Abstract
Pantothenamides have been the subject of much study as potential inhibitors of CoA and carrier protein dependent biosynthetic pathways. Based on an initial observation of growth inhibition in E. coli by 3, we have synthesized a small panel of pantetheine analogues and reexamined the inhibitory properties of this class of antibiotics with an emphasis on understanding the ability of these compounds to act as substrates of native CoA and carrier protein utilizing biosynthetic pathways. Our findings suggest a secondary structure-activity relationship is an important factor in the antibiotic activity of these compounds.
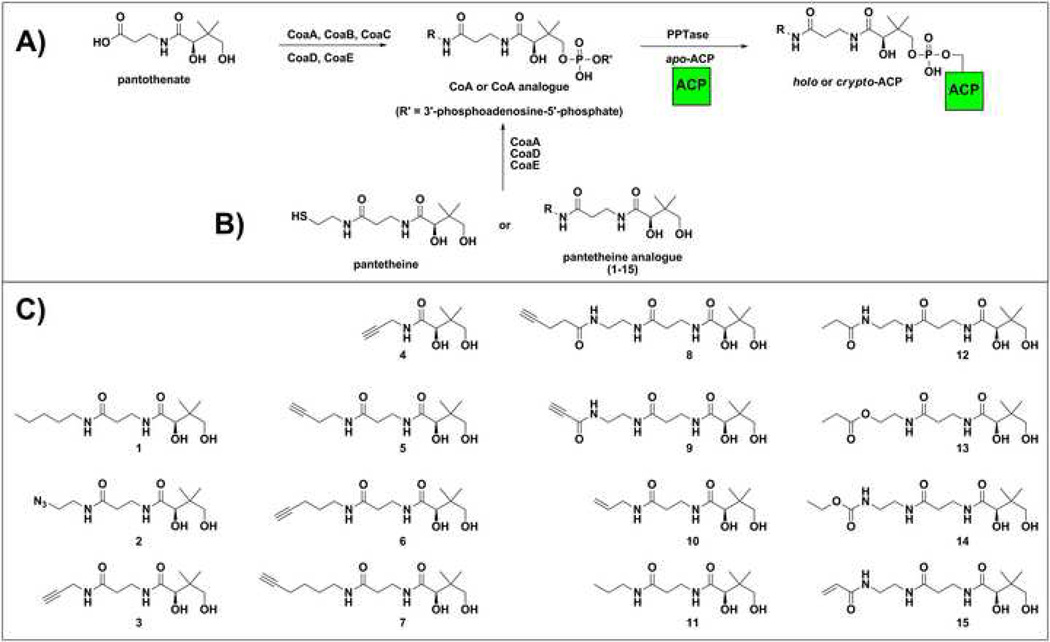
Coenzyme A (CoA) is an essential cofactor in living systems, functioning as the major acyl group carrier in numerous metabolic pathways.1 The bacterial CoA biosynthetic pathway was first elucidated in Escherichia coli, where it was shown that five enzymes (CoaA, CoaB, CoaC, CoaD, and CoaE) are responsible for the conversion of pantothenate (vitamin B5) to CoA (Figure 1A).2 While this pathway also exists in humans, comparison of the individual enzymes of the prokaryotic and eukaryotic CoA biosynthetic pathways has shown the two demonstrate very little sequence homology.3 With reports of bacterial resistance to traditional antibiotics becoming increasingly common, the design of selective inhibitors of bacterial CoA biosynthesis presents a potentially novel antibiotic target.4
Figure 1.
A) Pathway for CoA biosynthesis and posttranslational modification of carrier proteins. B) Pantetheine analogues can act as alternate substrates for these pathways to form CoA antimetabolites. These CoA analogues can be processed by PPTases to form crypto-carrier proteins. C) Structures of pantothenate and pantetheine analogues (1–15) examined in this study.
