or vitamin E are insufficient to conclude that they “prevented or limited neurotoxicity of platinum drugs among human patients.”
Importantly, we do not question the use of cisplatin in the treatment of metastatic TC, because this drug is still the cornerstone of the chemotherapy that rendered this cancer a model for a curable neoplasm.3,4 Whether carboplatin would have less long-term toxicity is unknown; however, carboplatin would decrease the cure rate.63 Nonetheless, we demonstrate that long-term serum levels of platinum correlate with the severity of neurotoxicity 5 to 20 years after chemotherapy in TCSs. Although chemotherapy-induced neuropathies are not life threatening, symptoms can have a debilitating effect on patients' quality of life and functioning. Increasing knowledge about the long-term effects of circulating platinum underscores the importance of interventional strategies to minimize platinum-related neurotoxicity and ototoxicity and potentially fatal events such as myocardial infarction and secondary malignancies. To validate our findings and to elucidate the underlying mechanisms of toxicities related to the effect of reactive platinum, we emphasize the importance of further studies on the impact of residual platinum in an independent large cohort of platinum-treated survivors of cancer.
Appendix
Treatment Details for Survivors of Testicular Cancer During the Study Period, 1980-1994
All men in the study sample had received cisplatin (n = 169). The initial regimen was usually cisplatin in combination with etoposide and bleomycin (BEP; 46.8% of the men) or cisplatin in combination with vinblastine and etoposide (CVB; 40.8% of the men). The standard BEP and CVB regimens were given with cisplatin 20 mg/m2 on days 1 through 5 per cycle and bleomycin 30,000 IU on days 2, 5, and 15. In addition, etoposide 100 mg/m2 was administered on days 1 through 5 for BEP, and vinblastine 6 mg/m2 was administered on days 1 and 2 for CVB. However, within certain research protocols, patients could be randomly assigned to so-called dose-intensive regimens, with cisplatin given at the same dose over the course of fewer days than the standard BEP (BEP 20), or with higher doses of cisplatin given over fewer or the same number of days as the standard BEP. Some of our patients were included in the Medical Research Council (MRC) TE09 protocol, which allocated half the patients to four cycles of carboplatin, etoposide, and bleomycin (CEB) and the other half to four cycles of the BEP schedule, with cisplatin given as a total dose of 100 mg/m2 over either 2 days (BEP 50) or 5 days (BEP 20).37 In the MRC TE13/European Organisation for Research and Treatment of Cancer (EORTC) 30895 protocol, patients could be randomly assigned to three cycles of bleomycin, vincristine, and cisplatin (BOP) at 10-day intervals followed by three cycles of etoposide, ifosfamide, cisplatin, and bleomycin (VIP-B) at 3-week intervals (BOP/VIP-B regimen). Cisplatin 50 mg/m2 was administered on days 1 and 2 for three cycles of BOP with a 10-day interval, followed by cisplatin 20 mg/m2 on days 1 through 5 for VIP-B, with three schedules administered every third week. Vincristine 1.4 mg/m2 (total dose per day not exceeding 2 mg) was administered on day 1 of BOP. Bleomycin 30,000 IU was given on day 1 of BOP and on days 8 and 15 for the VIP-B cycles. Etoposide 100 mg/m2 was given on days 1, 3, and 5, and ifosfamide 1,000 mg/m2 was given on days 1 through 5 for VIP-B.29 The Swedish-Norwegian testicular cancer project (SWENOTECA) explored the effect of high-dose cisplatin on a BEP schedule with 60 mg/m2 for 3 consecutive days, etoposide 120 mg/m2 on days 1 through 3, and bleomycin 30,000 IU on days 2, 9, and 16 every third week in patients presumed to have poor prognosis.31 Other dose-intensive regimens were BEP 40 (cisplatin 40 mg/m2 on days 1 through 5, etoposide 100 mg/m2 on days 1 through 5, and bleomycin 30,000 IU on days 1, 5, and 15) and CVB 60 (cisplatin 60 mg/m2 on days 1 through 3, vinblastine 4 to 5 mg/m2 on days 1 and 2, and bleomycin 30,000 IU on days 1, 5, and 15). Number of schedules could vary according to primary stages and response during chemotherapy. A total of 31 of our patients received dose-intensive regimens as specified above.
