Abstract
Objectives
Preeclampsia is associated with reduced trophoblast placenta growth factor (PGF) expression, elevated soluble fms-like tyrosine kinase-1 (sFlt-1) and decreased bioactivity of nitric oxide (NO). Elevated sFlt-1 reduces bioavailability of PGF and vascular endothelial growth factor (VEGF) leading to maternal endothelial dysfunction. Although NO can regulate gene expression, its ability to regulate trophoblast expression of angiogenic growth factors is not known.
Study Design
Human primary term trophoblast and JEG-3 choriocarcinoma cells were cultured under 21%O2 or 1%O2 conditions in the presence or absence of NO donor (SNP) or inhibitor (L-NAME). Effects on PGF, VEGF and Flt-1 isoform mRNA expression were determined by quantitative real time PCR. Changes in expression of soluble protein isoforms of FLT-1 was monitored by ELISA.
Results
Hypoxia decreased PGF mRNA but increased VEGF, sFlt-1 and Flt-1 mRNA expression in the trophoblast. Generation of NO in trophoblast under 1%O2 culture conditions significantly reversed sFlt-1 mRNA and protein expression, independent of mFlt-1. Conversely NO generation in hypoxic trophoblast increased VEGF and PGF mRNA expression.
Conclusions
NO production in primary human trophoblast cultures had divergent effects on pro-angiogenic (PGF, VEGF) versus anti-angiogenic (sFlt-1) mRNA expression, resulting in an enhanced pro-angiogenic gene expression environment in-vitro.
Keywords: placenta growth factor (PGF), soluble fms-like tyrosine kinase-1 (sFlt-1), vascular endothelial growth factor (VEGF), trophoblast, nitric oxide, hypoxia
Introduction
Preeclampsia remains one of the most common obstetrical complications in humans[1]. The etiology of preeclampsia is not known, but shallow trophoblast invasion of maternal spiral arteries early in gestation is thought to contribute to the development of a relatively hypoxic placenta later in gestation[2]. Preeclampsia results in an imbalance in placental production of pro- versus anti-angiogenic factors[3]. Numerous reports indicate that preeclampsia is associated with reduced maternal systemic levels of placenta growth factor (PGF) protein and elevated presence of a soluble form of its receptor, fms-like tyrosine kinase-1 (sFlt-1)[4-7]. Both of these proteins are prominently expressed by human trophoblast and are differentially regulated by low oxygen tension[8;9]. When elevated, sFlt-1 binds and reduces circulating levels of bioactive PGF and vascular endothelial growth factor (VEGF) which is thought to manifest in peripheral endothelial dysfunction[3;10]. sFlt-1 inhibits endothelial cell tube formation and PGF/VEGF induced vasodilation of renal microvessels in vitro[11]. Presence of functional membrane-spanning Flt-1(mFlt-1) receptors on human trophoblast establishes an autocrine loop that that can regulate apoptosis, differentiation and nitric oxide production[12;13]. Numerous reports now confirm that experimentally increased sFlt-1 induces preeclampsia-like symptoms (elevated blood pressure, proteinuria, glomerular endotheliosis) in pregnant animal models[11], [14-16].
Nitric oxide (NO) is synthesized from L-arginine by nitric oxide synthases (NOS) and is essential for maintaining vascular tone[17]. Although findings have not always been consistent, decreased bioactivity of this potent vasodilator and/or reduced NO serum levels are thought to be common in preeclampsia (see[2;17]) which is substantiated by recent studies[18;19]. Superoxide, a reactive oxygen species, is also produced by NOS [20] and is normally metabolized by superoxide dismutase (SOD). However, the reaction of NO with superoxide to form peroxynitrite out-competes SOD for detoxifying superoxide. Since superoxide can serve as a sink for the bio-availability of NO which results in peroxynitrite production, increasing amounts of superoxide depletes accessible NO within endothelial cells[20]. Elevated levels of superoxide and peroxynitrite are associated with preeclampsia[20] and decreased bioactivity of NO is generally associated with endothelial dysfunction, vasoconstriction and reduced trophoblast invasion[2].
We have shown that hypoxia significantly reduces human trophoblast PGF gene transcription and mRNA expression[8;21], and increases sFlt-1 expression and VEGF expression in vitro[8;22]. Nitric oxide differentially regulates expression of many genes including hypoxia inducible factor-1 (HIF-1), a principle regulatory transcription factor mediating hypoxia gene responses[23]. However, the ability of NO to regulate human trophoblast expression of hypoxia-responsive angiogenesis factors is unknown. We investigated the effects of NO generation in primary human syncytiotrophoblast on PGF, VEGF, mFlt-1 and sFlt-1 gene expression under conditions of varying oxygen tensions in vitro. Low oxygen tension (1%O2) significantly decreased PGF, but increased VEGF, mFlt-1 and especially sFlt-1 mRNA expression. Increased generation of NO within hypoxic primary syncytiotrophoblast tended to increase PGF and VEGF expression and significantly reduced sFlt-1 mRNA and protein expression. NO had little effect on mFlt-1 expression in trophoblast. These results suggest that increasing NO generation at 1%O2 is likely a compensatory response to restore a pro-angiogenic environment in hypoxic trophoblast in-vitro.
