Abstract
Purpose
Adolescents (age 15 to 21 years) compared with younger children with mature B-cell non-Hodgkin's lymphoma (NHL) have been historically considered to have an inferior prognosis. We therefore analyzed the impact of age and other diagnostic factors on the risk of treatment failure in children and adolescents treated on the French-American-British Mature B-Cell Lymphoma 96 (FAB LMB 96) trial.
Patients and Methods
Patients were divided by risk: group A (limited), group B (intermediate), and group C (advanced), as previously described. Prognostic factors analyzed for event-free survival (EFS) included age (< 15 v ≥ 15 years), stage (I/II v III/IV), primary site, lactate dehydrogenase (LDH), bone marrow/CNS (BM/CNS) involvement, and histology (diffuse large B-cell lymphoma v mediastinal B-cell lymphoma v Burkitt lymphoma or Burkitt-like lymphoma).
Results
The 3-year EFS for the whole cohort was 88% ± 1%. Age was not associated as a risk factor for increased treatment failure in either univariate analysis (P = .15) or multivariate analysis (P = .58). Increased LDH (≥ 2 × upper limit of normal [ULN] v < 2 × ULN), primary site, and BM-positive/CNS-positive disease were all independent risk factors associated with a significant increase in treatment failure rate (relative risk, 2.0; P < .001, P < .012, and P < .001, respectively).
Conclusion
LDH level at diagnosis, mediastinal disease, and combined BM-positive/CNS-positive involvement are independent risk factors in children with mature B-cell NHL. Future studies should be developed to identify specific therapeutic strategies (immunotherapy) to overcome these risk factors and to identify the biologic basis associated with these prognostic factors in children with mature B-cell NHL.
INTRODUCTION
Mature B-cell non-Hodgkin's lymphoma (NHL), including Burkitt lymphoma (BL), Burkitt leukemia, diffuse large B-cell lymphoma (DLBCL), and primary mediastinal B-cell lymphoma (PMBL) make up approximately 60% of all malignant NHLs that occur in children and adolescents.1,2 Multidisciplinary pediatric cooperative group collaborations over the past 25 years have reported a 99% survival rate in limited-risk patients, a 90% survival rate in intermediate-risk patients, and an approximate 70% to 80% overall survival (OS) rate in children with advanced-risk mature B-cell NHL.3–16
Several risk factors have been associated with influencing event-free survival (EFS) in children with mature B-cell NHL. Advanced stage (Murphy classification; ie, stages III and IV v stages I and II) has been associated with a decrease in EFS in children and adolescents with mature B-cell NHL.12–14,16 Increased lactate dehydrogenase (LDH) at diagnosis, either ≥ 2 × upper limit of normal (ULN) or ≥ 1,000 IU has also been associated with a significant decrease in EFS in children and adolescents with mature B-cell NHL.6,12–14,16 CNS involvement has also been an independent poor-risk factor on EFS in children with mature B-cell NHL.6,12–14,16 Response to reduction therapy following a reductive phase of chemotherapy in children and adolescents with mature B-cell NHL has also been associated with a significantly inferior EFS, particularly in patients with intermediate and advanced risk.6,12,13
Age, particularly those in the adolescent age group (15 to 21 years), has been suggested to be an additional potential independent prognostic risk factor in EFS in children and adolescents with mature B-cell NHL. Malignant lymphomas are the most common malignancy in the adolescent age group, representing approximately 26% of all malignancies.17 The first NHL treatment protocol—CCG-551—in the Children's Cancer Group (CCG) from 1977 to 1982 demonstrated that adolescents versus children younger than 15 years of age with BL treated with either cyclophosphamide, vincristine, methotrexate, and prednisone (COMP) or with LSA2 L2 therapy (cyclophosphamide, vincristine, methotrexate, daunomycin, prednisone, cytarabine, thioguanine, asparaginase, methotrexate, and carmustine) had a significantly inferior EFS (25% v 70%; P < .033).18–20 Subsequently, the CCG performed a retrospective review of all consecutive CCG studies between 1977 and 1994 that treated children and adolescents with BL or Burkitt-like lymphoma (BLL) and demonstrated a significant decrease in 5-year EFS in adolescents versus younger children treated on similar therapy.9 Similarly, Patte et al13 reported that the LMB 89 mature B-cell lymphoma protocol results demonstrated a significantly increased risk of relapse in patients 15 years of age or older. We previously reported the primary results of the French-American-British Mature B-Cell Lymphoma 96 (FAB LMB 96) study.6,10,12 In this report, we investigated the prognostic risk of adolescent age (15 to 21 years) and other prognostic factors on 5-year EFS and OS in a combined cohort of 1,111 patients with mature B-cell NHL registered and treated on this uniform international cooperative group protocol, which used modern, short, and intensive multiagent chemotherapy.