Seminoma
For men with early-stage seminoma I or IIA, infradiaphragmatic radiotherapy was the treatment of choice. Cisplatin-based chemotherapy was administered to patients who presented with more advanced disease or those who relapsed after initial radiotherapy. Our population included a total of 32 patients with seminoma (initial stages I through IV), of whom 15 received both chemotherapy and abdominal radiation. During the treatment period, the increasing awareness of the potential long-term consequences of radiation treatment resulted in a gradual reduction of target dose and volume for stage I to IIA patients (ie, from 36 to 40 Gy in the early 1980s to 25.2 to 30 Gy in the mid-1990s; Fossa SD, et al: J Clin Oncol 17:1146, 1999). Residual postchemotherapy masses were either observed or treated by surgery or irradiation (Duchesne GM, et al: Eur J Cancer 33:829-835, 1997).
Nonseminoma
Until approximately 1990, a modified bilateral or ipsilateral retroperitoneal lymph node dissection (RPLND) represented standard of care for patients with early-stage (I to IIA) nonseminoma. In patients with tumor cells identified in RPLND specimens, two or three cycles of cisplatin-based adjuvant chemotherapy were routinely applied. After 1990, nonseminoma stage I patients either received one to two cycles of adjuvant chemotherapy (BEP) or were followed by surveillance only (Cullen MH, et al: J Clin Oncol 14:1106-1113, 1996; Fosså SD, et al: Br J Cancer 70:1156-1160, 1994). Before 1990, initial treatment for patients with metastatic nonseminoma consisted of four to six cycles of chemotherapy followed by surgery, most often RPLND. After 1990, three or more cycles of cisplatin-based chemotherapy represented the standard of care for patients with stage IIA to IV nonseminoma [No authors listed] J Clin Oncol 15:594-603, 1997), with nerve-sparing RPLND typically performed after chemotherapy (Jacobsen KD, et al: Br J Cancer 80:249-255, 1999). For relapsing patients, second-line chemotherapy was applied and would routinely include cisplatin (Fosså SD, et al: Br J Cancer 80:1392-1399, 1999).
Table A1.
Items and Scoring of the Validated SCIN39
| Subscales | Items | Not at All | A Little | Quite a Bit | Very Much |
|---|---|---|---|---|---|
| Paresthesias hands/fingers | 1a. Have you suffered from pain and tingling in your feet/toes? | 0 | 1 | 2 | 3 |
| Paresthesias feet/toes | 1b. Have you suffered from pain and tingling in your hands/fingers? | 0 | 1 | 2 | 3 |
| Raynaud's phenomenon hands/fingers | 2a. Have you suffered from numb or cold feet or toes? | 0 | 1 | 2 | 3 |
| Raynaud's phenomenon feet/toes | 2b. Have you suffered from numb or cold hands or fingers? | 0 | 1 | 2 | 3 |
| Tinnitus | 3a. Have you suffered from ringing in your ears? | 0 | 1 | 2 | 3 |
| Impaired hearing | 3b. Have you suffered from reduced hearing? | 0 | 1 | 2 | 3 |
Abbreviation: SCIN, Scale for Chemotherapy-Induced Neurotoxicity.
Footnotes
Supported by Grants No. 1 UL1 RR024160-01 (L.B.T.) from the National Center for Research Resources of the National Institutes of Health, No. 5U56CA118635 (T.H.D., R.E.H., and C.B.) from the National Institutes of Health, and No. 39247 (M.S.) from the South-Eastern Norway Regional Health Authority.