Materials and Methods
Cell Culture and ELISAs
Primary human term trophoblast from uncomplicated deliveries were isolated [24] and allowed to form syncytium for 48hrs as we have described previously[8]. Collection of placentae was approved by the IRB committees at Southern Illinois University and the University of Alberta. JEG-3 choriocarcinoma cells (ATCC, Manassas, VA) were maintained in DMEM containing 10% FBS (Atlanta Biologicals, Atlanta, GA). Primary trophoblast were cultured in KGM-2 (Lonza, Walkersville, MD) supplemented with the supplied growth media kit (bovine pituitary extract, human epidermal growth factor, insulin, hydrocortisone, transferrin, epinephrine, gentamicin sulfate) and 10%FBS. Cells were plated at 5×105 cells per cm2 of culture vessel and were treated with 100μM of a spontaneous nitric oxide donor, sodium nitroprusside dihydrate (SNP) (EMD Chemicals, Gibbstown, NJ) or an inhibitor of NO synthase, NG-nitro-L-arginine methyl ester (L-NAME) (Sigma-Aldrich, St. Louis, MO) under 21% or 1% O2 for 24hrs. Supernatants were collected and stored at −80°C. Concentration of the stable bi-product of NO, nitrite (NO2), was used as a surrogate marker for quantitative measurement of nitric oxide production[17]. Nitrite concentration was quantified using Griess reagents (Invitrogen, Carlsbad, CA). Capture ELISAs for human sFlt1 and PGF from cell culture supernatants were performed as instructed (R&D Systems, Minneapolis, MN).
RNA Isolation and Real-Time PCR Analysis
RNA was isolated using TRI Reagent (Sigma-Aldrich) or RNeasy Mini Kit (Qiagen, Valencia, CA) as instructed. 50-100ng of RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and relative changes in PGF, VEGF, mFlt-1 and sFlt-1 mRNA quantified by real-time RT-PCR. Primers for VEGF were: VEGF-A (F): 5′-ATCACGAAGTGGTGAAGTTC-3′ and (R) 5′-TGCTGTAGGAAGCTCATCTC-3′ producing a 265bp product. We verified sFlt-1 mRNA using 2 different sets of primers to confirm that changes in relative expression were identical using both primer sequence: sFlt-1: (F) 5′-GGCCCCAGGGGTGCAAGATG-3′ and (R) 5′-CGCATGAGAGGAGGGAGGGGA-3′ producing a 108bp product and sFlt-1 (F) 5′-TGGCCATCACTAAGGAGCACT-3′ and (R) 5′-TCCGAGCCTGAAAGTTAGCAA-3′ which produced a 314bp amplicon[25]. Both sequences target the most highly conserved and expressed splice variant of sFlt-1 produced in human trophoblast as recently described[25]. All data for sFlt-1 expression were verified with both sFlt-1 primer sets. Primers specific for mFlt-1 were (F)5′-GTCGACACAGTGGCCATCAGCAGTT-3′ and (R)5′-TTCCACAGAGCCCTTCTGGTT-3′ producing a 219bp product as described[25]. Appropriately sized amplicons for all genes of interest were verified by gel electrophoresis and melting curve analyses (data not shown). PGF primers and cycling conditions were utilized as previously described[26]. Ribosomal protein (RPL32) mRNA, which is not altered under hypoxia[26] or TaqMan RNaseP (Applied Biosystems, Carlsbad, CA) were used as internal control genes for normalization. RPL32 was amplified with: (F) 5′-CCCAAGATCGTCAAAAAGA-3′ and (R) 5′-TCAATGCCTCTGGGTTT-3′[21]. Target gene expression was normalized to RPL32 or RNaseP expression within each experiment, and relative changes in expression calculated by the 2−ΔΔCT formula [26].
Statistical Analyses
The data are presented as mean (±SEM). Analyses were performed using GraphPad InStat 3 statistical software (GraphPad Software, San Diego, CA). To control for inter-assay variations, all data were normalized within individual experiments relative to control and relative changes in expression reported as % of control. Normalized data were analyzed via 2-tailed, 1 sample t-test with the hypothetical mean = 100%. Expression ratios of sFlt1/mFlt1 were calculated using normalized CT values and analyzed with one-way analysis of variance (ANOVA) and Bonferroni Multiple comparisons post test following log10 transformation of each ratio. A p≤0.05 was considered statistically significant.