PATIENTS AND METHODS
FAB LMB 96 was an international study from 161 treatment centers by three cooperative groups: Children's Oncology Group (COG; former CCG institutions in the United States, Canada, and Australia), the United Kingdom Children's Cancer Study Group (UKCCSG), and Societe Francaise d'Oncologie Pediatrique (SFOP; institutions in France and some centers in Belgium and the Netherlands). The protocol was approved by all of the local institutional review boards, and written informed consent was obtained in accordance with the Declaration of Helsinki. The study opened in May 1996 and closed to patient accrual in June 2001.
Eligibility
Children and adolescents with newly diagnosed mature B-lineage NHL with BL, DLBCL, or BLL, according to the Revised European-American Lymphoma (REAL) classification, were eligible.21 Staging was performed as previously described by Murphy et al.22 Risk classification was defined as low risk (group A) with resected stage I and abdominal completely resected stage II, high risk (group C) with bone marrow (BM) involvement (L3 blasts ≥ 25% and/or CNS disease), and intermediate risk (group B) was all others.6,10,12 Exclusion criteria included congenital or acquired immunodeficiency, prior malignancy, or prior chemotherapy. Therapy for group A involved a nonrandomized confirmatory study of brief chemotherapy10; therapy for groups B and C involved an open randomized trial that investigated the reduction of treatment.6,12
Treatment
Group A.
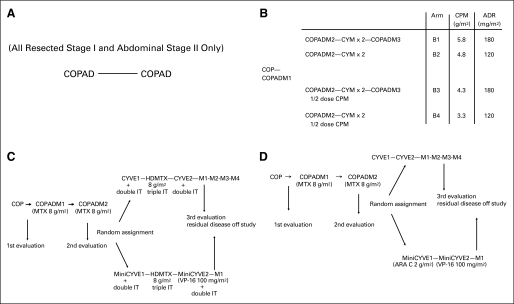
Patients assigned to group A following resection and diagnostic workup received two courses of cyclophosphamide, vincristine, prednisone, and doxorubicin (COPAD) without intrathecal (IT) chemotherapy, as we have previously described (Fig 1A).10
Fig 1.
Overall experimental design of (A) group A therapy, (B) group B therapy, (C) group C therapy for CNS-positive patients, (D) group C therapy for CNS-negative patients. The number after the regimen name indicates the cycle number. ADR, doxorubicin; ARA C, cytarabine; COP, cyclophosphamide, vincristine, and prednisone; COPAD, cyclophosphamide, vincristine, prednisone, and doxorubicin; COPADM, COPAD with high-dose methotrexate (HD-MTX; 8 g/m2); CPM, cyclophosphamide; CYM, cytarabine and HD-MTX; CYVE, cytarabine 2 g/m2 and etoposide 100 mg/m2; IT, intrathecal; M1, maintenance cycle 1; M2, maintenance cycle 2; M3, maintenance cycle 3; M4, maintenance cycle 4; VP-16, etoposide.
Group B.
The details of treatment and random assignment have been described previously.12 Patients received 7-day, low-dose, prophase cyclophosphamide, vincristine, and prednisone (COP) therapy. Induction therapy consisted of two cycles of fractionated COPAD and high-dose methotrexate 3 g/m2 (HD-MTX; COPADM). Patients then received two consolidation cycles of cytarabine and HD-MTX (CYM). Treatment concluded with one maintenance phase of COPADM (COPADM-3). Patients received IT chemotherapy prophylaxis during all phases of the therapy. As previously described, patients who did not progress during the first induction course were randomly assigned to therapy reduction with 50% cyclophosphamide delivered in the second induction cycle and/or the elimination of maintenance therapy in a four-arm stratified random assignment. Patients with less than a 20% response on day 7 of COP and patients with residual disease after CYM-1 (that is, the first cycle of CYM) were transferred to rescue group C therapy as outlined below in Figure 1B.6,12
Group C.
The details of treatment and random assignment are as previously reported.6 Patients received 7-day low-dose prophase COP. Induction therapy consisted of two cycles of COPADM (with HD-MTX 8 g/m2). Consolidation consisted of high-dose and continuous cytarabine with etoposide (CYVE). Patients with CNS disease did not receive cranial radiation but received additional IT therapy as well as an additional HD-MTX course between consolidation courses (Figs 1C and 1D, respectively). The first maintenance cycle consisted of COPADM, and three additional maintenance cycles followed in the standard arm of therapy. Patients with favorable disease reassessments were randomly assigned to reduction in chemotherapy during the consolidation phase (CYVE) and the elimination of the three maintenance arms in a two-arm random assignment.6
Definition, Eligibility, and Random Assignment of Adolescents
The upper age limit of enrollment was 21 years at diagnosis in COG institutions and 18 years at diagnosis in SFOP and UKCCSG institutions. The definition of adolescence for subsequent analysis included patients age ≥ 15 years at study enrollment. In the randomized analysis, patients were randomly assigned within cooperative groups and strata defined by all combinations of cooperative group, histology (DLBCL or not), stage and LDH level (group B), and CNS positivity (group C). No a priori stratification occurred on the basis of age at enrollment. The distribution of adolescents in the randomized arms was not planned or stratified in advance.