In addition to functioning directly as an acyl group carrier, CoA is also the source of the 4’-phosphopantetheine arm used for acyl group transfer in fatty acid biosynthesis (Figure 1A).5 In this pathway, a phosphopantetheinyltransferase (PPTase) enzyme functions to transfer the 4’-phosphopantetheine arm of CoA to a conserved serine residue of an apo-acyl carrier protein (ACP). The free thiol of this posttranslational modification is then used as the site of acyl-intermediate tethering during the loading, condensation, and reduction reactions necessary for production of fatty acids. ACPs and peptidyl carrier proteins (PCP) are used similarly in polyketide and nonribosomal peptide biosynthesis.6
In the past several years, it has been shown that many PPTases, most notably Sfp from the surfactin synthetase pathway in B. subtilis, have a relaxed substrate specificity which allows for the modification of ACPs with CoA analogues in vitro.7 When derivatized with fluorescence or affinity tags, this property can be used for the selective visualization and isolation of carrier protein utilizing biosynthetic enzymes.8 Our group has had a long-standing interest in using PPTase promiscuity as a strategy for the investigation of primary and secondary biosynthetic pathways in bacterial organisms, which has lead us to investigate methods for the manipulation of intracellular CoA pool as a means of labeling carrier proteins. As CoA analogues cannot cross the cell-membrane due to their strong negative charge, we have examined the utility of CoA precursors as in vivo carrier protein labels.9
Perhaps the most thoroughly investigated CoA precursors to date have been the antibacterial pantothenamides.10 This class of antibiotics, typified by N5-Pan (1), has been shown to inhibit E. coli and Staphylococcus aureus growth.11 Originally postulated as inhibitors of the pantothenate kinase (CoaA) enzyme, it has since been shown these compounds act as competitive substrates of CoaA and are believed to exert their antibacterial activity through interference with fatty acid biosynthesis by labeling of the E. coli fatty acid ACP (Figure 1B).12–13
During our own studies of CoA precursors we encountered an interesting phenomenon relevant to the continued development and study of this antibiotic class. Through the synthesis and evaluation of a large number of CoA precursors (henceforth referred to as pantetheine analogues), we identified a single compound (2) capable of modification the E. coli fatty acid ACP in the native organism (Figure 1B).14 Further, these studies showed that compound 2 was non-toxic to E. coli grown in minimal media at concentrations > 1 mM, while a structurally similar alkynyl pantetheine analogue (3) possessed cytotoxic activity near equivalent to that of 1 (Table 3). These observations ran contrary to expectations, as it was anticipated that the most toxic pantetheine analogues would be those which labeled the E. coli fatty acid ACP most efficiently, according to the proposed mode of action of these compounds.13
Table 3.
Minimum inhibitory concentrations of pantetheine analogues 1–15 to E. coli grown in different media and effect of additives.
| Media Compound # |
M9 | Tryptone | M9 + Pana | M9 + β-Alab |
|---|---|---|---|---|
| MIC (µM) | MIC (µM) | MIC (µM) | MIC (µM) | |
| 1 | 6 | 50 | 500 | 250 |
| 2 | >500 | >500 | ndc | nd |
| 3 | 4 | >500 | >500 | >500 |
| 4 | 500 | >500 | nd | nd |
| 5 | 7.5 | >500 | >500 | >500 |
| 6 | 15 | >500 | >500 | >500 |
| 7 | 500 | >500 | nd | nd |
| 8 | >500 | >500 | nd | nd |
| 9 | >500 | >500 | nd | nd |
| 10 | 3 | >500 | 250 | 125 |
| 11 | 1 | 500 | 500 | 250 |
| 12 | >500 | >500 | nd | nd |
| 13 | 500 | >500 | nd | nd |
| 14 | >500 | >500 | nd | nd |
| 15 | 500 | >500 | nd | nd |
Pan = addition of 1 mM pantothenate to growth medium.
β-Ala – addition of 1 mM β-alanine to growth medium.
nd = not determined.
These findings lead us to synthesize a small panel of pantetheine analogues in order to examine in detail the influence of chain length (4–9), oxidation state (10–11), and functionality (12–15) on the ability serve as CoA antimetabolites and inhibit growth in E. coli (Figure 1C).15 We first tested these compounds as substrates of CoaA, the first enzyme of CoA biosynthetic pathway and the rate-limiting step for the accumulation of CoA metabolites.16 In past studies we have correlated good turnover by CoaA with in vivo CoA production and carrier protein modification.17 As can be seen in Table 1, many of the analogues exhibit good turnover with some (1–3, 5, 10–11, 13) approaching the kinetic efficiency of pantothenate. When examining the effect of chain length on turnover of alkynyl pantetheines, 4 shows very poor catalytic efficiency due mainly to its high Km. This is indicative of weak binding, a conclusion consistent with the crystal structure of E. coli CoaA which shows the terminal acid of pantothenate having an important role in binding. This can be replaced by interaction with an amide which is missing in compound 4.4 In contrast 3 and 5 show catalytic efficiencies in the range of the native substrate, while 6–9 show a decrease in efficiency that correlates to the length of the analog. This decrease in efficiency is from both binding and turnover, as Km increases and Vmax decreases with the growing length of the alkynyl pantetheine substrates. Compounds 8 and 9 highlight another interesting trend seen in this panel, which is the detrimental effect of an amide bond at the natural thiol position of pantetheine on CoaA turnover. This effect is most readily observed by comparison of 12, 13, and 14, which demonstrate a steady decrease in catalytic efficiency with decreasing polarization of the carbonyl (amide < carbamate < ester) at this position.