Results
Effects of Hypoxia on Expression of Angiogenic Growth Factors in Trophoblast
Hypoxia (1%O2) significantly reduced PGF mRNA in primary syncytiotrophoblast to 17.67% (±3.41%) of control cultures (p<0.0001) (Figure 1). The effect was specific to PGF, as VEGF expression increased significantly (365.7% ± 47.1%, p<0.005) at 1%O2 (Figure 1). Similar trends in expression were evident in JEG-3 choriocarcinoma cells in that hypoxia reduced PGF expression 44% (±12.7%; p < 0.05) and increased VEGF mRNA expression (∼50%) at 1%O2 (data not shown). The relatively lower increase in VEGF expression may be explained by the substantially higher endogenous levels (∼4 fold) of VEGF mRNA in the choriocarcinoma cell line compared to primary syncytiotrophoblast. Culturing at 1%O2 also significantly increased sFlt-1 mRNA expression (256.0% ± 21.7%, p<0.005) in syncytiotrophoblast (Figure 1). Baseline sFlt-1 mRNA expression was considerably lower in JEG-3 cells than syncytiotrophoblast (∼20 fold), however, a similar trend for increased sFlt-1 mRNA expression occurred in JEG-3 cells (data not shown).
Figure 1.
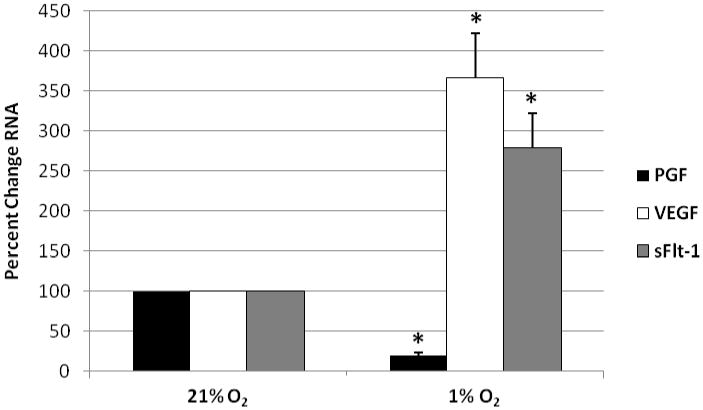
Effects of hypoxia on PGF, VEGF and sFlt-1 mRNA expression in syncytiotrophoblast. Cells were cultured under 21%O2 or 1%O2 for 24 hrs, RNA was isolated and relative changes in gene expression determined by qRT-PCR. Data were normalized to controls at 21%O2; set to 100% and plotted; n = 7 cultures, *=p< 0.01.
Effects of Nitric Oxide Donor/Inhibitor on PGF, VEGF, mFlt-1 and sFlt-1 mRNA
For both cell types, nitrite levels were at or below the limit of detection (0-1μM) within control supernatants of cells cultured at 21% or 1%O2. These data suggest that hypoxia does not affect nitric oxide production by syncytiotrophoblast under these culture conditions. In contrast, nitrite levels in cultures treated with SNP increased to 32.9 ± 5.4μM at 21% or 1%O2.
At 1%O2, NO generation with SNP significantly increased PGF mRNA expression (183% ± 29%, p<0.05) in syncytiotrophoblast (Figure 2A) when compared to the reduced level of PGF at 1%O2 (set = 100). Similarly, NO generation under 1%O2 further increased VEGF mRNA expression by ∼30% (±12.5, p= 0.05). In contrast, SNP treatment significantly reduced sFlt-1 mRNA expression by ∼70% (31.4 ± 6.3, p<0.0001, Figure 2A) in syncytiotrophoblast. Generation of NO in hypoxic JEG-3 cells had similar affects on PGF,VEGF & sFlt-1 mRNA (data not shown). Inhibition of NO synthesis with L-NAME during hypoxia did not significantly affect expression of PGF, VEGF or sFlt-1 in hypoxic syncytiotrophoblast (Figure 2A) or JEG-3 cells compared to hypoxia alone. This result is not unexpected since 1%O2 did not detectably increase NO production by these cells (see above). At 21%O2 culture conditions, neither generation nor inhibition of NO synthesis significantly affected expression of PGF, VEGF or sFlt-1 in either cell type.
Figure 2.