Hematopathology
The morphology and immunophenotype from the initial diagnostic material from each patient was independently evaluated by each of the six hematopathologists from the three national cooperative groups (SFOP: M. Raphael, M.J. Terrier-Lacombe; CCG: M.A. Lones, S.L. Perkins; UKCCSG: K. McCarthy, K.A. Wotherspoon) to establish a diagnosis. The initial standard immunophenotyping panel included antibodies to the following CD antigens: CD20, CD79a, CD3, CD45RO, TDT, CD30, and p80, as described previously.23,24 The protocol cases were classified according to the criteria described in the REAL and WHO classifications.23,24 At initial evaluation, only clinical information on biopsy site, age, and sex were known. All mediastinal cases were reviewed again by the pathology group at a multiheaded microscope, with full knowledge of all available clinical and cytogenetic information and with careful attention to morphologic features of sclerosis, clear-cell change, and immunophenotype. Because of the limited amount of tissue available, design of the protocol, and the availability of antibodies at the time of review, additional immunohistochemical staining could not be performed, Results of morphologic and immunophenotypic evaluations, as well as diagnosis, were recorded on a standard form for entry into a computer database. A national consensus diagnosis was established for each patient on the basis of independent agreement by the group of hematopathologists or following review by the national group on a multiheaded microscope. A final consensus diagnosis was established for each patient when at least two of the three national consensus diagnoses were in agreement or following review on a multiheaded microscope by all members of the reviewing committee. If morphology was ambiguous between DLBCL and BL leading to discordance by the reviewers, BCL-2 and MIB-1 stains were performed to aid in diagnosis, although this was necessary in less than 10% of cases.
Statistical Methods
The primary end point for analysis was EFS, which was defined as the minimum time to death from any cause, relapse, progressive disease, second malignant neoplasm, or biopsy-positive residual disease at the end of the group C consolidation phase. The secondary end point was OS, which was the time to death from any cause measured from the start of therapy. Product-limit estimates of EFS and OS probabilities are reported along with Greenwood SEs. The log-rank test and multivariate Cox regression analysis were used to identify significant factors. All reported P values are two-sided. Statistical computations were performed by using STATA version 11 (STATA, College Station, TX).
RESULTS
Demographics
There were 1,111 patients registered on FAB LMB 96 from May 1996 to June 2001. There were 132, 744, and 235 patients treated on group A, group B, and group C therapy, respectively. Fifteen percent of patients (n = 166) were 15 years old or older. Patients up to age 21 were permitted on study in CCG, but not in the other cooperative groups. Thus, 21% of CCG registered patients were 15 years of age or older compared with 7% for SFOP and 13% for UKCCSG (P < .001). The frequency of males was 3.3 times higher than that for females in the entire cohort.
Patient demographics and disease characteristics are summarized separately for adolescents (age ≥ 15 years) and younger children (age < 15 years) in Table 1. There was a difference in the distribution of disease site (P < .001) with a higher frequency of patients with abdominal/retroperitoneal and head and neck disease among younger children and a higher frequency of patients with primary mediastinal and peripheral node disease among adolescents. The distribution of pathology subtypes was also different (P < .001); patients with BL/BLL were more frequent among younger children although patients with DLBCL and mediastinal disease were more frequent among adolescents. There was a difference in distribution of disease stage (P = .003), with a higher percentage of patients younger than age 15 years in stage III. There was also a difference in distribution of BM and CNS positivity (P = .01), with a lower frequency of adolescents being BM-positive compared with younger children (13% v 22%), but frequency was similar for involvement of the CNS in the two age groups (11% v 10%). There was a lower proportion of patients with LDH ≥ 2 × institutional upper limit of normal (ULN) among adolescents compared with children younger than age 15 years (34% v 45%; P = .009).
Table 1.