Presented as a poster presentation at the 21st Meeting of the European Association for Cancer Research, Oslo, Norway, June 26-29, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas H. Darrah, Sophie D. Fosså, Lois B. Travis
Financial support: Robyn E. Hannigan, Clair Beard, Sophie D. Fosså, Lois B. Travis
Administrative support: Sophie D. Fosså
Provision of study materials or patients: Sophie D. Fosså
Collection and assembly of data: Mette Sprauten, Thomas H. Darrah, M. Ellen Campbell, Robyn E. Hannigan, Hege S. Haugnes, Sophie D. Fosså, Jan Oldenburg
Data analysis and interpretation: Mette Sprauten, Thomas H. Darrah, Derick R. Peterson, Milada Cvancarova, Clair Beard, Hege S. Haugnes, Sophie D. Fosså, Jan Oldenburg, Lois B. Travis
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: A 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, editor. Cancer in Norway 2008. Cancer Registry of Norway, Institute of Population-Based Research. 2008 [Google Scholar]
- 3.Einhorn LH. Testicular cancer as a model for a curable neoplasm: The Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1981;41:3275–3280. [PubMed] [Google Scholar]
- 4.Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99:4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haugnes HS, Aass N, Fosså SD, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. 2007;18:241–248. doi: 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 6.Meinardi MT, Gietema JA, van der Graaf WT, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. 2000;18:1725–1732. doi: 10.1200/JCO.2000.18.8.1725. [DOI] [PubMed] [Google Scholar]
- 7.Nuver J, Smit AJ, van der Meer J, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005;23:9130–9137. doi: 10.1200/JCO.2005.01.4092. [DOI] [PubMed] [Google Scholar]
- 8.Kollmannsberger C, Hartmann JT, Kanz L, et al. Therapy-related malignancies following treatment of germ cell cancer. Int J Cancer. 1999;83:860–863. doi: 10.1002/(sici)1097-0215(19991210)83:6<860::aid-ijc32>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Andersson M, Gospodarowicz M, et al. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92:1165–1171. doi: 10.1093/jnci/92.14.1165. [DOI] [PubMed] [Google Scholar]
- 10.Haugnes HS, Wethal T, Aass N, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J Clin Oncol. 2010;28:4649–4657. doi: 10.1200/JCO.2010.29.9362. [DOI] [PubMed] [Google Scholar]
- 11.Travis LB, Beard C, Allan JM, et al. Testicular cancer survivorship: Research strategies and recommendations. J Natl Cancer Inst. 2010;102:1114–1130. doi: 10.1093/jnci/djq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 13.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 14.Dearnaley D, Huddart R, Horwich A. Regular review: Managing testicular cancer. BMJ. 2001;322:1583–1588. doi: 10.1136/bmj.322.7302.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brydøy M, Oldenburg J, Klepp O, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101:1682–1695. doi: 10.1093/jnci/djp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuxen MK, Hansen SW. Neurotoxicity secondary to antineoplastic drugs. Cancer Treat Rev. 1994;20:191–214. doi: 10.1016/0305-7372(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 17.Bokemeyer C, Berger CC, Kuczyk MA, et al. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol. 1996;14:2923–2932. doi: 10.1200/JCO.1996.14.11.2923. [DOI] [PubMed] [Google Scholar]
- 18.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 19.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aass N, Kaasa S, Lund E, et al. Long-term somatic side-effects and morbidity in testicular cancer patients. Br J Cancer. 1990;61:151–155. doi: 10.1038/bjc.1990.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldenburg J, Kraggerud SM, Brydøy M, et al. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldenburg J, Kraggerud SM, Cvancarova M, et al. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 23.Brouwers EE, Huitema AD, Beijnen JH, et al. Long-term platinum retention after treatment with cisplatin and oxaliplatin. BMC Clin Pharmacol. 2008;8:7. doi: 10.1186/1472-6904-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerl A, Schierl R. Urinary excretion of platinum in chemotherapy-treated long-term survivors of testicular cancer. Acta Oncol. 2000;39:519–522. doi: 10.1080/028418600750013447. [DOI] [PubMed] [Google Scholar]