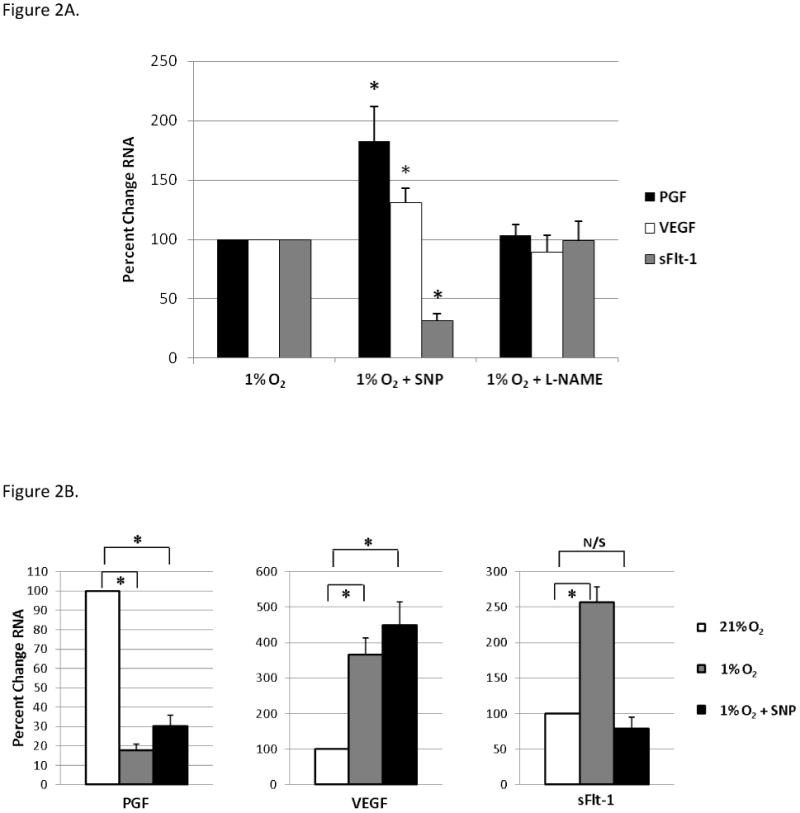
Nitric oxide generation significantly increases PGF and VEGF mRNA, but decreases sFlt-1 mRNA in hypoxic syncytiotrophoblast. (A.) Primary syncytiotrophoblast were treated with SNP or L-NAME and subjected to 1%O2 for 24hrs. Relative changes in RNA expression was determined by qRT-PCR, normalized to values at 1%O2; set to 100% and plotted; n=7, *=p<0.05. (B.) Divergent effects of NO generation under hypoxia on PGF, VEGF and sFlt-1 mRNA in primary syncytiotrophoblast. Relative changes in RNA for each treatment were determined by qRT-PCR, normalized to controls at 21%O2; set to 100% and plotted, n=7, *=p<0.005.
Comparisons between treatment groups normalized to 21% O2 culture conditions highlights the differential effects NO had on each gene product (Figure 2B). At 1%O2 NO generation increased PGF mRNA expression, although not to levels comparable to 21%O2. NO generation further augmented VEGF expression in the hypoxic trophoblast. In contrast, SNP treatment at 1%O2 inhibited sFlt-1 mRNA expression by >3.0 fold to levels not significantly different from 21%O2 culture conditions (p=0.22). Thus, under 1% O2 culture conditions, NO production had divergent effects on pro-angiogenic (PGF, VEGF) versus anti-angiogenic (sFlt-1) mRNA expression in primary syncytiotrophoblast.
Nitric oxide selectively alters sFlt-1 mRNA expression in hypoxic trophoblast
It was possible that NO was influencing sFlt-1 expression during hypoxia by regulating flt1 gene transcription. We then re-analyzed the cDNA samples using primers that differentiated membrane (m)Flt-1 from sFlt-1[25]. Hypoxia tended to increase mFlt-1 mRNA expression (167% ± 34%) compared to 21%O2, although this increase was not statistically significant (p=0.09) (Figure 3A). However, in sharp contrast to the effects on sFlt-1, increased NO generation during hypoxia had no effect on mFlt-1 mRNA expression (166%± 39%, p=0.82). Analyses of normalized CT ratios (Figure 3B) showed that under standard culture conditions, primary trophoblast express relatively more sFlt-1 (∼3.7 fold) than mFlt-1 mRNA. Low oxygen tension greatly increased expression of sFlt-1 mRNA which significantly increased the sFlt1/mFlt1 ratio (p<0.01). SNP generation of NO at 1%O2 preferentially reduced sFlt-1 mRNA but had little effect on mFlt-1 resulting in a sFlt-1/mFlt-1 ratio significantly (p< 0.001) lower than at 1%O2 alone and similar to the ratio at 21%O2 (p>0.05).
Figure 3.