Demographic Characteristics and Risk Factors
| Characteristic | Adolescents (older than age15 years) |
Children (younger than age 15 years) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Total No. of patients | 166 | 15 | 945 | 85 | |
| Sex | .26 | ||||
| Male | 122 | 73 | 732 | 77 | |
| Female | 44 | 27 | 213 | 23 | |
| Male:female ratio | 2.8:1 | 3.4:1 | .21 | ||
| Prognostic group | |||||
| A | 23 | 14 | 109 | 12 | |
| B | 116 | 70 | 628 | 66 | |
| C | 27 | 16 | 208 | 22 | |
| Stage (Murphy) | .003 | ||||
| I | 27 | 16 | 93 | 10 | |
| II | 27 | 16 | 200 | 21 | |
| III | 84 | 51 | 405 | 43 | |
| IV | 28 | 17 | 247 | 26 | |
| Primary site | < .001 | ||||
| Peripheral node | 32 | 19 | 88 | 9 | |
| Mediastinal | 32 | 19 | 22 | 2 | |
| Abdominal/retroperitoneal | 57 | 34 | 517 | 55 | |
| Head and neck | 13 | 9 | 177 | 19 | |
| Other | 32 | 19 | 141 | 15 | |
| Pathology | < .001 | ||||
| BL/BLL | 79 | 48 | 718 | 74 | |
| DLBCL | 75 | 45 | 174 | 18 | |
| Other | 12 | 7 | 53 | 8 | |
| BM/CNS | .01 | ||||
| BM negative/CNS negative | 137 | 83 | 696 | 74 | |
| BM positive/CNS negative | 10 | 6 | 151 | 16 | |
| BM negative/CNS positive | 7 | 4 | 39 | 4 | |
| BM positive/CNS positive | 11 | 7 | 57 | 6 | |
| LDH | .009 | ||||
| < 2 × institutional ULN | 107 | 66 | 504 | 55 | |
| > 2 × institutional ULN | 54 | 34 | 406 | 45 | |
| Unknown | 5 | 35 | |||
Abbreviations: BL, Burkitt lymphoma; BLL, Burkitt-like lymphoma; DLBCL, diffuse large B-cell lymphoma; LDH, lactate dehydrogenase; ULN, upper limit of normal.
EFS and OS
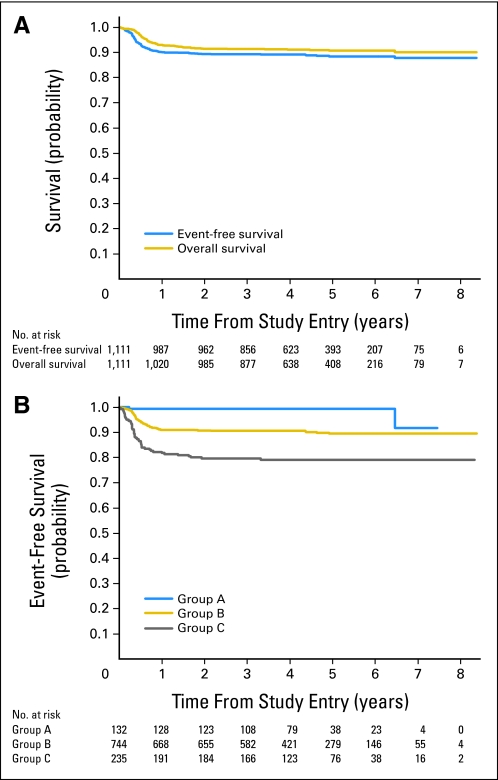
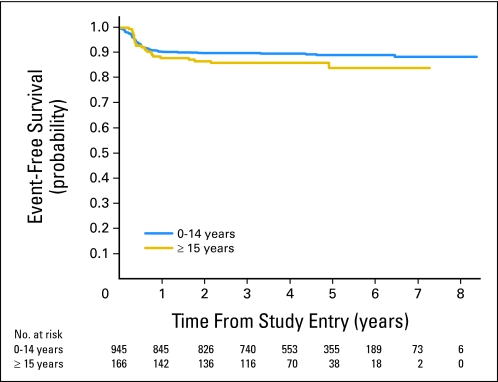
Median follow-up in patients not experiencing an event was 4.5 years. The estimated 3-year EFS and OS in the entire cohort of patients (N = 1,111) with newly diagnosed mature B-cell NHL treated in the FAB LMB 96 study was 88% ± 1.0% and 90% ± 0.91%, respectively (Fig 2A). The estimated 3-year EFS in patients who had group A, B, and C therapy was 99% ± 0.75%, 89% ± 1.2%, and 79% ± 2.7%, respectively (P < .001; Fig 2B). The estimated 3-year EFS in children younger than age 15 years was similar to that of adolescent patients (89% ± 1.0% v 84% ± 3.4%; P = .15; Fig 3). There was also no significant difference in the estimated 3-year OS between the two age groups (< 15 v ≥ 15 to 21 years; 91% ± 0.93% v 85% ± 3.2%; P = .083).
Fig 2.
(A) Probability of overall survival and event-free survival of entire cohort. (B) Probability of event-free survival in patients treated with group A, group B, or group C therapy.
Fig 3.
Probability of event-free survival in patients treated in the French-American-British Mature B-Cell Lymphoma 96 (FAB LMB 96) study, grouped by age.
Risk Factors for EFS
The log-rank analysis for EFS identified several risk factors that were significant, including prognostic group (P < .001; Fig 4A), LDH ≥ 2 × ULN (P < .001), BM/CNS status at diagnosis (P < .001, Fig 4B), stage III/IV (P < .001), and primary site (P < .001; Fig 4C).
Fig 4.