Table 1.
Kinetic data for CoaA turnover of natural substrate (pantothenate) as well as pantetheine analogues 1–15.a
| Compound # | Km (µM) | Vmax (µM s−1) | kcat (min−1) | kcat/Km (mM−1 min−1) |
|---|---|---|---|---|
| Pantothenate | 35.81±6.00 | 0.31 (±0.01) | 0.76±(0.03) | 21.13±1.93 |
| 1 | 33.98 (±7.11) | 0.34± (0.02) | 0.84 (±0.05) | 24.59 (±2.88) |
| 2 | 43.51 (±3.4) | 0.28±(.002) | 0.69(±0.01) | 16.1(±0.53) |
| 3 | 36.04 (±6.05) | 0.31± (0.02) | 0.77 (±0.04) | 21.38 (±2.11) |
| 4 | 396.50(±123.7) | 0.26± (0.05) | 0.64 (±0.12) | 1.62 (±0.61) |
| 5 | 34.04 (±10.72) | 0.33± (0.03) | 0.81 (±0.07) | 23.78 (±4.23) |
| 6 | 40.34 (±12.95) | 0.29± (0.03) | 0.72 (±0.07) | 17.95 (±3.41) |
| 7 | 40.00 (±14.26) | 0.29± (0.03) | 0.72 (±0.07) | 17.97 (±3.75) |
| 8 | 47.80 (±14.62) | 0.23± (0.02) | 0.56 (±0.05) | 11.70 (±2.22) |
| 9 | 48.49 (±10.99) | 0.22± (0.02) | 0.54 (±0.04) | 11.11 (±1.53) |
| 10 | 41.16 (±5.94) | 0.38± (0.02) | 0.94 (±0.04) | 22.85 (±1.96) |
| 11 | 36.91 (±7.52) | 0.37± (0.02) | 0.91 (±0.05) | 24.65 (±2.88) |
| 12 | 34.57 (±6.07) | 0.22± (0.01) | 0.53 (±0.03) | 15.43 (±1.52) |
| 13 | 27.78 (±5.21) | 0.26± (0.01) | 0.65 (±0.03) | 23.49 (±2.33) |
| 14 | 33.70 (±3.98) | 0.25± (0.01) | 0.62 (±0.02) | 18.29 (±1.20) |
| 15 | 57.97 (±12.01) | 0.26± (0.02) | 0.64 (±0.04) | 10.96 (±1.49) |
Values are means of three experiments, standard deviation is given in parentheses.
In comparing what effect degree of unsaturation has on turnover of pantetheine analogues by CoaA, the overall trend appears to indicate marginally improved turnover for fully saturated alkyl-pantetheine analogues. While this effect is slight among those pantetheine analogues terminating in propyl-derived chains (3, 10, 11), it can be clearly observed upon comparison of 1 to 6. A similar effect is observed on comparison of amide-bond extended analogues 12 and 15.
While kinetic values of pantetheine analogues with CoaA are a good predictor of in vivo activity, many other factors, including cell permeability and susceptibility to efflux pumps, impact the performance of antimetabolites when administered to living cells. To analyze the ability of these compounds to be processed by the CoA biosynthetic pathway in vivo and to interact with carrier proteins as CoA analogues, we utilized an in vivo assay.17 This assay provides a qualitative measure of the ability of pantetheine analogues to be processed by the endogenous CoA biosynthetic enzymes of E. coli by coupling CoA analogue production to the modification of a carrier protein. To facilitate detection and isolate CoA biosynthesis from variables such as carrier protein expression and PPTase promiscuity, E. coli are first transformed with expression plasmids for a carrier protein, in this case the Fren-ACP from the frenocylin polyketide synthase, as well as the PPTase Sfp, which is known to have a very broad substrate specificity. After growth to mid-log phase, the pantetheine analogue (1–15) is added at the same time as IPTG, which induces expression of the reporter system.15 Compounds that exhibit uptake and processing by the native E. coli CoA biosynthetic pathway produce modified ACPs which demonstrate a mass shift characteristic of posttranslational modification by each analogue, and can be observed by MALDI-TOF.