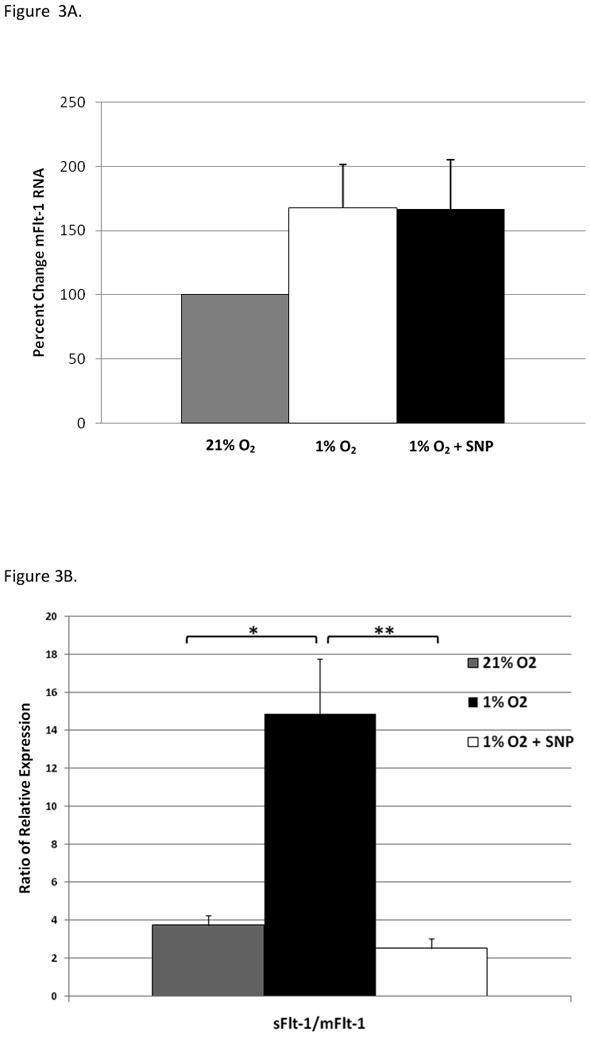
NO generation decreases sFlt-1/mFlt-1 mRNA expression ratios in hypoxic syncytiotrophoblast. (A.) Relative changes in mFlt-1 mRNA for each culture condition were determined by qRT-PCR and normalized data plotted relative to values at 21% O2; set =100%, n=7. (B.) Relative sFlt-1/mFlt-1 expression ratios in syncytiotrophoblast under hypoxia and hypoxia with SNP-induced NO-production shows preferential decrease in sFlt-1 mRNA expression with SNP treatment during hypoxia. ANOVA p<0.0001; *=p< 0.01; **=p<0.001, n=7 each.
Effects of Nitric Oxide Donor/Inhibitor on sFlt-1 protein expression
Presence of sFlt-1 proteins in syncytiotrophoblast supernatants were analyzed to confirm that protein concentrations reflected the changes in mRNA expression. Similarly to mRNA expression, SNP treatment significantly reduced sFlt-1 protein to 38.1% ±1.4% (p<0.005) of hypoxic cultures (Figure 4). In contrast, L-NAME treatment did not significantly (p= 0.8) alter sFlt-1 protein levels. These protein findings corroborate sFlt-1 mRNA findings and confirm that NO can significantly decrease sFlt-1 production in hypoxic primary human trophoblast. PGF protein was also measured in these same cell culture supernatants. Despite the modest (∼2 fold) increase of PGF mRNA following SNP treatment of hypoxic trophoblast (Figure 2B), we did not detect a significant change in free PGF protein in the supernatants (data not shown). However, these results may be confounded by the presence of sFlt-1 protein in the supernatants.
Figure 4.
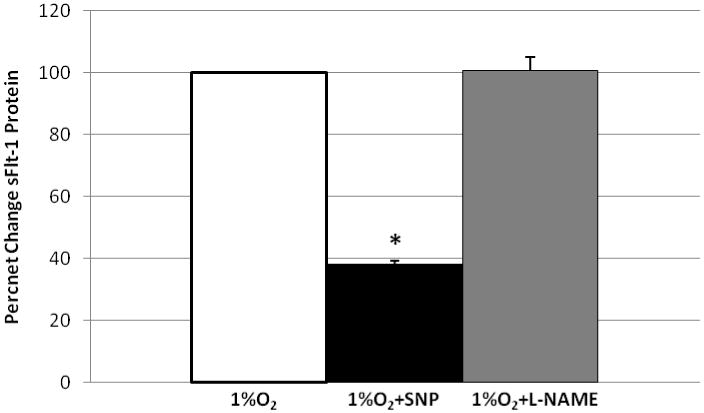
Nitric oxide generation significantly inhibits sFlt-1 protein release in hypoxic syncytiotrophoblast. Relative changes in sFlt-1 protein were determined by capture ELISA and data were normalized to control values from trophoblast cultured at 1% O2; set = 100% and plotted, n=3 each, *=p< 0.005.
Discussion
Aside from hypoxia, factors that regulate trophoblast expression of PGF, VEGF and sFlt-1 are not well understood. We are the first to report that NO generation mediated a significant reduction in sFlt-1 mRNA and protein expression in primary human syncytiotrophoblast cultured at 1%O2, but had little effect on mFlt-1 expression. Furthermore, generation of NO tended to increase PGF mRNA and further augment VEGF mRNA expression in syncytiotrophoblast cultured at 1%O2. To our knowledge, this is the first report of NO-mediated regulation of these pro- and anti-angiogenic genes in primary human trophoblast. Assuming similar results occur with hypoxic preeclamptic trophoblast in vivo, these results suggest that increased trophoblast NO production could enhance the bio-availability of PGF and VEGF angiogenic factors which may help ameliorate the anti-angiogenic environment commonly associated with this complication.