Probability of event-free survival in patients treated in the French-American-British Mature B-Cell Lymphoma 96 (FAB LMB 96) study, stratified by (A) lactate dehydrogenase (LDH) at diagnosis (< 2 × institutional upper limit of normal (ULN) v ≥ 2 × institutional ULN), (B) bone marrow (BM)/CNS involvement, and (C) primary site. Abd/retro, abdominal/retroperitoneal; H&N, head and neck; Med, mediastinal; PN, peripheral node.
A Cox multivariate regression analysis was performed that included age (≥ 15 v < 15 years), prognostic group, stage, primary site, pathology, BM/CNS involvement, and LDH ≥ 2 × ULN. The relative failure rate (RFR) estimates, confidence intervals, and P values from this analysis are summarized in Table 2. Age, prognostic group, stage, and pathology were not significant in this analysis. However, several other variables were significant. LDH ≥ 2 × ULN had a relative risk (RR) of 2.0 (P = .003). Primary site was significant (P < .012) primarily because of higher treatment failure rate associated with mediastinal disease and abdominal/retroperitoneal disease (RFR, 4.5 and 2.7, respectively, v patients with peripheral node primaries). BM-positive/CNS-positive status was significant (P < .001), primarily because of the higher treatment failure rate associated with combined BM and CNS involvement (RFR, 4.9 v patients with neither BM nor CNS involvement).
Table 2.
Significant Risk Factors Associated With Relapse/Progression on French-American-British Mature B-Cell Lymphoma 96 (FAB LMB 96) Study Univariate and Multivariate Analysis
| Risk Factor | Univariate Analysis |
Multivariate Analysis |
|||
|---|---|---|---|---|---|
| 3-Year EFS (% ± SE) | Log-Rank P | RFR | 95% CI | P | |
| Age, years | .15 | .58 | |||
| < 15 | 89 ± 1.0 | 1.0 | |||
| ≥ 15 | 84 ± 3.4 | 1.2 | 0.70 to 1.9 | ||
| Prognostic group | < .001 | .90 | |||
| A | 99 ± 0.75 | 1.0 | |||
| B | 89 ± 1.2 | 2.0 | 0.38 to 11 | ||
| C | 79 ± 2.7 | 2.6 | 0.36 to 19 | ||
| Stage (Murphy) | < .001 | .082 | |||
| I/II | 98 ± 1.1 | 1.0 | |||
| III/IV | 84 ± 1.4 | 2.4 | 0.90 to 6.4 | ||
| Primary site | < .001 | .012 | |||
| Peripheral node | 97 ± 2.0 | 1.0 | |||
| Mediastinal | 72 ± 6.2 | 4.5 | 1.2 to 17 | ||
| Abdominal/retroperitoneal | 87 ± 1.4 | 2.7 | 0.83 to 9.0 | ||
| Head and neck | 94 ± 2.0 | 1.2 | 0.32 to 4.4 | ||
| Other | 85 ± 2.8 | 1.2 | 0.35 to 4.3 | ||
| Pathology | .92 | .24 | |||
| BL/BLL | 89 ± 1.1 | 1.0 | |||
| DLBCL | 87 ± 2.5 | 1.6 | 0.92 to 2.7 | ||
| Other | 87 ± 4.2 | 1.0 | 0.49 to 2.1 | ||
| BM/CNS | < .001 | < .001 | |||
| BM negative/CNS negative | 91 ± 1.1 | 1.0 | |||
| BM positive/CNS negative | 88 ± 2.6 | 1.1 | 0.43 to 2.7 | ||
| BM negative/CNS positive | 83 ± 5.6 | 1.8 | 0.50 to 6.6 | ||
| BM positive/CNS positive | 61 ± 6.0 | 4.9 | 1.6 to 15 | ||
| LDH | < .001 | .003 | |||
| < 2 × institutional ULN | 94 ± 1.1 | 1.0 | |||
| ≥ 2 × institutional ULN | 81 ± 1.9 | 2.0 | 1.3 to 3.2 | ||
Abbreviations: BL, Burkitt lymphoma; BLL, Burkitt-like lymphoma; DLBCL, diffuse large B-cell lymphoma; EFS, event-free survival; RFR, relative failure rate; ULN, upper limit of normal.