- 25.Tothill P, Klys HS, Matheson LM, et al. The long-term retention of platinum in human tissues following the administration of cisplatin or carboplatin for cancer chemotherapy. Eur J Cancer. 1992;28A:1358–1361. doi: 10.1016/0959-8049(92)90519-8. [DOI] [PubMed] [Google Scholar]
- 26.Gietema JA, Meinardi MT, Messerschmidt J, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet. 2000;355:1075–1076. doi: 10.1016/s0140-6736(00)02044-4. [DOI] [PubMed] [Google Scholar]
- 27.Wethal T, Haugnes HS, Kjekshus J, et al. C-reactive protein; A potential marker of second cancer and cardiovascular disease in testicular cancer survivors? Eur J Cancer. 2010;46:3425–3433. doi: 10.1016/j.ejca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Peckham MJ, McElwain TJ, Barrett A, et al. Combined management of malignant teratoma of the testis. Lancet. 1979;2:267–270. doi: 10.1016/s0140-6736(79)90288-5. [DOI] [PubMed] [Google Scholar]
- 29.Kaye SB, Mead GM, Fossa S, et al. Intensive induction-sequential chemotherapy with BOP/VIP-B compared with treatment with BEP/EP for poor-prognosis metastatic nonseminomatous germ cell tumor: A Randomized Medical Research Council/European Organization for Research and Treatment of Cancer study. J Clin Oncol. 1998;16:692–701. doi: 10.1200/JCO.1998.16.2.692. [DOI] [PubMed] [Google Scholar]
- 30.Lewis CR, Fossà SD, Mead G, et al. BOP/VIP: A new platinum-intensive chemotherapy regimen for poor prognosis germ cell tumours. Ann Oncol. 1991;2:203–211. doi: 10.1093/oxfordjournals.annonc.a057906. [DOI] [PubMed] [Google Scholar]
- 31.Aass N, Klepp O, Cavallin-Stahl E, et al. Prognostic factors in unselected patients with nonseminomatous metastatic testicular cancer: A multicenter experience. J Clin Oncol. 1991;9:818–826. doi: 10.1200/JCO.1991.9.5.818. [DOI] [PubMed] [Google Scholar]
- 32.Klepp O, Flodgren P, Maartman-Moe H, et al. Early clinical stages (CS1, CS1Mk+ and CS2A) of non-seminomatous testis cancer: Value of pre- and post-orchiectomy serum tumor marker information in prediction of retroperitoneal lymph node metastases—Swedish-Norwegian Testicular Cancer Project (SWENOTECA) Ann Oncol. 1990;1:281–288. doi: 10.1093/oxfordjournals.annonc.a057749. [DOI] [PubMed] [Google Scholar]
- 33.Ozols RF, Behrens BC, Ostchega Y, et al. High dose cisplatin and high dose carboplatin in refractory ovarian cancer. Cancer Treat Rev. 1985;12:59–65. doi: 10.1016/0305-7372(85)90019-2. [DOI] [PubMed] [Google Scholar]
- 34.Lokich J, Anderson N. Carboplatin versus cisplatin in solid tumors: An analysis of the literature. Ann Oncol. 1998;9:13–21. doi: 10.1023/a:1008215213739. [DOI] [PubMed] [Google Scholar]
- 35.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 36.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 37.Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: A Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844–1852. doi: 10.1200/JCO.1997.15.5.1844. [DOI] [PubMed] [Google Scholar]
- 38.O'Sullivan JM, Huddart RA, Norman AR, et al. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol. 2003;14:91–96. doi: 10.1093/annonc/mdg020. [DOI] [PubMed] [Google Scholar]
- 39.Oldenburg J, Fosså SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): Psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res. 2006;15:791–800. doi: 10.1007/s11136-005-5370-6. [DOI] [PubMed] [Google Scholar]
- 40.Fosså SD, Moynihan C, Serbouti S. Patients' and doctors' perception of long-term morbidity in patients with testicular cancer clinical stage I: A descriptive pilot study. Support Care Cancer. 1996;4:118–128. doi: 10.1007/BF01845761. [DOI] [PubMed] [Google Scholar]
- 41.Brouwers EE, Tibben MM, Pluim D, et al. Inductively coupled plasma mass spectrometric analysis of the total amount of platinum in DNA extracts from peripheral blood mononuclear cells and tissue from patients treated with cisplatin. Anal Bioanal Chem. 2008;391:577–585. doi: 10.1007/s00216-008-2034-8. [DOI] [PubMed] [Google Scholar]
- 42.Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, et al. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009;1:479–488. doi: 10.1039/b905145g. [DOI] [PubMed] [Google Scholar]
- 43.Brouwers EE, Tibben MM, Rosing H, et al. Sensitive inductively coupled plasma mass spectrometry assay for the determination of platinum originating from cisplatin, carboplatin, and oxaliplatin in human plasma ultrafiltrate. J Mass Spectrom. 2006;41:1186–1194. doi: 10.1002/jms.1087. [DOI] [PubMed] [Google Scholar]