Despite the significant role of sFlt-1 in preeclampsia, there is limited information concerning the regulation of sFlt-1 expression in human trophoblast. Under 1%O2, trophoblast increase Flt-1 expression[27], and this may be mediated at least in part by HIF-1[28]. NO may regulate HIF-1 stability[29] but our studies show that hypoxic regulation of VEGF, a classic HIF-1-mediated response, was not inhibited by NO generation in trophoblast at 1%O2. In addition, we found that NO, reported to increase HIF-1 activity at 21%O2[29], increased neither sFlt-1 nor VEGF expression at this oxygen level (data not shown). Thus, it is not likely that HIF-1 is involved in NO regulation of sFlt-1 production. An alternative explanation is that NO may regulate trophoblast sFlt-1 accumulation under hypoxia independently of transcription. Production of sFlt-1 arises via alternative splicing of mature Flt-1 mRNA, our data (Figure 3B) confirms that human trophoblast favor sFlt-1 production, especially under 1%O2[25]. Our data further implies that generation of NO alters post-transcriptional processing of mature Flt-1 mRNA to produce less of the sFlt-1 isoform and/or increase sFlt-1 mRNA degradation. We propose that one posttranscriptional mechanism involves Jumonji domain-containing protein 6 (Jmjd6), an oxygen sensitive lysyl hydroxylase, recently shown to regulate alternative splicing of Flt-1 mRNA in HUVECs[30]. Potential sFlt-1 transcriptional and post-transcriptional control mechanisms regulated by NO in human trophoblast warrants future investigation.
Although NO generation did not alter trophoblast VEGF expression at 21%O2, the combination of hypoxia plus NO generation tended to further elevate VEGF mRNA expression in trophoblast. This effect may also be independent of HIF-1α in trophoblast as NO is generally thought to destabilize this subunit under hypoxia[29]. However, the effect of NO on hypoxia-induced HIF-1α accumulation is highly variable and depends upon the level of NO generated and the O2 tension[29]. Most importantly, there are cell type specific differences in the ability of cells to sense hypoxia[29] as exemplified by the unique trophoblast responses with PGF production[21].
NO generation at 1%O2 significantly increased PGF mRNA expression when compared to PGF levels at 1%O2. However, the mechanism involved appears independent of GCM-1, one principle regulator of PGF expression in trophoblast [26]. NO generation at 1%O2 did not effect GCM-1 expression from 1%O2 alone. Perhaps increased PGF mRNA was mediated via NO through metal responsive transcription factor-1 (MTF-1) interaction with its response elements within the PGF promoter[31]. MTF-1 is activated in cultured sheep pulmonary artery endothelial cells following treatment with a NO donor[31]. Interestingly, MTF-1 expression is decreased under hypoxic conditions in trophoblast and has been implicated in the hypoxic regulation of PGF[32]. The potential effects of NO modulation on MTF-1 expression and/or function in trophoblast are unknown.
Our results provide mechanistic insights into the association between hypoxia, NO and preeclampsia. Systemic inhibition of NO synthesis with L-NAME in pregnant rats selectively increased sFlt-1 levels and maternal hypoxia selectively decreased PGF while the combination resulted in severe fetal growth restriction[33]. Our studies suggest that one of the biological mechanisms by which NO inhibition affects fetal outcome in this model may involve trophoblast. Conceptually, during hypoxic stress in vivo trophoblast increase sFlt-1 production and concomitant L-NAME treatment to inhibit NO would further increase sFlt-1 mRNA and protein production. Indeed, there is an inverse relationship between high levels of sFlt-1 and lower levels of NO production in preeclamptic patients[34]. Both VEGF and PGF act through mFlt-1 receptors to induce NO production in endothelial cells[35] and trophoblast [36]. Treatment of trophoblast cells with PGF augmented NO production and PGF binding to mFlt-1 receptors [36], which we hypothesize would result in a selective decrease in sFlt-1 production via alternative splicing of flt1 by trophoblast at 1%O2. The effects of PGF treatment on NO production at varying oxygen concentrations has not been examined to our knowledge. While low oxygen tension does not affect NO production quantified in the supernatants of trophoblast, it has recently been shown that hypoxia reduces eNOS protein expression [37]. Thus, decreases in trophoblast PGF production concomitant with increased sFlt-1 production, of which both are apparent in a hypoxic trophoblast environment, could act in paracrine and autocrine fashions to limit NO production and trophoblast function. Regulating angiogenesis and vasodilation at the maternal-fetal interface is complex, and many mechanisms act through the NO pathway [38]. In the context of trophoblast, we suggest that decreasing NO production in trophoblast perpetuates a further prominence of an anti-angiogenic state by increased sFlt-1 production.