DISCUSSION
This trial was the largest multinational cooperative group study in children and adolescents with newly diagnosed mature B-cell NHL. Malignant lymphomas are the most common cancer in adolescents (age 15 to 19 years) and represent almost one in four of all malignant tumors during in this age group.17,25–27 Furthermore, successful treatment of adolescent cancer has been significantly hampered by several contributing factors, including labile emotional well-being, lack of parental guidance, poor participation in clinical trials, decreased medical insurance coverage, lack of economic resources, and few multidisciplinary programs.27–29
Historically, there was general consensus that mature B-cell NHL, especially BL, occurring in adolescence was an independent risk factor for a poorer EFS compared with that occurring in children younger than age 15 years.9,13 In the CCG retrospective review of 470 children with BL treated from 1977 to 1994 on front-line CCG B-cell NHL trials, adolescents age ≥ 15 years had a significantly inferior survival compared with children younger than age 15 years (35% v 55% to 60%; P < .002).9 Similarly, Patte et al13 demonstrated in patients with intermediate-risk disease given group B therapy in LMB 89 that adolescents had an RR of 6.7 (range, 2.2 to 20.4) for relapse (P < .006) compared with younger patients (younger than age 15 years) with mature B-cell NHL. Burkhardt et al26 reviewed the outcome of all adolescents with NHL treated on Berlin-Frankfurt-Münster (BFM) 86, BFM 90, and BFM 95 and demonstrated in a multivariate analysis a significantly inferior outcome in adolescents (age 15 to 18 years) with mediastinal large B-cell lymphoma (P < .054) and a trend in females age ≥ 15 years with DLBCL. However, our current, more intensive study demonstrated that age ≥ 15 years (adolescents) was not an independent risk factor for inferior EFS, nor was there any indication of a differential effect of age within patient subgroups defined by morphology or sex.
However, the current study did demonstrate that LDH level, mediastinal disease, and BM-positive/CNS-positive disease are independent risk factors for outcome in children and adolescents with mature B-cell NHL treated on modern, short but intensive therapy such as that in FAB LMB 96. A major risk factor identified in this study associated with an inferior outcome was primary site (P < .012 in multivariate analysis), especially in patients with mediastinal disease (RR of 4.5 relative to patients with peripheral node primaries). Although mediastinal disease represents less than 2% of all NHLs in children younger than age 15 years, its incidence in adolescence increases to approximately 5% to 7%.26 In an earlier CCG study, Lones et al23 reported a 75% EFS in children with NHL arising in the mediastinum in which the predominance and histology was PMBL. Similarly, Burkhardt et al26 reported for the BFM, for NHL studies BFM-86, BFM-90, and BFM-95, an approximately 65% ± 8% EFS in children and adolescents with mediastinal large B-cell lymphoma. Gene expression profiles in adults with PMBL are significantly different from those of other common histologic subtypes of DLBCL.23,30–32 These findings suggest that short but intensive mature B-cell NHL therapy without radiotherapy, such as that in the FAB LMB 96 study, may not be the optimal therapy for mediastinal disease in children and adolescents. We are currently investigating the role of systemic rituximab with FAB LMB group B therapy in children with advanced mature B-cell NHL, including those with mediastinal disease.33,34
Increase in LDH (≥ 2 × ULN) was also associated with a significant decrease in EFS in children and adolescents with mature B-cell NHL treated on the FAB LMB 96 trial (RR, 2.0; P < .001). Advanced-stage disease has previously been demonstrated by several pediatric cooperative groups, including CCG, SFOP, BFM, and the Italian Association of Pediatric Hematology Oncology (AEIOP) to be associated with an inferior outcome7,9,13,14,35,36 In this most recent study, advanced stage was not an independent risk factor for relapse or progression. However, increased LDH at diagnosis as defined differently by different cooperative groups has been historically associated with an inferior outcome in children and adolescents with mature B-cell NHL.7,13,14,35 Recent studies by Woessman et al16 have demonstrated that HD-MTX (5 g/m2) over 24- versus 4-hour infusion in patients with advanced-stage disease and/or increased LDH levels at diagnosis in children and adolescents with mature B-cell NHL treated on BFM NHL 95 is superior and is associated with a 93% EFS. Similarly, the addition of rituximab to the FAB MB B4 chemotherapy backbone in children and adolescents with mature B-cell NHL with advanced-stage disease with or without increased LDH levels is safe and well-tolerated.33,34 Recently, Meinhardt et al37 reported good response rates with rituximab in children with intermediate and advanced mature B-cell NHL in a single-dose phase II window design. Randomized and prospective studies will be required to determine whether these and other strategies will significantly increase the EFS in children and adolescents with newly diagnosed advanced-stage mature B-cell NHL and/or with increased LDH levels at diagnosis.
In summary, this large and prospective FAB LMB 96 trial in children and adolescents with newly diagnosed mature B-cell NHL demonstrated that adolescent age (≥ 15 years) is not an independent risk factor for inferior outcome in either univariate or multivariate analysis. Further, increased LDH level (≥ 2 × institutional ULN), mediastinal disease, and combined BM and CNS disease at diagnosis were each independently associated with an increased risk of treatment failure in children and adolescents with mature B-cell NHL who were treated with modern, short but intensive therapy such as that in the FAB LMB 96 study. Other biologic features such as cytogenetics and/or molecular genetics and/or minimal residual disease may also be associated with an increased risk of treatment failure in children and adolescents with mature B-cell NHL.36,38 Future studies will be required to determine whether different therapeutic strategies can overcome the poor prognostic risk factors discussed herein in children and adolescents with mature B-cell NHL. The results of this analysis will hopefully form the basis of the next risk-adapted childhood and adolescent mature B-cell NHL study.