Having confirmed that the majority of these compounds are capable of formation of CoA analogues in vivo, we sought to correlate our findings with their antibacterial activity in native E. coli. To investigate the effects additives present in the media might have on antibiotic activity, we determined the MIC values for 1–15 using E. coli K12 grown in both minimial (M9) media, as well as in a richer, 1% tryptone broth which had been used to determine MIC values in an earlier study of pantothenamides.11, 13
Inspecting the results, all of the analogues tested showed greater growth inhibition in minimal media compared to rich media (Table 3). These results show a direct correlation between toxicity and CoaA kinetic profile for these compounds. This is to be expected, as it has previously been shown that CoaA is the rate-limiting step for CoA biosynthesis in vivo, and the antibacterial activity of these compounds is believed to be dependent on their in vivo transformation to CoA analogues. The major outliers in this respect are 2 and 13, which possess good kinetics but do not show inhibition of E. coli at concentrations up to 500 µM even in minimal media. Additional evidence that these compounds act as CoA antimetabolites was provided by the observation that the inhibitory effects of the most toxic members of this panel (1, 3, 5–6, 10–11) were greatly decreased by addition of the CoA precursors pantothenate and β-alanine to the growth medium (Table 3). Among the alkynyl analogues which initially inspired this study (3, 5–9), an increasing MIC value is observed with growing chain length, mirroring the decline in catalytic efficiency observed among this group. Interestingly, among alkyl pantetheine analogues of the same chain-length (3 and 11, 1 and 6), changing the oxidation state from an alkyne to a saturated alkyl chain lowers the MIC by a factor of two to four. However, while 11 is six-fold more active than 1 in minimal media, administration of these same compounds to E. coli grown on rich media shows 11 to be at least 10× less toxic under these conditions.
The kinetic profiles, in vivo analysis, and inhibitory data generated here all support the previously held hypothesis that the antibiotic activity of pantetheine analogues is due to the production of CoA analogues in vivo.12 However, the finding that saturated and unsaturated pantetheine analogues demonstrate rates of CoaA turnover within error of one another (i.e. 3 and 11), yet show drastically different MICs suggests that CoA analogue production alone is not sufficient for antibacterial activity. Based on our results it appears that of the pantetheine analogues processed efficiently by CoaA, those terminating in fully saturated alkyl groups are ideal for activity, while substitution by unsaturated alkynyl chains and polar terminal groups on the pantetheine chain (i.e. 2, 13) results in decreased or no growth inhibition. This suggests a secondary structure-activity relationship for pantetheine analogue inhibition, in which one set of structural characteristics is necessary for biosynthetic processing and formation of CoA or ACP-analogues in vivo, while the identity of the terminal group facilitates interaction of the CoA or ACP-analogue with a biologically relevant target.
In E. coli, fatty acid ACP is estimated to be present in the cytosol at concentrations approaching 1 mM. This abundance may explain the observation that 2 is capable of ACP modification in native E. coli without toxicity. Why then, do alkyl pantetheine analogues 1 and 10–11, show increased inhibitory properties? Based on their kinetics with CoaA, these analogues seem unlikely to modify ACP at substantially higher levels than 2 in vivo. More likely, and in agreement with the secondary structural features of antimicrobial pantetheine analogues observed here, is the hypothesis that the activity of alkyl pantetheine analogues is due to the differential activity of alkyl-ACP-prodrugs to bind and disrupt the associated lower abundance enzymes of fatty acid biosynthesis. Further elucidation of this process may have important implications for design of new members of this antibiotic class.
Supplementary Material
Table 2.