Reducing systemic sFlt-1 protein by increasing ligand availability reverses PE-like conditions in animal models[11;39]. Conceptually, our results suggest that it might be possible to decrease the systemic effects of inverted pro- to anti-angiogenic gene products expression via manipulation of NO levels in preeclampsia. Indeed, a recent large scale study found that L-arginine supplementation during pregnancy significantly decreases the incidence of preeclampsia in a high risk population [40]. However, benefits of NO donors in pregnancy have not been uniform and additional clinical studies are needed (see [41]). Given the clinical heterogeneity of preeclampsia and the potential for patient-specific responses to NO-altering approaches (see[42]), the variability in results is not surprising. Studies assessing the effects of NO augmentation on serum levels of PGF/VEGF and sFlt-1 during human preeclampsia are lacking.
Clinical management approaches such as anti-hypertensive drugs to control blood pressure in preeclampsia have been shown to reduce maternal serum and placental production of sFlt-1[43]. However, many anti-hypertensive agents have no effect on lowering sFlt-1 production from human placenta explants in vitro[44]. Non-pharmacological approaches, such as regular exercise during pregnancy, has been shown to increase serum PGF while simultaneously decrease sFlt-1 and soluble endoglin during the third trimester and reduces the risk of developing preeclampsia[45]. The molecular explanations behind these observations are not known but continued investigation into mechanisms to re-establish expression of pro-angiogenic while limiting anti-angiogenic gene expression in trophoblast may provide novel treatment avenues for obstetrical complications like preeclampsia and/or fetal growth restriction.
Acknowledgments
Supported in part by NIH 5RO1HD36830 (DST).
Footnotes
Presented in part at the 30TH Annual American Society for Reproductive Immunology Meeting, Nemacolin, PA, May 17-20, 2010.
Reference List
- 1.ACOG: ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia Number 33, January 2002 American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 2.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 3.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 5.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Urinary placental growth factor and risk of preeclampsia. Jama. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–72. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 7.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–44. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 8.Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–65. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of Placental Vascular Endothelial Growth Factor (VEGF) and Placenta Growth Factor (PlGF) and Soluble Flt-1 by Oxygen- A Review. Placenta. 2000;21:S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 10.Khankin EV, Royle C, Karumanchi SA. Placental vasculature in health and disease. Semin Thromb Hemost. 2010;36:309–320. doi: 10.1055/s-0030-1253453. [DOI] [PubMed] [Google Scholar]
- 11.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo J, Torry RJ, Torry DS. Deferential Regulation of Placenta Growth Factor (PlGF)-Mediated Signal Transduction in Human Primary Term Trophoblast and Endothelial Cells. Placenta. 2004;25:379–86. doi: 10.1016/j.placenta.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–23. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, da Costa Gonzalves AC, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2009 Jun 16; doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22:564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M, Suzuki M, Sato Y. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension. 2009;54:1129–1135. doi: 10.1161/HYPERTENSIONAHA.109.134668. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Jaramillo P, Arenas WD, Garcia RG, Rincon MY, Lopez M. The role of the L-arginine-nitric oxide pathway in preeclampsia. Ther Adv Cardiovasc Dis. 2008;2:261–275. doi: 10.1177/1753944708092277. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res. 2010;36:239–247. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Sharma D, Raghunandan C, Bhattacharjee J. Role of inflammatory cytokines and eNOS gene polymorphism in pathophysiology of pre-eclampsia. Am J Reprod Immunol. 2010 Mar 1;63:244–251. doi: 10.1111/j.1600-0897.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010 31:S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobble RM, Groesch KA, Chang M, Torry RJ, Torry DS. Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta. 2009;30:869–875. doi: 10.1016/j.placenta.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torry DS, Hinrichs M, Torry RJ. Determinants of placental vascularity. Am J Reprod Immunol. 2004;51:257–268. doi: 10.1111/j.1600-0897.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 23.Brune B, Zhou J. Hypoxia-inducible factor-1alpha under the control of nitric oxide. Methods Enzymol. 2007;435:463–478. doi: 10.1016/S0076-6879(07)35024-6. [DOI] [PubMed] [Google Scholar]