- 43a.McLaughlin MP, Darrah TH, Holland PL. Palladium(II) and platinum(II) bind strongly to an engineered blue copper protein. Inorg Chem. 2011;50:11294–11296. doi: 10.1021/ic2017648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouwers EE, Tibben M, Rosing H, et al. The application of inductively coupled plasma mass spectrometry in clinical pharmacological oncology research. Mass Spectrom Rev. 2008;27:67–100. doi: 10.1002/mas.20159. [DOI] [PubMed] [Google Scholar]
- 45.Long GL, Winefordner JD. Limit of detection: A closer look at the IUPAC definition. Anal Chem. 1983;55:712A–724A. [Google Scholar]
- 46.Hansen SW, Helweg-Larsen S, Trojaborg W. Long-term neurotoxicity in patients treated with cisplatin, vinblastine, and bleomycin for metastatic germ cell cancer. J Clin Oncol. 1989;7:1457–1461. doi: 10.1200/JCO.1989.7.10.1457. [DOI] [PubMed] [Google Scholar]
- 47.Vogelzang NJ, Bosl GJ, Johnson K, et al. Raynauds phenomenon: A common toxicity after combination chemotherapy for testicular cancer. Ann Intern Med. 1981;95:288–292. doi: 10.7326/0003-4819-95-3-288. [DOI] [PubMed] [Google Scholar]
- 48.Krarup-Hansen A, Rietz B, Krarup C, et al. Histology and platinum content of sensory ganglia and sural nerves in patients treated with cisplatin and carboplatin: An autopsy study. Neuropathol Appl Neurobiol. 1999;25:29–40. doi: 10.1046/j.1365-2990.1999.00160.x. [DOI] [PubMed] [Google Scholar]
- 49.Stewart DJ, Mikhael NZ, Nanji AA, et al. Renal and hepatic concentrations of platinum: Relationship to cisplatin time, dose, and nephrotoxicity. J Clin Oncol. 1985;3:1251–1256. doi: 10.1200/JCO.1985.3.9.1251. [DOI] [PubMed] [Google Scholar]
- 50.Poirier MC, Reed E, Litterst CL, et al. Persistence of platinum-ammine-DNA adducts in gonads and kidneys of rats and multiple tissues from cancer patients. Cancer Res. 1992;52:149–153. [PubMed] [Google Scholar]
- 51.Dikhoff TG, De Goeij JJ, Mcvie JG. Long-term body retention and tissue distribution of platinum in cisplatin treated cancer patients. J Radioanal Nucl Chem. 1998;236:81–86. [Google Scholar]
- 52.von Schlippe M, Fowler CJ, Harland SJ. Cisplatin neurotoxicity in the treatment of metastatic germ cell tumour: Time course and prognosis. Br J Cancer. 2001;85:823–826. doi: 10.1054/bjoc.2001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fosså SD, de Wit R, Roberts JT, et al. Quality of life in good prognosis patients with metastatic germ cell cancer: A prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20) J Clin Oncol. 2003;21:1107–1118. doi: 10.1200/JCO.2003.02.075. [DOI] [PubMed] [Google Scholar]
- 54.Vaughn DJ, Palmer SC, Carver JR, et al. Cardiovascular risk in long-term survivors of testicular cancer. Cancer. 2008;112:1949–1953. doi: 10.1002/cncr.23389. [DOI] [PubMed] [Google Scholar]
- 55.Woods JS. Altered porphyrin metabolism as a biomarker of mercury exposure and toxicity. Can J Physiol Pharmacol. 1996;74:210–215. [PubMed] [Google Scholar]
- 56.Gagnon ZE, Newkirk C, Hicks S. Impact of platinum group metals on the environment: A toxicological, genotoxic and analytical chemistry Study. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:397–414. doi: 10.1080/10934520500423592. [DOI] [PubMed] [Google Scholar]
- 57.Pingree SD, Simmonds PL, Rummel KT, et al. Quantitative evaluation of urinary porphyrins as a measure of kidney mercury content and mercury body burden during prolonged methylmercury exposure in rats. Toxicol Sci. 2001;61:234–240. doi: 10.1093/toxsci/61.2.234. [DOI] [PubMed] [Google Scholar]
- 58.Nuver J, Smit AJ, Sleijfer DT, et al. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur J Cancer. 2004;40:701–706. doi: 10.1016/j.ejca.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Huddart RA, Norman A, Shahidi M, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 60.Wanderås EH, Fosså SD, Tretli S. Risk of subsequent non-germ cell cancer after treatment of germ cell cancer in 2006 Norwegian male patients. Eur J Cancer. 1997;33:253–262. doi: 10.1016/s0959-8049(96)00458-3. [DOI] [PubMed] [Google Scholar]
- 61.Chemotherapy-induced peripheral neuropathy. Meeting of the National Cancer Institute Symptom Management and Quality of Life Steering Committee; March 23, 2009; Rockville, MD. [Google Scholar]
- 62.Albers JW, Chaudhry V, Cavaletti G, et al. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2011;2:CD005228. doi: 10.1002/14651858.CD005228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with ‘good-risk' metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015–1021. doi: 10.1093/oxfordjournals.annonc.a010493. [DOI] [PubMed] [Google Scholar]