Acknowledgment
We thank the members of the Data Safety Monitoring Committee, Michael Link, MD, Alfred Reiter, MD, David Harrington, PhD, and Robert Souhami, MD, for diligent oversight of this study; Ian Waxman, MD, and Jing Fan, MS, for their help with data analysis; and Roxanne Escobedo and Erin Morris, RN, for editorial assistance.
Presented in part at the 40th Congress of the International Society of Paediatric Oncology, Berlin, Germany, October 2-6, 2008.
Footnotes
Written on behalf of the French-American-British Mature B-Cell Lymphoma 96 (FAB LMB 96) International Study Committee.
Supported by Grant No. U10 CA98543 from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, by the Cancer Research Campaign, and by the Association pour la Recherche contre le Cancer, La Ligue Nationale Contre le Cancer, Institut-Gustave-Roussy.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002757.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mitchell S. Cairo, Richard Sposto, Mary Gerrard, Anne Auperin, Ross Pinkerton, Catherine Patte
Administrative support: Mitchell S. Cairo
Provision of study materials or patients: Mitchell S. Cairo, Ross Pinkerton, Catherine Patte
Collection and assembly of data: Mitchell S. Cairo, Anne Auperin, Lauren Harrison, Ross Pinkerton, Martine Raphael, Keith McCarthy, Sherrie L. Perkins, Catherine Patte
Data analysis and interpretation: Mitchell S. Cairo, Richard Sposto, Anne Auperin, Stanton C. Goldman, Ross Pinkerton, Martine Raphael, Keith McCarthy, Sherrie L. Perkins, Catherine Patte
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Cairo MS, Raetz E, Perkins SL. Non-Hodgkin's lymphoma in children. In: Kufe DW, Bast RC, Hait WN, et al., editors. Cancer Medicine. ed 7. Hamilton, Ontario, Canada: BC Decker; 2005. pp. 1962–1976. [Google Scholar]
- 2.Perkins SL, Segal GH, Kjeldsberg CR. Classification of non-Hodgkin's lymphomas in children. Semin Diagn Pathol. 1995;12:303–313. [PubMed] [Google Scholar]
- 3.Atra A, Imeson JD, Hobson R, et al. Improved outcome in children with advanced stage B-cell non-Hodgkin's lymphoma (B-NHL): Results of the United Kingdom Children's Cancer Study Group (UKCCSG) 9002 protocol. Br J Cancer. 2000;82:1396–1402. doi: 10.1054/bjoc.1999.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman WP, Shuster JJ, Cook B, et al. Improved survival for children with B-cell acute lymphoblastic leukemia and stage IV small noncleaved-cell lymphoma: A pediatric oncology group study. J Clin Oncol. 1996;14:1252–1261. doi: 10.1200/JCO.1996.14.4.1252. [DOI] [PubMed] [Google Scholar]
- 5.Brecher ML, Schwenn MR, Coppes MJ, et al. Fractionated cylophosphamide and back to back high dose methotrexate and cytosine arabinoside improves outcome in patients with stage III high grade small non-cleaved cell lymphomas (SNCCL): A randomized trial of the Pediatric Oncology Group. Med Pediatr Oncol. 1997;29:526–533. doi: 10.1002/(sici)1096-911x(199712)29:6<526::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairo MS, Krailo MD, Morse M, et al. Long-term follow-up of short intensive multiagent chemotherapy without high-dose methotrexate (‘Orange') in children with advanced non-lymphoblastic non-Hodgkin's lymphoma: A Children's Cancer Group report. Leukemia. 2002;16:594–600. doi: 10.1038/sj.leu.2402402. [DOI] [PubMed] [Google Scholar]
- 8.Cairo MS, Sposto R, Hoover-Regan M, et al. Childhood and adolescent large-cell lymphoma (LCL): A review of the Children's Cancer Group experience. Am J Hematol. 2003;72:53–63. doi: 10.1002/ajh.10262. [DOI] [PubMed] [Google Scholar]
- 9.Cairo MS, Sposto R, Perkins SL, et al. Burkitt's and Burkitt-like lymphoma in children and adolescents: A review of the Children's Cancer Group experience. Br J Haematol. 2003;120:660–670. doi: 10.1046/j.1365-2141.2003.04134.x. [DOI] [PubMed] [Google Scholar]
- 10.Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin's lymphoma: Results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- 11.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 12.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: It is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patte C, Auperin A, Michon J, et al. The Société Française d'Oncologie Pédiatrique LMB89 protocol: Highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 14.Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94:3294–3306. [PubMed] [Google Scholar]
- 15.Spreafico F, Massimino M, Luksch R, et al. Intensive, very short-term chemotherapy for advanced Burkitt's lymphoma in children. J Clin Oncol. 2002;20:2783–2788. doi: 10.1200/JCO.2002.08.088. [DOI] [PubMed] [Google Scholar]
- 16.Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: A report of the BFM Group Study NHL-BFM95. Blood. 2005;105:948–958. doi: 10.1182/blood-2004-03-0973. [DOI] [PubMed] [Google Scholar]