MALDI-TOF data for in vivo modificaton of Fren-ACP by compounds 1–15.
| Compound # | apo | modified | difference | expected |
|---|---|---|---|---|
| None (control) | 8663 | 9000a | 337a | 342a |
| 1 | 8660 | 9011 | 351 | 351 |
| 2 | 8663 | 9018 | 355 | 358 |
| 3 | 8663.7 | 8982 | 318.3 | 319 |
| 4 | 8661.1 | -- | -- | 248 |
| 5 | 8664.9 | 8996 | 331.1 | 333 |
| 6 | 8662.2 | 9008 | 345.8 | 347 |
| 7 | 8665.8 | 9025.9 | 360.1 | 361 |
| 8 | 8663.4 | 9065 | 401.6 | 404 |
| 9 | 8666 | 9042 & 9350b | 376b | 376 |
| 10 | 8658 | 8979 | 321 | 321 |
| 11 | 8660 | 8982 | 322 | 323 |
| 12 | 8660 | 9042 | 382 | 380 |
| 13 | 8660 | 9046 | 386 | 381 |
| 14 | 8660 | 9058 | 398 | 396 |
| 15 | 8666 | 9039 | 373 | 376 |
Modification by native CoA of E. coli results in the expected mass shift.
Compound 9 gives a large peak at 9350 in addition to the expected peak at 9042, possibly due to matrix interactions with the unsaturated activated alkyne.
Acknowledgements
This work was funded by NIH Grant RO1075797.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Leonardi R, Zhang YM, Rock CO, Jackowski S. Prog. Lipid Res. 2005;44:125. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Strauss E, Kinsland C, Ge Y, McLafferty FW, Begley TP. J. Biol. Chem. 2001;276:13513. doi: 10.1074/jbc.C100033200. [DOI] [PubMed] [Google Scholar]
- 3.Gerdes SY, Scholle MD, D’Souza M, Bernal A, Baev MV, Farrell M, Kurnasov OV, Daugherty MD, Mseeh F, Polanuyer BM, Cmapbell JW, Anantha S, Shatalin KY, Chowdhury SA, Fonstein MY, Osterman AL. J. Bacteriol. 2002;184:4555. doi: 10.1128/JB.184.16.4555-4572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivey RA, Zhang YM, Virga KG, Hevener K, Lee RE, Rock CO, Jackowski S, Park HW. J. Biol. Chem. 2004;279:35622. doi: 10.1074/jbc.M403152200. [DOI] [PubMed] [Google Scholar]
- 5.Mercer AC, Burkart MD. Nat. Prod. Rep. 2007;24:750. doi: 10.1039/b603921a. [DOI] [PubMed] [Google Scholar]
- 6.Fischbach MA, Walsh CT. Chem. Rev. 2006;106:3468. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 7.Quadri LE, Weinreb PH, Lei M, Nakano MM, Zuber P, Walsh CT. Biochemistry. 1998;37:1585. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 8.La Clair JJ, Foley TL, Schegg TR, Regan CM, Burkart MD. Chem. Biol. 2004;11:195. doi: 10.1016/j.chembiol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Clarke KM, Mercer AC, La Clair JJ, Burkart MD. J. Am. Chem. Soc. 2005;127:11234. doi: 10.1021/ja052911k. [DOI] [PubMed] [Google Scholar]
- 10.Spry C, Kirck K, Saliba KJ. FEMS Microbiol. Rev. 2008;32:56. doi: 10.1111/j.1574-6976.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 11.Virga KG, Zhang YM, Leonardi R, Ivey RA, Hevener K, Park HW, Jackowski S, Rock CO, Lee RE. Bioorg. Med. Chem. 2006;14:1007. doi: 10.1016/j.bmc.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Strauss E, Begley TP. J. Biol. Chem. 2002;277:48205. doi: 10.1074/jbc.M204560200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YM, Frank MW, Virga KG, Lee RE, Rock CO, Jackowski S. J. Biol. Chem. 2004;279:50969. doi: 10.1074/jbc.M409607200. [DOI] [PubMed] [Google Scholar]
- 14.Mercer AC, Meier JL, Torpey JW, Burkart MD. Submitted. [Google Scholar]
- 15.See supporting information for synthetic procedures and data as well as assay details
- 16.Jackowski S, Rock CO. J. Bacteriol. 1981;148:926. doi: 10.1128/jb.148.3.926-932.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier JL, Mercer AC, Rivera H, Jr, Burkart MD. J. Am. Chem. Soc. 2006;128:12174. doi: 10.1021/ja063217n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.