- 24.Guilbert LJ, Winkler-Lowen B, Sherburne R, Rote NS, Li H, Morrish DW. Preparation and functional characterization of villous cytotrophoblasts free of syncytial fragments. Placenta. 2002;23:175–183. doi: 10.1053/plac.2001.0756. [DOI] [PubMed] [Google Scholar]
- 25.Thomas CP, Raikwar NS, Kelley EA, Liu KZ. Alternate processing of Flt1 transcripts is directed by conserved cis-elements within an intronic region of FLT1 that reciprocally regulates splicing and polyadenylation. Nucleic Acids Res. 2010 Apr 12;38:5130–5140. doi: 10.1093/nar/gkq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang M, Mukherjea D, Gobble RM, Groesch KA, Torry RJ, Torry DS. Glial cell missing 1 regulates placental growth factor (PGF) gene transcription in human trophoblast. Biol Reprod. 2008;78:841–851. doi: 10.1095/biolreprod.107.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26:210–7. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, Caniggia I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2006 Oct 1;291:R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007 Jul 15;75:275–282. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Boeckel JN, Guarani V, Koyanagi M, Roexe T, Lengeling A, Schermuly RT, Gellert P, Braun T, Zeiher A, Dimmeler S. Jumonji domain-containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF-receptor 1. Proc Natl Acad Sci U S A. 2011 Feb 22;108:3276–3281. doi: 10.1073/pnas.1008098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stitt MS, Wasserloos KJ, Tang X, Liu X, Pitt BR, St Croix CM. Nitric oxide-induced nuclear translocation of the metal responsive transcription factor, MTF-1 is mediated by zinc release from metallothionein. Vascul Pharmacol. 2006;44:149–155. doi: 10.1016/j.vph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Nishimoto F, Sakata M, Minekawa R, Okamoto Y, Miyake A, Isobe A, Yamamoto T, Takeda T, Ishida E, Sawada K, Morishige K, Kimura T. Metal transcription factor-1 is involved in hypoxia-dependent regulation of placenta growth factor in trophoblast-derived cells. Endocrinology. 2009;150:1801–1808. doi: 10.1210/en.2008-0949. [DOI] [PubMed] [Google Scholar]
- 33.Bahtiyar MO, Buhimschi C, Ravishankar V, Copel J, Norwitz E, Julien S, Guller S, Buhimschi IA. Contrasting effects of chronic hypoxia and nitric oxide synthase inhibition on circulating angiogenic factors in a rat model of growth restriction. Am J Obstet Gynecol. 2007;196:72–76. doi: 10.1016/j.ajog.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008;52:402–407. doi: 10.1161/HYPERTENSIONAHA.108.115006. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le NF, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006 Sep 29;99:715–722. doi: 10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]
- 36.Angelucci C, Lama G, Iacopino F, Maglione D, Sica G. Effect of placenta growth factor-1 on proliferation and release of nitric oxide, cyclic AMP and cyclic GMP in human epithelial cells expressing the FLT-1 receptor. Growth Factors. 2001;19:193–206. doi: 10.3109/08977190109001086. [DOI] [PubMed] [Google Scholar]
- 37.Park MH, Galan HL, Arroyo JA. Effect of hypoxia on endothelial nitric oxide synthase, NO production, intracellular survival signaling (p-ERK1/2 and p-AKT) and apoptosis in human term trophoblast. Am J Reprod Immunol. 2011;65:407–414. doi: 10.1111/j.1600-0897.2010.00886.x. [DOI] [PubMed] [Google Scholar]
- 38.Valdes G, Corthorn J. Review: The angiogenic and vasodilatory utero-placental network. Placenta. 2011;32:S170–S175. doi: 10.1016/j.placenta.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves AC, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14:1857–1867. doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vadillo-Ortega F, Perichart-Perera O, Espino S, vila-Vergara MA, Ibarra I, Ahued R, Godines M, Parry S, Macones G, Strauss JF. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meher S, Duley L. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007:CD006490. doi: 10.1002/14651858.CD006490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hale SA, Jones CW, Osol G, Schonberg A, Badger GJ, Bernstein IM. Sildenafil increases uterine blood flow in nonpregnant nulliparous women. Reprod Sci. 2010;17:358–365. doi: 10.1177/1933719109354648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalil A, Muttukrishna S, Harrington K, Jauniaux E. Effect of antihypertensive therapy with alpha methyldopa on levels of angiogenic factors in pregnancies with hypertensive disorders. PLoS One. 2008;3:e2766. doi: 10.1371/journal.pone.0002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu B, Thornton C, Tooher J, Ogle R, Lim S, Makris A, Hennessy A. Effects of anti-hypertensive drugs on production of soluble fms-like tyrosine kinase 1 and soluble endoglin from human normal and pre-eclamptic placentas in vitro. Clin Exp Pharmacol Physiol. 2009;36:839–842. doi: 10.1111/j.1440-1681.2009.05155.x. [DOI] [PubMed] [Google Scholar]
- 45.Weissgerber TL, Davies GA, Roberts JM. Modification of angiogenic factors by regular and acute exercise during pregnancy. J Appl Physiol. 2010;108:1217–1223. doi: 10.1152/japplphysiol.00008.2010. [DOI] [PubMed] [Google Scholar]