- 17.Bleyer WA, O'Leary M, Barr R, et al. Bethesda, MD: National Cancer Institute; 2006. Cancer Epidemiology in Older Adolescents and Young Adults 15-29 Years of Age, Including SEER Incidence and Survival, 1975-2000. NIH publication 06-5767. [Google Scholar]
- 18.Anderson JR, Jenkin RD, Wilson JF, et al. Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin's lymphoma: A report of CCG-551 from the Children's Cancer Group. J Clin Oncol. 1993;11:1024–1032. doi: 10.1200/JCO.1993.11.6.1024. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JR, Wilson JF, Jenkin DT, et al. Childhood non-Hodgkin's lymphoma: The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2) N Engl J Med. 1983;308:559–565. doi: 10.1056/NEJM198303103081003. [DOI] [PubMed] [Google Scholar]
- 20.Cairo MS. Treatment and outcome in adolescents and young adults (AYA) with intermediate and aggressive non-Hodgkin's Lymphoma (NHL) Ann Oncol. 2005;16:v41. abstr 035. [Google Scholar]
- 21.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 22.Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: Dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- 23.Lones MA, Perkins SL, Sposto R, et al. Large-cell lymphoma arising in the mediastinum in children and adolescents is associated with an excellent outcome: A Children's Cancer Group report. J Clin Oncol. 2000;18:3845–3853. doi: 10.1200/JCO.2000.18.22.3845. [DOI] [PubMed] [Google Scholar]
- 24.Miles RR, Raphael M, McCarthy K, et al. Pediatric diffuse large B-cell lymphoma demonstrates a high proliferation index, frequent c-Myc protein expression, and a high incidence of germinal center subtype: Report of the French-American-British (FAB) International Study Group. Pediatr Blood Cancer. 2008;51:369–374. doi: 10.1002/pbc.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Percy CL, Smith MA, Linet M, et al. Lymphomas and reticuloendothelial neoplasms. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, Seer Program; 1999. pp. 35–50. [Google Scholar]
- 26.Burkhardt B, Zimmermann M, Oschlies I, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39–49. doi: 10.1111/j.1365-2141.2005.05735.x. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg J, Waxman IM, Kelly KM, et al. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: State of the science. Br J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 28.Albritton K, Bleyer WA. The management of cancer in the older adolescent. Eur J Cancer. 2003;39:2584–2599. doi: 10.1016/j.ejca.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Patte C, Bleyer WA, Cairo MS. Non-Hodgkin lymphoma. In: Bleyer A, Barr R, editors. Cancer in Adolescents and Young Adults. Heidelberg, Germany: Springer-Verlag; 2008. pp. 127–151. [Google Scholar]
- 30.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feuerhake F, Kutok JL, Monti S, et al. NFkappaB activity, function, and target-gene signatures in primary mediastinal large B-cell lymphoma and diffuse large B-cell lymphoma subtypes. Blood. 2005;106:1392–1399. doi: 10.1182/blood-2004-12-4901. [DOI] [PubMed] [Google Scholar]
- 32.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 33.Cairo MS, Lynch J, Harrison L, et al. Safety, efficacy and rituximab levels following chemoimmunotherapy (rituximab + FAB chemotherapy) in children and adolescents with mature B-cell non-Hodgkin lymphoma (B-NHL): A Children's Oncology Group report. Blood. 2008;112:838a. abstr 838. [Google Scholar]
- 34.Cairo MS, Lynch JC, Harrison L, et al. Safety, kinetics, and outcome following rituximab (R) in combination with FAB chemotherapy in children and adolescents (C+A) with stage III/IV (Group B) and BM+/CNS+ (Group C) mature B-NHL: A Children's Oncology Group Report. J Clin Oncol. 2010;28(suppl):687s. abstr 9536. [Google Scholar]
- 35.Pillon M, Di Tullio MT, Garaventa A, et al. Long-term results of the first Italian Association of Pediatric Hematology and Oncology protocol for the treatment of pediatric B-cell non-Hodgkin lymphoma (AIEOP LNH92) Cancer. 2004;101:385–394. doi: 10.1002/cncr.20382. [DOI] [PubMed] [Google Scholar]
- 36.Mussolin L, Pillon M, Conter V, et al. Prognostic role of minimal residual disease in mature B-cell acute lymphoblastic leukemia of childhood. J Clin Oncol. 2007;25:5254–5261. doi: 10.1200/JCO.2007.11.3159. [DOI] [PubMed] [Google Scholar]
- 37.Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- 38.Poirel HA, Cairo MS, Heerema NA, et al. Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin's lymphoma: Results of the FAB/LMB 96 international study. Leukemia. 2009;23:323–331. doi: 10.1038/leu.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]